Abstract
Western flower thrips (Frankliniella occidentalis) has become a key insect pest of agricultural and horticultural crops worldwide. Little is known about host plant resistance to thrips. In this study, we investigated thrips resistance in chrysanthemum (Dendranthema grandiflora). We identified thrips-resistant chrysanthemums applying bioassays. Subsequently, nuclear magnetic resonance (NMR)-based metabolomics was applied to compare the metabolome of thrips-resistant and -susceptible chrysanthemums. NMR facilitates wide-range coverage of the metabolome. We show that thrips-resistant and -susceptible chrysanthemums can be discriminated on basis of their metabolomic profiles. Thrips-resistant chrysanthemums contained higher amounts of the phenylpropanoids chlorogenic acid and feruloyl quinic acid. Both phenylpropanoids are known for their inhibitory effect on herbivores as well as pathogens. Thus, chlorogenic and feruloyl quinic acid are the compounds of choice to improve host plants resistance to thrips in ornamentals and crops. The effect of chlorogenic acid on thrips was further studied in bioassays with artificial diets. These experiments confirmed the negative effects on thrips. Our results prove NMR to be an important tool to identify different metabolites involved in herbivore resistance. It constitutes a significant advance in the study of plant-insect relationships, providing key information on the implementation of herbivore resistance breeding strategies in plants.
Western flower thrips (Frankliniella occidentalis) is a vital pest of chrysanthemum (Dendranthema grandiflora), an economically important ornamental for the Dutch horticultural industry (Mantel and van de Vrie, 1988). Thrips have piercing-sucking mouthparts, which enable them to feed on different types of plant cells (Hunter and Ullman, 1989). Western flower thrips can cause two types of feeding damage in chrysanthemum. Feeding on developing tissue leads to growth damage including distortion, reduction in plant growth, and eventually yields loss. Feeding on expanded tissue results in the characteristic silver damage, which affects product appearance and reduces market quality (de Jager et al., 1995a). Indirect damage is caused by transmission of tospoviruses (Maris et al., 2003).
Occurrence of host-plant resistance to thrips is sparse and little is known about the underlying mechanisms. Thrips preferentially feed on the older chrysanthemum leaves (de Jager et al., 1995a). Morphological plant characters were not involved in resistance to western flower thrips in chrysanthemum (de Jager et al., 1995a). Instead, resistance was influenced by the chemical composition of host plants, although until recently no specific compounds were identified (de Jager et al., 1995b, 1996). A novel isobutylamide was suggested to be associated with host-plant resistance to western flower thrips in chrysanthemum (Tsao et al., 2005). Overexpression of Cys protease inhibitors in transgenic chrysanthemum was not related with resistance to thrips (Annadana et al., 2002). In contrast, transgenic potato (Solanum tuberosum) multidomain Cys protease inhibitors were affiliated with thrips resistance (Outchkourov et al., 2004a, 2004b). Of late, two pyrrolizidine alkaloids jaconine- and jacobine-N-oxide and a flavanoid, kaempferol glucoside, were identified to be related to thrips resistance in hybrids of the wild plants Senecio jacobaea and Senecio aquaticus (Leiss et al., 2009).
However, as is generally the case in biological processes, it is very likely that not one but several compounds are involved in plant resistance, the identity of which are, a priori, unknown. The study of chemical host-plant resistance has so far, for technical reasons, been limited to the identification of single compounds. NMR spectroscopy allows the simultaneous detection of a wide range of metabolites, providing an instantaneous image of the metabolome of the resistant plant (Verpoorte et al., 2007). So far, NMR has been used to study the effect of pathogen infection on host plants such as phytoplasmas in Catharanthus roseus (Choi et al., 2004) and Tobacco mosaic virus in tobacco (Nicotiana tabacum; Choi et al., 2006). The effect of herbivores on plants has been studied with NMR spectroscopy for Plutella xylostella and Spodoptora exigua in Brassica rapa (Widarto et al., 2006) and Arabidopsis (Arany et al., 2008). We successfully applied NMR to study chemical host-plant resistance to western flower thrips in Senecio hybrids (Leiss et al., 2009).
In this study we investigated the metabolomic basis of thrips resistance in chrysanthemum. We first conducted an in vivo bioassay to identify thrips-resistant and -susceptible chrysanthemum cultivars, on which subsequently NMR-based metabolomics was applied. We then used an in vitro bioassay with artificial diets to confirm our findings.
RESULTS
Metabolomics
Identification of Metabolites by One- and Two-Dimensional NMR Spectroscopy
The signals of a number of metabolites present in the leaf extract of chrysanthemum, including amino acids, organic acids, carbohydrates, phenylpropanoids, and flavonoid glycosides were attributed in the 1H-NMR spectra (Table I). The identification of those metabolites was based on the analysis of one- and two-dimensional NMR experiments (COSY, J-resolved, HSQC, HMBC), together with the comparison of reference compounds and previously reported data (Choi et al., 2006). In particular, the two-dimensional J-resolved experiment improved the resolution of 1H-NMR spectra, allowing the attribution of the characteristic protons of phenylpropanoids in the region of phenolic signals (δ 6.0–8.5). By analysis of J-resolved and COSY spectra the signals from the two trans-olefinic protons of chlorogenic acid (H-7′ at δ 7.58, H-8′ at δ 6.34), 3-O-caffeoyl quinic acid (H-7′ at δ 7.60, H-8′ at δ 6.32), and feruloyl quinic acid (H-7′ at δ 7.62, H-8′ at δ 6.44) were clearly distinguished and attributed. In addition, two-dimensional NMR techniques allowed the assignment of the signals of a kaempferol derivative. The signals of three flavone derivatives were identified in the leaf extracts of cultivars 2 and 4 by integration of the proton signals of H-6 (δ 6.54, 6.55, and 6.56, d, J = 2), H-8 (δ 6.88, 6.89, and 6.90, d, J = 2), and H-3 (δ 6.75, 6.71, and 6.67, s). In the region of anomeric protons of carbohydrates, α-Glc and Suc were identified. The signal of Glc needs a special note, as the α-form of the molecule was present at very low concentration in the NMR spectra, while the β-Glc signal (δ 4.59, d), although the most representative in nature, was not detectable. We considered the very low amount of Glc in the extract, together with the vicinity of the residual water signal hampering the detection of β-Glc. Moreover, signals from Ala, Gln, together with those from choline, succinic acid, malic acid, and α-linolenic acid were identified in the region between 3.0 and 0.8 ppm.
Table I.
Characteristics of 1H chemical shifts (δ) and coupling constants in metabolites of chrysanthemum leaves identified using one- and two-dimensional NMR spectra
| Metabolite | Chemical Shifts (ppm) and Coupling Constant (Hz) |
|---|---|
| Ala | 1.48 (H-3, d, J = 7.3) |
| α-Linolenic acid | 0.96 (H-ω, t, J = 7.5), 1.31 (CH2, brs) |
| Gln | 2.13 (H-4, m), 2.34 (H-3, m) |
| Choline | 3.23 (s) |
| Malic acid | 2.53 (H-β, dd, J = 5.5, 15.4), 2.78 (H-β, dd, J = 3.0, 15.7), 4.28 (H-α, dd, J = 3.0, 6.5) |
| Succinic acid | 2.54 (s) |
| Suc | 4.13 (H-1′, d, J = 8.5), 5.40 (H-1, d, J = 3.6) |
| α-Glc | 5.24 (H-1, d, J = 3.7) |
| Fumaric acid | 6.52 (s) |
| Chlorogenic acid (5-O-caffeoyl quinic acid) | 6.34 (H-8′, d, J = 16.0), 6.84 (H-5′, d, J = 8.4), 7.03 (H-6′, dd, J = 8.5, 1.8), 7.13 (H-2′, d, J = 1.8), 7.58 (H-7′, d, J = 16.0) |
| 3-O-caffeoyl quinic acid | 6.32 (H-8′, d, J = 16.0), 7.60 (H-7′, d, J = 16.0) |
| 5-O-feruloyl quinic acid | 6.44 (H-8′, d, J = 16.0), 6.83 (H-5′, d, J = 8), 7.02 (H-6′, dd, J = 8.3, 1.5), 7.14 (H-2′, d, J = 1.8), 7.62 (H-7′, d, J = 16.0) |
| Kaempferol glycoside | 7.12 (H-3′ and H-5′, d, J = 8), 8.01 (H-2′ and H-6′, d, J = 8) |
| Flavone derivative | 6.54 (H-6, d, J = 2), 6.75 (H-3, s), 6.88 (H-8, d, J = 2) |
Data Reduction
Metabolomics experiments produce a large number of data. Multivariate data analysis can deal with the large number of data by reducing the dimensionality of a data set. Principal component analysis (PCA) is an unsupervised method that reduces the dimensionality of a given data set by producing new linear combinations of the original variables. PCA allows the visualization of trends, clustering, similarity/dissimilarity among the samples, thus giving a macroscopic metabolic differentiation within the data set. Previous to data reduction, raw NMR data have to be bucketed to align the signals and avoid possible fluctuations. In this study, the signals in the region between δ 0.3 and δ 10.0 were bucketed every 0.04 ppm, generating 243 variables. The generated NMR spectra were submitted to PCA analysis and thus reduced to a few principal components that explain the variation among the samples in terms of metabolic changes. A PCA model constituted by nine principal components explaining 88% of the total variance was investigated. Although not clearly separating the group of susceptible cultivars from the group of resistant lines, PCA highlighted the clustering of cultivars Oxford and Polar together, differentiating them from the rest of the samples (data not shown). According to the loading plot, the levels of chlorogenic acid, of its isomer, 3-O-caffeoyl quinic acid, and of feruloyl quinic acid were higher in the two resistant cultivars than in the susceptible ones and, to a minor extent, compared to the other resistant cultivars. The content of malic acid, succinic acid, Ala, and apigenin glycoside was instead higher in susceptible leaves samples (data not shown).
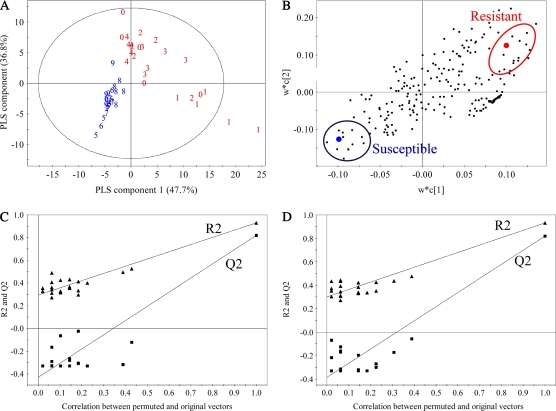
To distinguish resistant leaves from susceptible ones, the analysis was extended to partial least square-discrimination analysis (PLS-DA), a supervised multivariate data technique. In contrast to PCA that only uses the information of the metabolomic matrix, PLS-DA also takes into account the resistance matrix (Berrueta et al., 2007). PLS-DA uses a discrete class matrix (in this case 0 for the susceptible and 1 for the resistant group). The separation of PLS-DA is achieved by the covariance of the two datasets. When PLS-DA was applied the separation of thrips-resistant and -susceptible cultivars considerably improved (Fig. 1A). For the resistant group the validation of the PLS-DA model by permutation tests through 20 applications resulted in a variance R2 of the model of 0.93 and a predictive ability Q2 of the model of 0.82. For the susceptible group R2 and Q2 values were 0.94 and 0.79, respectively. Q2 values greater than 0.5 are generally accepted as good (Bailey et al., 2004). All Q2 values of the permuted Y vectors were lower than the original ones and the regression of Q2 lines intersected at below zero (Fig. 1, C and D). Intercepts below 0.05 indicate a valid model (Eriksson et al., 2001).
Figure 1.
Score (A), loading plot (B), and permutation validation (20 permutations with three components) plot for the resistant group (C) and the susceptible group (D) of PLS-DA based on 1H-NMR spectra of crude extracts from Polar (0), Oxford (1), Dublin (2), Penny Lane (3), Biarritz (4), Baykal (5), Bradford (6), Cuenca (7), Samos (8), and Super Pink Pompon (9). The PLS-DA was based on two classes of thrips-resistant (red circle) and -susceptible (blue circle) chrysanthemum lines. The ellipse in the score plot represents the Hotelling T2 with 95% confidence interval in the model (Eriksson et al., 2001). In the loading plot each dot represents the chemical shifts from bucketed 1H-NMR data. The circles evidence the chemical shifts of chlorogenic acid and feruloyl quinic acid contained in the resistant samples (on the top right) and the chemical shifts of succinic acid and malic acid contained in the susceptible samples (on the bottom left). In the validation plots R2 stands for variance and Q2 stands for predictive ability of the model. [See online article for color version of this figure.]
According to the loading plot (Fig. 1B), chlorogenic acid and feruloyl quinic acid characterized the resistant cultivars in comparison to the susceptible ones. Susceptible cultivars showed higher contents of malic and succinic acid, together with higher NMR signals from other aliphatic compounds (in the crowded region of carbohydrates, at δ 3.0–4.5).
A t test was applied to the data set to determine statistically significant signals between the resistant and susceptible groups. From 243 1H-NMR signals 81 were significant (P < 0.05). Among them, the identified signals of chlorogenic acid, 3-O-caffeoyl quinic acid, feruloyl quinic acid, Ala, and malic acid, which were identified by PLS-DA, were confirmed as significantly differentiating the two groups of samples.
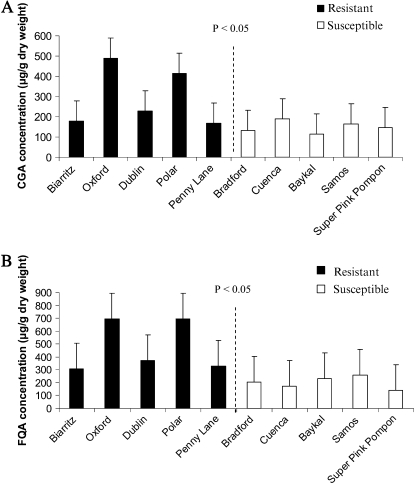
One of the advantages of applying NMR to metabolomics studies is that metabolites can be quantified based on their signal intensity relative to internal standard (IS; trimethylsilylpropionic acid [TMSP]). The content of the two discriminating metabolites, chlorogenic acid and feruloyl quinic acid, was thus determined by manual integration of known protons in the molecules. The signals of the two olefinic protons (H-7′ and H-8′) at 7.58 and 6.34 ppm for chlorogenic acid, and at 7.62 and 6.44 ppm for feruloyl quinic acid were considered for the calculations. Resistant chrysanthemum cultivars contained significantly more chlorogenic acid (F = 8.46, df = 1, P = 0.017; Fig. 2A) and feruloyl quinic acid (F = 8.031, df = 1, P = 0.020; Fig. 2B) compared to the susceptible cultivars. While there were no differences in the amount of these metabolites among the different susceptible cultivars, the resistant cultivars Oxford and Polar contained significantly more chlorogenic acid (F = 14.64, df = 4, P = 0.000) and feruloyl quinic acid (F = 43.22, df = 4, P = 0.000) compared to the other resistant cultivars. Both chlorogenic acid (r = −0.780, n = 10, P = 0.008) and feruloyl quinic acid (r = −0.791, n = 10, P = 0.006) were significantly negatively correlated with silver damage. They respectively explained 43% and 45% of the total variance in thrips resistance. The data for chlorogenic acid were confirmed by HPLC analysis following the method of Shao and Zhuang (2004) using the commercially available 5-O-caffeoyl quinic acid as standard. The extracts used for NMR analysis were thereafter used for HPLC measurement. Chlorogenic acid data measured by NMR and HPLC (data not presented) were highly conform as expressed by their significant positive correlation (r = 0.950, P ≤ 0.001, n = 10).
Figure 2.
Quantification of chlorogenic acid (CGA; A) and feruloyl quinic acid (FQA; B) calculated on the peak intensities in 1H-NMR spectra relative to an IS. Data are based on five replicates per cultivar. Means and se of the mean are presented. Data were analyzed by ANOVA. Significant differences between resistant and susceptible cultivars are designated as P ≤ 0.05.
Thrips Bioassays
In Vivo Thrips Bioassays
As expected, silver damage in chrysanthemum cultivars that were previously described as thrips resistant (mean of 3.56 ± 0.77) was significantly lower (F = 15.74, df = 1, P = 0.004) compared to cultivars that were previously described as susceptible (mean of 68.44 ± 20.18; Table II). Cultivar Polar showed the lowest silver damage (0.6 ± 0.6) within the resistant cultivars (F = 6.28, df = 4, P = 0.002) while cultivar Super Pink Pompon showed the highest silver damage (251 ± 41) within the susceptible cultivars (F = 22.44, df = 4, P = 0.000). Even when taking cultivar Super Pink Pompon out of the analysis silver damage was still significantly different between resistant and susceptible cultivars (F = 25.19, df = 1, P = 0.002). Growth damage did not differ between thrips-resistant (mean of 1.0 ± 0.3) and -susceptible (mean of 0.9 ± 0.2) chrysanthemum cultivars (F = 0.003, df = 1, P = 0.955). Number of leaves, as indicator of plant growth, was not significantly different between resistant and susceptible cultivars (F = 0.481, df = 1, P = 0.508). Resistant cultivars had on average 24.3 ± 0.9 and susceptible cultivars 23.0 ± 1.0 leaves, respectively.
Table II.
Silver damage (mm2) of thrips-resistant and -susceptible chrysanthemum cultivars
Data represent means and SEs of five replicates.
| Resistant or Susceptible | Cultivar | Silver Damage |
|---|---|---|
| Resistant | Polar | 0.6 ± 0.6 |
| Oxford | 2 ± 0.7 | |
| Dublin | 2 ± 0.9 | |
| Penny Lane | 5 ± 2 | |
| Biarritz | 7 ± 2 | |
| Susceptible | Baykal | 16 ± 3 |
| Bradford | 23 ± 5 | |
| Cuenca | 24 ± 7 | |
| Samos | 30 ± 5 | |
| Super Pink Pompon | 251 ± 41 |
In Vitro Thrips Bioassays
In two bioassays the relative growth rate of first instar thrips larvae was significantly reduced (F = 5.12, df = 2, P = 0.008 and F = 3.43, df = 2, P = 0.037) in the medium containing 5% chlorogenic acid (Fig. 3A). The mean of the relative growth rate was close to 1 (1.01 ± 0.003 and 1.04 ± 0.003), indicating no further growth. Also thrips survival was significantly reduced in both bioassays when adding 5% chlorogenic acid to the medium (χ2 = 14.32, df = 2, P = 0.0008 and χ2 = 7.26, df = 2, P = 0.026; Fig. 3B). In choice experiments first instar larvae showed a significant preference for the control over both concentrations of 1% and 5% chlorogenic (Fig. 4). This preference became significant after 1 h in the diet containing 5% chlorogenic acid and 3 h in the diet containing 1% chlorogenic acid.
Figure 3.
In vitro bioassay with first instar larvae of western flower thrips on artificial diets with 0%, 1%, and 5% chlorogenic acid (CGA). Means and se of relative growth rate (A) and survival (B) are presented. For each diet 30 larvae were tested. The bioassay was performed twice. Asterisks indicate significant differences with ** P ≤ 0.01 and * P ≤ 0.05. Exp 1, Experiment 1; Exp 2, experiment 2.
Figure 4.
In vitro choice tests with first instar larvae of western flower thrips. There was a choice between an artificial diet without chlorogenic acid (CGA) and an artificial diet containing 1% (A) and 5% (B) chlorogenic acid. For each diet 30 larvae were tested. The mean and se of the percentage larvae having made a choice for one of the diets is presented. Asterisks indicate significant differences with *** P ≤ 0.001. Choice tests were performed twice with the same result. Here only data from the first bioassay are shown.
DISCUSSION
In our study both the in vivo as well as the in vitro bioassays showed that chlorogenic acid is related to thrips resistance. Chlorogenic acid belongs to the naturally occurring phenolic compounds, which are known for their role in plant defense (Bennett and Wallsgrove, 1994). Plant phenolics are maintained in the nontoxic reduced state by antioxidants and stored in the cell vacuoles (Miles, 1999). These are ingested by thrips being cell feeders. When ingested by thrips the antioxidants are not renewed and phenolics become free to autooxidase or become oxidized by salivary oxidases as has been shown for aphids (Miles, 1999). Upon oxidation quinones are formed, which copolymerase with proteins (van Fleet, 1954).
Chlorogenic acid thus functions as chemical defense against herbivores due to its prooxidant effect. Chlorogenic acid is oxidized to chlorogenoquinone, which binds to free amino acids and proteins. This leads to a reduced bioavailability of amino acids and a decreased digestibility of dietary proteins (Felton et al., 1989, 1991). This negative effect on herbivores has primarily been shown for caterpillars in vitro (Bernays et al., 2000; Beninger et al., 2004) and in vivo (Elliger et al., 1981; Huang and Renwick, 1995; Mallikarjuna et al., 2004). In addition a harmful effect of chlorogenic acid has been shown for different leaf beetles (Fulcher et al., 1998; Ikonen, et al., 2002; Jassbi, 2003) as well as for a leafhopper (Dowd and Vega, 1996) and for aphids (Miles and Oertli, 1993). Chlorogenic acid not only affects the primary but also the secondary trophic level. The predator performance of a stinkbug was negatively affected by chlorogenic acid-fed prey (Traugott and Stamp, 1997). However, larvae of tobacco hornworm (Manduca sexta) and tobacco budworm (Heliothis virescens) only showed a modest growth reduction when fed on Phe ammonia-lyase modified tobacco lines (Eichenseer, et al., 1998; Johnson and Felton, 2001).These lines showed elevated levels of phenylpropanoids including a 6-fold increase in chlorogenic acid. However, high levels of oxidizable phenolics in foliage are not necessarily prooxidant (Johnson and Felton, 2001). The net oxidative balance depends on the predominant types of phenolics and phenolases present. These can vary substantially among plant species. In willow (Salix spp.), the response of different leaf beetles on foliar chlorogenic acid was dependant on willow species (Ikonen et al., 2001, 2002).
Miles and Oertli (1993) already suggested that a balance of reduction and oxidation, i.e. a redox system, is important in the plant defense against sucking insects. They showed that addition of the antioxidant ascorbate enhanced the negative effect of chlorogenic acid on apple aphid (Aphis pomi). Improvement of reducing activity by adding an antioxidant preserved toxicity. Initial oxidation of chlorogenic acid to chlorogenoquinone expresses toxicity, while further oxidation considerably decreases it due to formation of phenolic oligomers (Felton and Duffey, 1991), which are much less toxic and may even act as feeding stimulants. At low concentrations the phenol catechin, a feeding deterrent in rose plants, was converted to phagostimulant polymers by salivary polyphenol oxidase of rose aphid (Macrosiphum rosae; Peng and Miles, 1991). This may explain why chlorogenic acid stimulated feeding of the leaf beetle Popillia japonica at low concentrations, while it deterred feeding at high concentrations (Fulcher et al., 1998).
Phenols may not only affect the plant dietary proteins but the proteins of the herbivores as well. Chewing insects have a peritrophic membrane that prevents many organic molecules from making immediate contact with the cells lining the midgut (Bernays, 1981). Plant-sucking insects lack such a membrane and their digestive system may thus be more vulnerable to copolymerization of proteins. However, it was suggested that salivary oxidases of aphids protect the digestive tract from copolymerization by oxidation of ingested phenolics while on their way to and within the gut (Miles, 1999).
Besides the negative effect of chlorogenic acid on herbivores it also affects fungi, bacteria, and virus such as Phytophtora capsicii (Lizzi et al., 1995), Pseudomonas syringae (Niggeweg et al., 2004), and baculovirus (Hoover et al., 1998), as well as nuclear polyhedrosis virus (Felton et al., 1986). In addition, chlorogenic acid is the most widespread natural plant dietary antioxidant (Niggeweg et al., 2004). As such chlorogenic acid is thought to prevent development of cancer and cardiovascular diseases in humans (Laranjinha et al., 1994; Sawa et al., 1999).
Another phenylpropanoid, feruloyl quinic acid, closely related to chlorogenic acid, was also identified to be involved in thrips resistance in chrysanthemum. Feruloyl quinic acid is an ester of quinic and ferulic acid. The latter is a precursor of lignin conferring rigidity to cell walls (Bennett and Wallsgrove, 1994). As such it is linked to the resistance against stem borers in maize (Zea mays; Santiago et al., 2006; Mao et al., 2007) and cotton (Gossypium hirsutum; Wang et al., 2006) as well as cereal aphids (Cabrera et al., 1995; Havlickova et al., 1996) and cereal midges (Ding et al., 2000; Abdel-Aal et al., 2001). It is also involved in the resistance to fungi such as Fusarium gramineum in maize (Bily et al., 2003), Sclerotium rolfsii in chick pea (Cicer arietinum; Sarma and Singh, 2003), and Puccinia coronata in smooth bromegrass (Bromus inermis; Delgado et al., 2002). Ferulic acid, being a strong antioxidant, has shown inhibiting effects on human cancer cell lines (Kampa et al., 2003; Lee, 2005).
Our approach combining in vitro and in vivo thrips bioassays with NMR was successful to show chlorogenic acid to be involved in resistance to western flower thrips in chrysanthemum. Next to chlorogenic acid, feruloyl quinic acid was implicated with thrips resistance. Both metabolites are not only implicated with the resistance to insects but also to fungi and in the case of chlorogenic acid to bacteria and virus. This may form the basis of a multiresistance breeding program. In addition, due to the unique combination of negative effects on thrips with positive effects on human health chlorogenic and feruloyl quinic acid are the compounds of choice to improve host plant resistance. Indeed, additive genetic inheritance of chlorogenic acid has been shown (Ky et al., 1999). Besides, Niggeweg et al. (2004), for dietary purposes, engineered tomatoes (Solanum lycopersicum) with a doubled amount of chlorogenic acid, without influencing other phenylpropanoids, by overexpression of hydroxycinnamoyl-CoA quinate hydroxycinnamoyl transferase. Our results prove NMR a promising tool to identify different metabolites involved in herbivore resistance. It constitutes a significant advance in the study of plant-insect relationships, providing key information on the implementation of herbivore resistance breeding strategies in plants.
MATERIALS AND METHODS
Plant Materials
Commercially produced cuttings of five chrysanthemum cultivars resistant for silver damage (cultivars Biarritz, Oxford, Dublin, Polar, and Penny Lane) and five cultivars susceptible to silver damage (cultivars Bradford, Cuenca, Baykal, Samos, and Super Pink Pompon) derived from the Dutch chrysanthemum breeder Deliflor were used for this study. Plants were described as thrips resistant or susceptible based on an earlier choice bioassay we conducted for Deliflor on a commercial basis. Plants were potted into 11 cm diameter pots filled with equal parts of dune sand and potting soil in spring 2008. Ten replicates of each cultivar were transferred to a growth chamber (L:D, 18:6, 20°C:15°C) and grown for 6 weeks. Five replicates were used for the thrips bioassay while the other five replicates were used for the NMR metabolomics.
In Vivo Thrips Bioassay
Five 6-week-old vegetative plants of each chrysanthemum cultivar were tested in a nonchoice bioassay. Each plant was placed into individual thrips-proof cages, consisting of plastic cylinders (80 cm height, 20 cm diameter), closed on both ends with displaceable rings of thrips-proof gaze. The cages were arranged in a fully randomized design and to each cage 10 adult western flower thrips were added and left for 1 week. Thereafter, silver damage, expressed as the leaf area damaged in mm2, and growth damage, expressed as the number of leaves with distortions, was visually scored for each plant. The number of leaves for each plant was counted. Silver damage did not follow a normal distribution and was, therefore, ln transformed. Data were analyzed using a nested ANOVA (Sokal and Rohlf, 1995) with cultivars nested within susceptible and resistant chrysanthemums, respectively.
Metabolomics
Extraction of Plant Material
Five plants each of the five resistant and the five susceptible chrysanthemum cultivars were used for NMR metabolomics. Plants were grown under standard conditions, described above, for 6 weeks. From each individual plant the third leaf from below was taken for analysis. Thus five replicate leaves per cultivar were used. The plant material was immediately frozen in liquid nitrogen upon harvesting and stored at −80°C until extraction. Each sample was ground under liquid N2 and freeze dried. Freeze-dried plant material (50 mg) was transferred to a 2-mL microtube. A volume of 1.5 mL of a mixture of KH2PO4 buffer (pH 6.0) in D2O containing 0.05% trimethyl silyl propionic acid sodium salt (w/v; TMSP) and methanol-d4 (1:1) was added to the plant samples. The mixture was vortexed at room temperature for 1 min, ultrasonicated for 20 min, and centrifuged at 13,000 rpm for 15 min. An aliquot of 0.8 mL was used for NMR analysis.
NMR Analysis
NMR spectra were recorded at 25°C on a 600 MHz Bruker DMX-600 spectrometer (Bruker) operating at a proton NMR frequency of 600.13 MHz. MeOH-d4 was used as the internal lock. Each 1H-NMR spectrum consisted of 256 scans requiring 8 min and 30 s acquisition time with the following parameters: 0.12 Hz/point, pulse width of 30 (11.3 μs), and relaxation delay of 1.5 s. A presaturation sequence was used to suppress the residual water signal with low power selective irradiation at the water frequency during the recycle delay. Free induction decay was Fourier transformed with a line broadening factor of 0.3 Hz. The resulting spectra were manually phased and baseline corrected, and calibrated to the IS trimethyl silyl propionic acid sodium salt (TMSP) at 0.0 ppm using TOPSPIN (version 2.0, Bruker). Two dimensional J-resolved NMR spectra were acquired using 32 scans per 64 increments for F1 and 1,638.4 k for F2 using spectral widths of 6,009.6 Hz in F2 (chemical shift axis) and 50 Hz in F1 (spin-spin coupling constant axis). A 1.5 s relaxation delay was employed. Datasets were zero filled to 512 points in F1 and both dimensions were multiplied by sine-bell functions (spinning sideband = 0) prior to double complex Fourier transform. J-resolved spectra were tilted by 45°, symmetrized about F1, and then calibrated to TMSP, using TOPSPIN (version 2.0, Bruker). The COSY spectra were acquired with a 1.0 s relaxation delay and 6,009.6 Hz spectral width in both dimensions. The window function for the COSY spectra was Qsine (spinning sideband = 2.0). The HSQC spectra were obtained with a 1.0 s relaxation delay and 6,009.15 Hz spectral width in F2 and 164 Hz in F1. The HMBC spectra were recorded with a 1.0 s relaxation delay and 31,692.7 Hz spectral width in F2 and 164 Hz in F1. The optimized coupling constants for HSQC and HMBC were 140 and 10 Hz, respectively.
Quantification
For the quantification of chrysanthemum metabolites using NMR spectroscopy, the peak area of selected proton signals belonging to the target compounds, and the peak area of IS, TMSP, were integrated manually for all the samples. The following equation was applied for the calculations (adjusted from van Beek et al. [1993]):
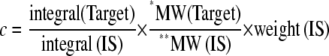 |
MW = Mr: *, divided by the number of protons involved in the target signal (Target); **, divided by the number of protons involved in the IS signal (IS). c = concentration (μg/50 mg). Weight is in micrograms.
The concentration of TMSP in each NMR tube was fixed as 1.55 μmol, from which the weight of IS (in μg) was calculated.
Data Analysis
Spectral intensities of 1H-NMR spectra were scaled to the intensity of the IS (TMSP, 0.05% [w/v]) and reduced to integrated regions of equal width (0.04) corresponding to the region of δ 0.4 to δ 10.0. The regions of δ 4.8 to δ 4.9 and δ 3.28 to δ 3.40 were excluded from the analysis because of the residual signal of water and MeOH. PCA and PLS-DA were performed with the SIMCA-P software (v. 11.0, Umetrics). The scaling method for PCA was Pareto and for PLS-DA the unit-variance method. The t test was performed by Multi Experiment Viewer (v. 4; Saeed et al., 2003). The PLS-DA model was validated using the permutation method through 20 applications, which is a default validation tool in the software package applied (SIMCA-P). Variance (R2) and cross-validated variance values (predictive ability of the model, Q2) of PLS-DA using five components were calculated.
In Vitro Thrips Bioassays
Special observation plates of clear plastic, as described by de Jager et al. (1995b) were used to study larval performance on liquid media with different chlorogenic acid concentrations. A general insect diet developed by Singh (1983) was modified, taking only the soluble ingredients, and used as liquid medium. The liquid medium was placed in small cups of a bottom plate and covered with stretched parafilm, through which thrips are able to feed (de Jager et al., 1995b). Bioassays and choice tests were performed. For the bioassays a middle plate with six wholes was placed on top of the parafilm to keep the thrips larvae separated. The middle plate was covered by a top plate. Bioassays were conducted with concentrations of 0%, 1%, and 5% chlorogenic acid added to the liquid medium. Thirty first instar larvae of western flower thrips were introduced on each medium and their length was measured. After 3 d, at the end of the first larval instar period, their length was remeasured and their relative growth rate calculated. Also the percentage of surviving larvae after 3 d was determined. For the choice tests one-half of the plates was filled with artificial diet without chlorogenic acid while the other half contained liquid medium to which chlorogenic acid in concentrations of 1% or 5% had been added. A middle plate with three arenas comprising one cup each of the different media was placed on top of the parafilm and covered by a glass plate. Sixty first instar larvae were introduced. The choice of the thrips larvae was recorded every hour up to 5 h after introduction of larvae. The in vitro tests were performed in a growth chamber under standard rearing conditions (L:D 12:12, 23°C:23°C). Each bioassay and each choice test was performed twice. A one-way ANOVA was performed to analyze relative growth rates in the bioassays, while survival was analyzed by a chi-square test. In the choice tests data were analyzed for each hour using a binomial test (Sokal and Rohlf, 1995).
Acknowledgments
We thank the Dutch chrysanthemum breeder Deliflor for providing the thrips-resistant and -susceptible chrysanthemum cultivars.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kirsten A. Leiss (k.a.leiss@biology.leidenuniv.nl).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open access articles can be viewed online without a subscription.
References
- Abdel-Aal ESM, Hucl P, Sosluski FW, Graf R, Gillot C, Pietrzak L (2001) Screening spring wheat for midge resistance in relation to ferulic acid content. J Agric Food Chem 49 3559–3566 [DOI] [PubMed] [Google Scholar]
- Annadana S, Kuiper G, Visser PB, de Kogel WJ, Udayakumar M, Jongsma MA (2002) Expression of potato multicystatin in florets of chrysanthemum and assessment of resistance to Western Flower Thrips, Frankliniella occidentalis. Acta Hortic 572 121–129 [Google Scholar]
- Arany AM, de Jong TJ, Kim HK, van Dam NM, Choi YH, Verpoorte R, van der Meijden E (2008) Glucosinulates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effect on a generalist and a specialist herbivore. Chemoecology 18 65–71 [Google Scholar]
- Bailey NJC, Wang Y, Sampson J, Davis W, Whitcombe I, Hylands PJ, Croft SL, Holmes E (2004) Prediction of anti-plasmodial activity of Artemisia annua extracts: application of 1H-NMR spectroscopy and chemometrics. J Pharm Biomed Anal 35 117–126 [DOI] [PubMed] [Google Scholar]
- Beninger CW, Abou-Zaid MM, Kistner ALE, Hallett RH, Iqbal MJ, Grodzinski B, Hall JC (2004) A flavanone and two phenolic acids from Crysanthemum morifolium with phytotoxic and insect growth regulating activity. J Chem Ecol 30 589–606 [DOI] [PubMed] [Google Scholar]
- Bennett RN, Wallsgrove RM (1994) Tansley review no. 72: secondary metabolites in plant defence mechanisms. New Phytol 127 617–633 [DOI] [PubMed] [Google Scholar]
- Bernays EA (1981) Plant tannins and insect herbivores: an appraisal. Ecol Entomol 6 353–360 [Google Scholar]
- Bernays EA, Oppenheim S, Chapman RF, Kwon H, Gould F (2000) Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: a behavioral test of the hypothesis with two closely related caterpillars. J Chem Ecol 26 547–563 [Google Scholar]
- Berrueta LA, Alonso-Salces RM, Heberger K (2007) Supervised pattern recognition in food analysis. J Chromatogr A 1158 196–214 [DOI] [PubMed] [Google Scholar]
- Bily AC, Reid LM, Taylor JH, Johnston D, Malouin C, Burt AJ, Bakan B, Regnault-Roger C, Pauls KP, Arnason JT, et al (2003) Dehydromers of ferulic acid in maize pericarp and aleurone: resistance factors to Fusarium gramineum. Phytopathology 93 712–719 [DOI] [PubMed] [Google Scholar]
- Cabrera HM, Munoz O, Zuniga GE, Corcuera LJ, Argandona VH (1995) Changes in ferulic acid and lipid content in aphid-infested barley. Phytochemistry 39 1023–1026 [Google Scholar]
- Choi YH, Casas Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JTJ, Brzin J, Zel J, Verpoorte R (2004) Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 135 2398–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Kim HK, Linthorst HJM, Hollander JG, Lefeber AWM, Erkelens C, Nuzillard JM, Verpoorte R (2006) NMR metabolomics to revisit the tobacco mosaic virus infection in Nicotiana tabacum leaves. J Nat Prod 69 742–748 [DOI] [PubMed] [Google Scholar]
- de Jager CM, Butôt RPT, Klinkhamer PGL, de Jong TJ, Wolff K, van der Meijden E (1995. a) Genetic variation in chrysanthemum for resistance to Frankliniella occidentalis. Entomol Exp Appl 77 277–287 [Google Scholar]
- de Jager CM, Butôt RPT, Klinkhamer PGL, van der Meijden E (1995. b) Chemical characteristics of chrysanthemum cause resistance to Frankliniella occidentalis (Thysanoptera: Thripidae). J Econ Entomol 88 1746–1753 [Google Scholar]
- de Jager CM, Butôt RPT, van der Meijden E, Verpoorte R (1996) The role of primary and secondary metabolites in chrysanthemum resistance to Frankliniella occidentalis. J Chem Ecol 22 1987–1999 [DOI] [PubMed] [Google Scholar]
- Delgado NJ, Casler MD, Grau CR, Jung HG (2002) Reactions of smooth bromegrass clones with divergent lignin or etherified ferulic acid concentration to three fungal pathogens. Crop Sci 42 1824–1831 [Google Scholar]
- Ding H, Lamb RJ, Ames N (2000) Inducible production of phenolic acids in wheat and antibiotic resistance to Sitodiplosis mosellana. J Chem Ecol 26 969–985 [Google Scholar]
- Dowd PF, Vega FE (1996) Enzymatic oxidation products of allelochemicals as a basis for resistance against insects: effects on the corn leafhopper Dalbulus maidis. Nat Toxins 4 85–91 [DOI] [PubMed] [Google Scholar]
- Eichenseer H, Bi JL, Felton GW (1998) Indiscrimination of Manduca sexta larvae to overexpressed and underexpressed levels of phenylalanine ammonia-lyase in tobacco leaves. Entomol Exp Appl 87 73–78 [Google Scholar]
- Elliger CA, Wong Y, Chan BG, Waiss AC, Jr (1981) Growth inhibitors in tomato (Lycopersion) to tomato fruitworm (Heliothis zea). J Chem Ecol 7 753–758 [DOI] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Wold S (2001) Multi- and Megavariate Data Analysis: Principles and Applications. Umetrics AB, Umea, Sweden
- Felton GW, Donato KK, Broadway RM, Duffey SS (1991) Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38 277–285 [Google Scholar]
- Felton GW, Donato K, Del Vecchio RJ, Duffey SS (1989) Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J Chem Ecol 15 2667–2694 [DOI] [PubMed] [Google Scholar]
- Felton GW, Duffey SS (1991) Protective action of midgut catalase in lepidopteran larvae against oxidative plant defences. J Chem Ecol 17 1715–1732 [DOI] [PubMed] [Google Scholar]
- Felton GW, Duffey SS, Vail PV, Kaya HK, Manning J (1986) Interaction of nuclear polyhedrosis virus with catechols: potential incompatibility for host-plant resistance against noctuid larvae. J Chem Ecol 13 947–957 [DOI] [PubMed] [Google Scholar]
- Fulcher AF, Ranney TG, Burton JD, Walgenbach JF, Danehower DA (1998) Role of foliar phenolics in host plant resistance of Malus taxa to adult Japanese beetle. HortScience 33 862–865 [Google Scholar]
- Havlickova H, Cvikrova M, Eder J (1996) Phenolic acids in wheat cultivars in relation to plant suitability for and response to cereal aphids. Z Pflanzenkr Pflanzenschutz 103 535–542 [Google Scholar]
- Hoover K, Alaniz SA, Yee JL, Rocke DM, Hammock BD, Duffey SS (1998) Dietary protein and chlorogenic acid effect on baculovirus disease of noctuid (Lepidoptera: Noctuidae) larvae. Environ Entomol 27 1264–1272 [Google Scholar]
- Huang XP, Renwick JAA (1995) Chemical and experimental basis for rejection of Tropaeolum majus by Pieris rapae larvae. J Chem Ecol 21 1601–1617 [DOI] [PubMed] [Google Scholar]
- Hunter WB, Ullman DE (1989) Analysis of mouthpart movements during feeding of Frankliniella occidentalis (Pergande) and F. schultzei Trybom (Thysanoptera: Thripidae). Int J Insect Morphol Embryol 18 161–171 [Google Scholar]
- Ikonen A, Tahvanainen J, Roininen H (2001) Chlorogenic acid as an antiherbivore defence of willows against leaf beetles. Entomol Exp Appl 99 47–54 [Google Scholar]
- Ikonen A, Tahvanainen J, Roininen H (2002) Phenolic secondary compounds as determinants of the host plant preferences of the leaf beetle Agelastica alni. Chemoecology 12 125–131 [Google Scholar]
- Jassbi AR (2003) Secondary metabolites as stimulants and antifeedants of Salix integra for the leaf beetle Plagiodera versicolora. Z Naturforsch [C] 58 573–579 [DOI] [PubMed] [Google Scholar]
- Johnson KS, Felton GW (2001) Plant phenolics as dietary antioxidants for herbivorous insects: a test with genetically modified tobacco. J Chem Ecol 27 2579–2597 [DOI] [PubMed] [Google Scholar]
- Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, Bakogeorgou E, Kouimtzoglou E, Blekas G, Boskou D, et al (2003) Antoproliferative and apoptotic effects or selective phenolic acids on 747D human breast cancer cells: potential mechansisms of action. Breast Cancer Res 6 R63–R74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ky CL, Louarn J, Guyot B, Charrier A, Hamon S, Noirot M (1999) Relations between and inheritance of chlorogenic acid contents in an interspecific cross between Coffea pseudozanguebariae and Coffea liberica var ‘dewevrei’. Theor Appl Genet 98 628–637 [Google Scholar]
- Laranjinha JA, Almeida LM, Madeira VM (1994) Reactivity of dietary phenolic acids with peroxyl radicals: antioxidant activity upon low density lipoprotein peroxidation. Biochem Pharmacol 48 487–494 [DOI] [PubMed] [Google Scholar]
- Lee YS (2005) Role of NADPH oxidase-mediated generation of reactive oxygen species in the mechanism of apoptosis induced by phenolic acids in HepG2 human heptoma cells. Arch Pharm Res 28 1183–1189 [DOI] [PubMed] [Google Scholar]
- Leiss KA, Choi YH, Abdel-Farid IB, Verpoorte R, Klinkhamer PGL (2009) NMR metabolomics of thrips resistance in wild Senecio hybrids. J Chem Ecol 35 219–229 [DOI] [PubMed] [Google Scholar]
- Lizzi Y, Roggero JP, Coulomb PJ (1995) Behaviour of the phenolic compounds on Capsicum annuum leaves infected with Phytophtora capsici. J Phytopathol 143 619–627 [Google Scholar]
- Mallikarjuna N, Kranthi KR, Jadhav DR, Kranthi S, Chandra S (2004) Influence of foliar chemical compounds on the development of Spodoptera litura (Fab.) in interspecific derivatives of groundnut. J Appl Entomol 128 321–328 [Google Scholar]
- Mantel WP, van de Vrie M (1988) De californische trips, Frankliniella occidentalis, een nieuwe schadelijke tripssoort in de tuinbouw onder glas in Nederland. Entomol Ber (Amst) 48 140–144 [Google Scholar]
- Mao JQ, Burt AJ, Ramputh AI, Simmonds J, Cass L, Hubbard K, Miller S, Altosaar I, Arnason JT (2007) Diverted secondary metabolism and improved resistance to european corn borer (Ostrinia nubilalis) in maize (Zea mays L.) transformed with wheat oxalate oxidase. J Agric Food Chem 55 2582–2589 [DOI] [PubMed] [Google Scholar]
- Maris PC, Joosten NN, Peters D, Goldbach RW (2003) Thrips resistance in pepper and its consequences for the acquisition and inoculation of Tomato spotted wilt virus by the Western Flower Thrips. Phytopathology 93 96–101 [DOI] [PubMed] [Google Scholar]
- Miles PW (1999) Aphid saliva. Biol Rev Camb Philos Soc 74 41–85 [Google Scholar]
- Miles PW, Oertli JJ (1993) The significance of antioxidants in the aphid-plant interaction: the redox hypothesis. Entomol Exp Appl 67 275–283 [Google Scholar]
- Niggeweg R, Michael AJ, Martin C (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol 22 746–754 [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, De Kogel WJ, Schuurman-de Bruin A, Abrahmson M, Jongsma MA (2004. a) Specific cysteine protease inhibitors act as deterrents of western flower thrips, Frankliniella occidentalis (Pergande), in transgenic potato. Plant Biotechnol J 2 439–448 [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, De Kogel WJ, Wiegers GL, Abrahmson M, Jongsma MA (2004. b) Engineered multidomain cysteine protease inhibitors yield resistance against western flower thrips (Frankliniella occidentalis) in greenhouse trials. Plant Biotechnol J 2 449–458 [DOI] [PubMed] [Google Scholar]
- Peng Z, Miles PW (1991) Oxidases in the gut of an aphid, Macrosiphym rosae (L.) and their relation to dietary phenolics. J Insect Physiol 37 779–787 [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34 374–378 [DOI] [PubMed] [Google Scholar]
- Santiago R, Butron A, Arnason JT, Reid LM, Souto XC, Malvar RA (2006) Putative role of pith cell wall phenylpropanoids in Sesamia nonagrioides (Lepidoptera: Noctuidae) resistance. J Agric Food Chem 54 2274–2279 [DOI] [PubMed] [Google Scholar]
- Sarma BK, Singh UP (2003) Ferulic acid may prevent infection of Cicer arietinum by Sclerotium rolfsii. World J Microbiol Biotechnol 19 123–127 [Google Scholar]
- Sawa T, Nakao M, Akaike T, Ono K, Maeda H (1999) Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem 47 397–402 [DOI] [PubMed] [Google Scholar]
- Shao X, Zhuang Y (2004) Determination of chlorogenic acid in plant samples by using near-infrared spectrum with wavelet transform preprocessing. Anal Sci 20 451–454 [DOI] [PubMed] [Google Scholar]
- Singh P (1983) A general purpose laboratory diet mixture for rearing insects. Insect Sci Appl 4 357–362 [Google Scholar]
- Sokal RR, Rohlf FJ (1995) Biometry, Ed 3. Freeman and Company, New York
- Traugott MS, Stamp NE (1997) Effects of chlorogenic acid-and tomatine-fed caterpillars on performance of an insect predator. Oecologia 109 265–272 [DOI] [PubMed] [Google Scholar]
- Tsao R, Marvin CH, Broadbent AB, Friesen M, Allen WR, McGarvey BD (2005) Evidence for an isobutylamide associated with host-plant resistance to western flower thrips, Frankliniella occidentalis, in chrysanthemum. J Chem Ecol 31 103–110 [DOI] [PubMed] [Google Scholar]
- van Beek TA, van Veldhuizen A, Lelyveld GP, Piron I, Lankhorst PP (1993) Quantitation of bilobalide and ginkolides A, B, C and J by means of nuclear magnetic resonance spectroscopy. Phytochem Anal 4 261–268 [Google Scholar]
- van Fleet DS (1954) The significance of the histochemical localisation of quinones in the differentiation of plant tissues. Phytomorphology 4 300–310 [Google Scholar]
- Verpoorte R, Choi YH, Kim HK (2007) NMR-based metabolomics at work in phytochemistry. Phytochem Rev 6 3–14 [Google Scholar]
- Wang Y, Cai QN, Zhang QW, Han Y (2006) Effect of secondary substances from wheat on the growth and digestive physiology of cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae). Eur J Entomol 103 255–258 [Google Scholar]
- Widarto HT, van der Meijden E, Lefeber AWM, Erkelens C, Kim HK, Choi YH, Verpoorte R (2006) Metabolomic differentiation of Brassica rapa following herbivory by different insect instars using two dimensional nuclear magnetic resonance spectroscopy. J Chem Ecol 32 2417–2428 [DOI] [PubMed] [Google Scholar]