Abstract
The galactolipid digalactosyldiacylglycerol (DGD) is an abundant thylakoid lipid in chloroplasts. The introduction of the bacterial lipid glucosylgalactosyldiacylglycerol (GGD) from Chloroflexus aurantiacus into the DGD-deficient Arabidopsis (Arabidopsis thaliana) dgd1 mutant was previously shown to result in complementation of growth, but photosynthetic efficiency was only partially restored. Here, we demonstrate that GGD accumulation in the double mutant dgd1dgd2, which is totally devoid of DGD, also complements growth at normal and high-light conditions, but photosynthetic efficiency in the GGD-containing dgd1dgd2 line remains decreased. This is attributable to an increased susceptibility of photosystem II to photodamage, resulting in reduced photosystem II accumulation already at normal light intensities. The chloroplasts of dgd1 and dgd1dgd2 show alterations in thylakoid ultrastructure, a phenotype that is restored in the GGD-containing lines. These data suggest that the strong growth retardation of the DGD-deficient lines dgd1 and dgd1dgd2 can be primarily attributed to a decreased capacity for chloroplast membrane assembly and proliferation and, to a smaller extent, to photosynthetic deficiency. During phosphate limitation, GGD increases in plastidial and extraplastidial membranes of the transgenic lines to an extent similar to that of DGD in the wild type, indicating that synthesis and transport of the bacterial lipid (GGD) and of the authentic plant lipid (DGD) are subject to the same mechanisms of regulation.
Higher plants contain two galactolipids, monogalactosyldiacylglycerol (MGD) and digalactosyldiacylglycerol (DGD), that constitute about 75 mol % of the thylakoid lipids in chloroplasts (Benson, 1971; Joyard et al., 1998; Dörmann and Benning, 2002). MGD and DGD are synthesized from UDP-Gal and diacylglycerol by MGD and DGD synthases in the chloroplast envelope membranes. While MGD is mostly produced from diacylglycerol originating from the plastid (“prokaryotic lipid”), DGD is largely derived from endoplasmic reticulum (ER) lipid precursors (“eukaryotic lipid”; Heinz and Roughan, 1983; Browse et al., 1986b). The lipid transport from the ER to the plastid is not well understood. However, recent results suggest that an ATP-binding cassette-type transport complex is involved in the transfer of eukaryotic lipids from the ER to the chloroplast (Xu et al., 2003). Galactolipids do not only establish the lipid bilayer into which the photosynthetic complexes are embedded. Structural analysis of crystallized protein complexes revealed that galactolipids are also found within the structures of PSI and PSII, light-harvesting complex II (LHCII), and cytochrome b6/f (Jordan et al., 2001; Stroebel et al., 2003; Liu et al., 2004; Loll et al., 2005; Jones, 2007). MGD and DGD play crucial roles in photosynthetic light reactions, and this is in agreement with results obtained from the analysis of galactolipid-deficient Arabidopsis (Arabidopsis thaliana) mutants (Härtel et al., 1997; Reifarth et al., 1997; Guo et al., 2005). The dgd1 mutant of Arabidopsis contains approximately one-tenth of the wild-type amount of DGD (Dörmann et al., 1995). The residual amount of DGD in dgd1 is synthesized by DGD2, and the double mutant dgd1dgd2 is totally devoid of DGD (Kelly et al., 2003). Detailed analyses of photosynthesis in the dgd1 mutant revealed that both PSI and PSII activities are affected, indicating that this lipid is crucial to maintain an optimal efficiency of photosynthetic electron flow. Thus, the strong growth retardation observed for dgd1 was suggested to be based on a shortage of photosynthetic capacity (Härtel et al., 1997).
In addition to its role in photosynthesis, DGD was shown to play an important function in cellular phosphate homeostasis. During phosphate deprivation, expression of the two DGD synthase genes DGD1 and DGD2 is induced in Arabidopsis, resulting in DGD accumulation in plastidial and extraplastidial membranes (Härtel et al., 2000; Kelly and Dörmann, 2002; Kelly et al., 2003; Jouhet et al., 2004; Andersson et al., 2005). The extra amount of DGD synthesized during phosphate limitation replaces phospholipids in the membranes and thus makes phosphate available for other important cellular processes. The extraplastidial fraction of DGD produced during phosphate limitation is believed to be predominantly synthesized by DGD2, and this DGD fraction shows a specific eukaryotic fatty acid pattern distinct from other prokaryotic or eukaryotic lipids (Härtel et al., 2000; Kelly and Dörmann, 2002). The mechanism of DGD export from the plastid to extraplastidial membranes, and the molecular basis for the selective transport of DGD, are unknown.
The role of the sugar moiety in the head group of DGD was previously studied after introducing a glucosyltransferase (GlcT) from the bacterium Chloroflexus aurantiacus involved in the synthesis of glucosylgalactosyldiacylglycerol (GGD) into the dgd1 mutant of Arabidopsis (Hölzl et al., 2006). Transgenic dgd1 plants transformed with the bacterial glucosyltransferase GlcT accumulate large amounts of GGD and show wild-type-like growth. Photosynthesis in the transformed dgd1 plants is partially complemented, indicating that the terminal Gal in the diglycosyl lipid can be replaced with Glc with no impact on growth but that Gal is the preferred sugar in glycolipids for the stabilization of the photosynthetic complexes. The transformed dgd1 plants still contain a small amount of DGD, and it remained unclear whether GGD can also functionally replace this residual DGD amount (Hölzl et al., 2006). Furthermore, the role of GGD during phosphate starvation in transgenic dgd1 plants (i.e. the question whether GGD accumulates during phosphate limitation) has not been addressed. Therefore, the Chloroflexus GlcT gene was transferred into the DGD-free dgd1dgd2 double mutant and photosynthesis was analyzed in plants grown at normal and high-light conditions. From these experiments, it became clear that the accumulation of GGD results in a complementation of growth. Furthermore, GGD measurements in transgenic plants revealed that the bacterial lipid increases upon phosphate deprivation and that it is also exported from the plastid to extraplastidial membranes.
RESULTS
Transfer of the Chloroflexus GGD Lipid into the dgd1dgd2 Mutant
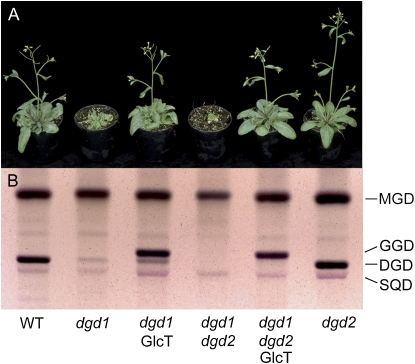
Introduction of the bacterial lipid GGD by transfer of the Chloroflexus GlcT gene into the DGD-deficient dgd1 mutant resulted in the complementation of growth deficiency, while photosynthesis was only partially rescued (Hölzl et al., 2006). The transgenic dgd1 plant accumulated GGD in amounts similar to the DGD content in the wild type, but it still contained a residual amount of DGD of about 1 mol % (Hölzl et al., 2006). To unravel the role of the residual DGD amount in photosynthesis, the bacterial glucosyltransferase GlcT was introduced into the dgd1dgd2 mutant, which is fully DGD deficient (Kelly et al., 2003). The transgenic plants (dgd1dgd2GlcT) grow similarly to dgd1GlcT and wild-type plants (Fig. 1A). They accumulate about 20 mol % of GGD, which is comparable to the amounts of the sum of GGD and DGD in dgd1GlcT and of DGD in the wild type (Figs. 1B and 2). Therefore, growth deficiency of the DGD-free line dgd1dgd2 can be compensated by transfer of the GGD lipid into this plant.
Figure 1.
Transfer of the Chloroflexus GGD lipid into the Arabidopsis dgd1dgd2 mutant results in growth complementation. A, Growth of the wild type (WT) and dgd1, dgd2, and dgd1dgd2 mutants and of lines transformed with the Chloroflexus GlcT gene. Plants were grown under normal light (120 μmol m−2 s−1) for 5 weeks. B, Glycolipid composition of Arabidopsis galactolipid mutants carrying the Chloroflexus GlcT gene. Glycolipids were separated by TLC and stained with α-naphthol.
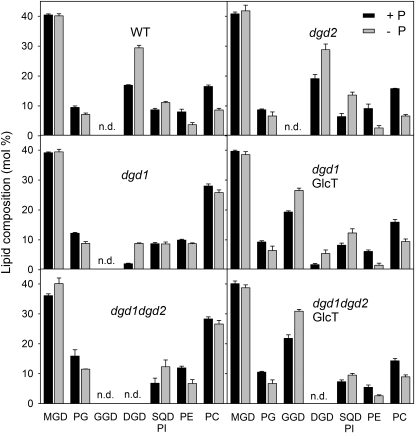
Figure 2.
Membrane lipid composition of DGD-deficient mutants carrying the Chloroflexus GlcT gene. Lipids were isolated from leaves of plants grown in the presence or absence of phosphate (+P, black bars; –P, gray bars) and separated by TLC. After transmethylation, fatty acid methyl esters were quantified by GC. SQD and phosphatidylinositol (PI) were not separated. The bars show lipid composition in mol % and indicate means ± sd of three measurements. n.d., Nondetectable (below 1 mol %); PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; WT, wild type.
GGD Accumulates in Transgenic Plants during Normal and Phosphate-Limiting Conditions
DGD was previously shown to play an important role in the replacement of phospholipids during growth under phosphate limitation (Härtel et al., 2000). The accumulation of DGD is mediated via induction of DGD1 and DGD2 expression (Kelly et al., 2003). As a consequence, the increase of DGD during phosphate limitation is attenuated in the dgd1 and dgd2 mutants (Fig. 2; Kelly et al., 2003). To study the impact of phosphate deprivation on GGD accumulation in transgenic GlcT lines, membrane lipids were isolated and measured in plants grown with or without phosphate. Figure 2 shows that GGD is strongly increased in lines dgd1GlcT and dgd1dgd2GlcT when the plants are grown without phosphate. The amount of total diglycosyl lipids in mutant and transgenic lines is similar. For example, the GGD content in dgd1dgd2GlcT plants, and the sum of GGD and DGD in the dgd1GlcT line, are very similar to the amount of DGD in the wild type, and the extra amount of diglycosyl lipid synthesized during phosphate deprivation is also in the same range in all lines analyzed. Therefore, the entire diglycosyl lipid pool (DGD in the wild type and DGD + GGD in GlcT lines) seems to be subject to the same mechanism of regulation under full phosphate and phosphate-deficient conditions.
Table I shows the fatty acid composition of the glycolipids MGD, DGD, and GGD in mutant and transgenic lines. Previous studies showed that the extra amount of DGD synthesized during phosphate deprivation was enriched in 16:0, and this was explained by the fact that this lipid is in part produced via the DGD2 pathway, mostly based on eukaryotic diacylglycerol produced at the ER (Härtel et al., 2000; Kelly et al., 2003). In agreement with this hypothesis, DGD of the dgd1 mutant is particularly high in 16:0, while DGD in dgd2 contains lower proportions of 16:0 (Table I; Kelly et al., 2003). The high 16:3 content of GGD in the GlcT lines grown under normal (+inorganic phosphate) conditions indicates that this lipid is partially derived from prokaryotic MGD, in contrast to DGD, which is mostly of eukaryotic origin (Table I). The amount of 16:0 in GGD of dgd1GlcT and dgd1dgd2GlcT is very low and hardly affected by phosphate limitation (Table I). Therefore, the extra amount of GGD synthesized during phosphate deprivation is different from the 16:0-rich MGD pool employed for DGD synthesis under phosphate limitation.
Table I.
Fatty acid composition of glycolipids in Arabidopsis galactolipid mutants expressing Chloroflexus GlcT
Fatty acid composition of glycolipids was determined by TLC and GC of fatty acid methyl esters. Values are given in mol % and indicate means of three measurements. sd was always below 2.5 mol %. n.d., Not detected (below 0.5%). Note that the lines dgd1dgd2 and dgd1dgd2GlcT do not contain DGD, while the wild type, dgd1, and dgd2 are devoid of GGD.
| Fatty Acid | MGD
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild Type
|
dgd1
|
dgd1GlcT
|
dgd1dgd2
|
dgd1dgd2GlcT
|
dgd2
|
|||||||
| +P | −P | +P | −P | +P | −P | +P | −P | +P | −P | +P | −P | |
| 16:0 | 1.3 | 2.7 | 5.7a | 10.2a | 4.2a | 7.7a | 3.6a | 10.9a | 3.6a | 6.8a | 1.5 | 3.7 |
| 16:1 | 1.0 | 0.6 | 0.6 | n.d. | 0.9 | 0.9 | 0.7 | 0.9 | n.d. | n.d. | n.d. | 0.5 |
| 16:3 | 33.7 | 31.7 | 7.7a | 3.4a | 15.4a | 12.0a | 13.4a | 2.6a | 14.1a | 10.3a | 32.4a | 25.8a |
| 18:0 | n.d. | n.d. | n.d. | 0.6 | n.d. | n.d. | n.d. | 0.7 | 0.5 | 0.5 | n.d. | n.d. |
| 18:1 | n.d. | 0.5 | 0.5 | 0.7 | 0.9 | 0.8 | n.d. | 1.1 | 0.5 | 0.8 | n.d. | 0.9 |
| 18:2 | 2.5 | 2.8 | 2.3 | 1.6 | 3.5 | 3.2 | 1.1 | 3.8 | 2.3a | 4.4a | 2.4a | 3.5a |
| 18:3 | 61.1 | 62.2 | 81.8 | 83.5 | 73.5 | 74.6 | 80.5 | 79.9 | 78.6 | 76.8 | 62.5 | 64.9 |
| Fatty Acid | DGD
|
GGD
|
||||||||||
| Wild Type
|
dgd1
|
dgd1GlcT
|
dgd2
|
dgd1GlcT
|
dgd1dgd2GlcT
|
|||||||
| +P | −P | +P | −P | +P | −P | +P | −P | +P | −P | +P | −P | |
| 16:0 | 13.0a | 24.6a | 24.6a | 33.3a | 22.8a | 29.3a | 10.6a | 13.2a | 3.9 | 7.6 | 2.8a | 4.8a |
| 16:1 | n.d. | 1.5 | 3.1 | n.d. | 1.7 | 1.0 | 0.7 | 0.6 | 0.8 | 1.0 | n.d. | 0.5 |
| 16:3 | 3.7 | 2.0 | 2.6 | 0.5 | 4.6 | 1.9 | 2.3 | 2.4 | 10.7 | 8.4 | 8.1 | 11.5 |
| 18:0 | 1.2 | 1.0 | 2.5 | 3.9 | 3.3 | 1.7 | 0.9 | n.d. | 0.5 | n.d. | n.d. | 0.5 |
| 18:1 | 1.6 | 0.9 | 2.7 | 0.5 | 1.8 | 2.1 | 0.8 | 1.0 | 0.7 | 0.6 | n.d. | n.d. |
| 18:2 | 5.3a | 6.9a | 8.8 | 7.9 | 9.7 | 9.3 | 4.5a | 5.4a | 2.7 | 3.2 | 2.0 | 2.2 |
| 18:3 | 75.1a | 62.2a | 54.0 | 53.8 | 54.4 | 54.5 | 79.7 | 77.1 | 79.6 | 78.3 | 86.1 | 80.1 |
Values greater than 2 mol % and significantly different between +P and –P according to Student's t test (P < 0.05).
During Phosphate Limitation, GGD Accumulates in Plastidial and Extraplastidial Membranes
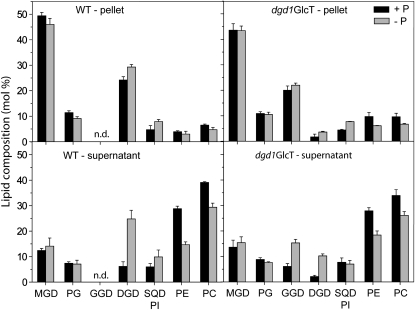
Under phosphate-limiting conditions, DGD also accumulates in extraplastidial membranes, including the plasma membrane, tonoplast, and mitochondrion (Härtel et al., 2000; Jouhet et al., 2004; Andersson et al., 2005). To analyze the subcellular localization of GGD synthesized during phosphate deprivation in dgd1GlcT plants, leaf extracts were separated by centrifugation into two fractions enriched in plastids (pellet) and extraplastidial membranes (supernatant) and employed for lipid analysis (Härtel et al., 2000). Figure 3 shows the contents of the two diglycosyl lipids, DGD and GGD. MGD and phosphatidylethanolamine represent lipids specific for the chloroplast and extraplastidial lipids, respectively. The ratio of the diglycosyl lipid to the plastidial marker lipid MGD can be taken as a measure for the increase of galactolipid during phosphate limitation. The ratio of DGD to MGD increases in the wild type from 0.49 to 0.64 and from 0.50 to 1.76 in plastidial and extraplastidial fractions, respectively. Due to the mutation in DGD synthesis, the amount of DGD is strongly reduced in dgd1GlcT. The ratio of DGD to MGD increases from 0.04 to 0.08 in the pellet fraction of dgd1GlcT (Fig. 3). In the supernatant, this value increases from 0.15 to 0.66, which can be attributed to the activity of the DGD2 enzyme (Härtel et al., 2000). Interestingly, the GGD-MGD ratio in line dgd1GlcT increases from 0.46 to 0.51 in the pellet and from 0.45 to 0.99 in the supernatant fraction. Therefore, similar to DGD, synthesis of the bacterial diglycosyl lipid GGD is increased in transgenic Arabidopsis plants, and GGD is transferred to the thylakoids and exported to extraplastidial membranes during phosphate limitation.
Figure 3.
Glycolipid export from the chloroplast during phosphate deprivation. Leaf extracts from plants grown with (+P) or without (−P) phosphate were separated into fractions enriched in plastidial (pellet) or extraplastidial membranes (supernatant). Lipids were separated by TLC and quantified by GC of fatty acid methyl esters. While MGD, a typical chloroplast lipid, is enriched in the pellet fraction, phosphatidylethanolamine (PE), an extraplastidial phospholipid, accumulates in the supernatant fraction. SQD and phosphatidylinositol (PI) were not separated. Data are means ± sd of three measurements. PC, Phosphatidylcholine; PG, phosphatidylglycerol; WT, wild type.
Introduction of GGD Results in Complementation of Altered Chloroplast Ultrastructure in DGD-Deficient Lines
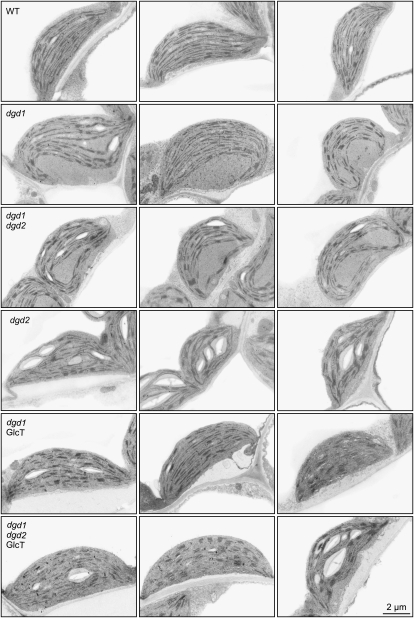
Electron microscopy revealed that the decrease in DGD results in alteration of dgd1 chloroplast ultrastructure (Dörmann et al., 1995). The thylakoids are bent, and large thylakoid-free areas can be observed in the stroma. To study the effect of changes in diglycosyl lipid composition in dgd1dgd2 and GlcT-expressing lines on chloroplast membrane ultrastructure, cross-sections of mesophyll cells were analyzed by transmission electron microscopy (Fig. 4). Representative chloroplasts of dgd1dgd2 are very similar to those of dgd1. Thylakoids of dgd1 (five of six chloroplasts analyzed) and of dgd1dgd2 (12 of 12 analyzed) are highly curved with large thylakoid-free areas in the stroma (Fig. 4; Dörmann et al., 1995). Therefore, the more severe reduction of DGD in dgd1dgd2 as compared with dgd1 has no additional consequences for chloroplast ultrastructure. The dgd2 mutant, which has a DGD content similar to the wild type, contains no dgd1-like chloroplasts (no chloroplasts with alterations of seven analyzed), similar to the wild type (none of 10 analyzed; Fig. 4). Chloroplasts of the lines dgd1GlcT and dgd1dgd2GlcT resemble wild-type chloroplasts because they have few dgd1-like thylakoid changes (six of 21 and one of seven chloroplasts analyzed for dgd1GlcT and dgd1dgd2GlcT, respectively; Fig. 4). Therefore, introduction of GGD results in complementation of growth and largely restores alterations of thylakoid structure in DGD-deficient lines.
Figure 4.
Ultrastructures of chloroplasts from DGD-deficient and GlcT-expressing lines. Representative leaf mesophyll chloroplasts (three each) of the lines wild type (WT), dgd1, dgd1dgd2, dgd2, dgd1GlcT, and dgd1dgd2GlcT are shown. Chloroplasts of DGD-deficient lines (dgd1 and dgd1dgd2) are characterized by bent thylakoids and large, thylakoid-free stroma areas. Introduction of the GlcT gene results in complementation of the alterations in thylakoid ultrastructure similar to chloroplasts from the wild-type or the dgd2 line. Bar = 2 μm.
DGD Is Required for PSII Integrity and Stability
The amount of chlorophyll in the mutant leaves and the maximal quantum efficiency of dark-adapted PSII were determined to study the impact of DGD deficiency and GGD accumulation on photosynthesis (Table II). Although the replacement of DGD by GGD leads to a full complementation of the growth deficiency of dgd1, neither the 25% reduction in chlorophyll content (per leaf area) nor the reduced photosynthetic efficiency observed in dgd1 was restored in dgd1GlcT plants (Fig. 5; Table II; Hölzl et al., 2006). Complete loss of DGD in dgd1dgd2 resulted in a 50% reduction in leaf chlorophyll content, which was partially compensated by GGD. The introduction of GGD into the double mutant dgd1dgd2 also led to a partial recovery of maximal PSII efficiency.
Table II.
Chlorophyll content, chlorophyll a/b ratio, and maximal PSII quantum efficiency of wild-type Arabidopsis and galactolipid mutants
| Variable | Wild Type | dgd1 | dgd1GlcT | dgd1dgd2 | dgd1dgd2GlcT |
|---|---|---|---|---|---|
| Chlorophyll (mg m−2) | 342.2 ± 14.9 | 259.2 ± 12.9 | 255.2 ± 19.9 | 178.4 ± 31.7 | 225.2 ± 6.5 |
| Chlorophyll a/b | 3.68 ± 0.07 | 3.12 ± 0.05 | 3.25 ± 0.07 | 2.92 ± 0.10 | 3.17 ± 0.17 |
| Maximal PSII quantum efficiencya | 0.81 ± 0.01 | 0.74 ± 0.01 | 0.72 ± 0.01 | 0.63 ± 0.02 | 0.68 ± 0.05 |
The maximal PSII quantum efficiency was determined after 15 min of dark adaptation of leaves, so that qE-type quenching was completely relaxed.
Figure 5.
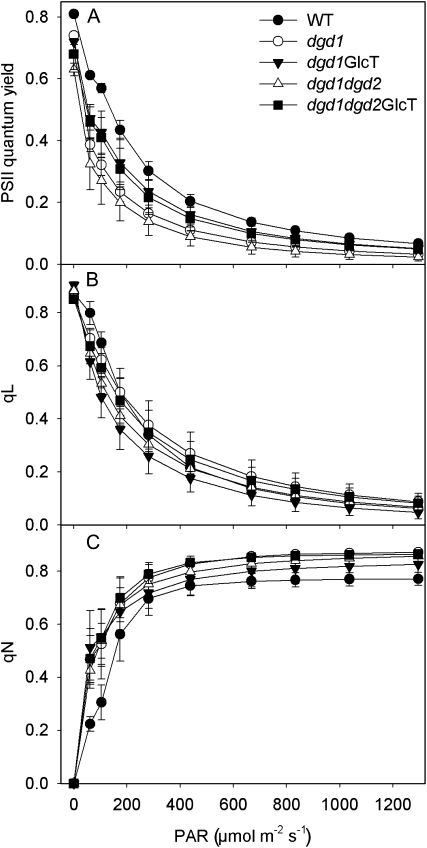
Light-response curves of chlorophyll a fluorescence parameters of Arabidopsis wild type (WT) and galactolipid mutants grown at normal light (120 μmol m−2 s−1) for 35 d. Plants were dark adapted for 15 min prior to the measurements and exposed for 3 min to each light intensity. A, Light-response curves of PSII quantum yield, showing the increase in PSII photosynthetic efficiency of the two GlcT-complemented lines relative to dgd1 and dgd1dgd2. B, Light-response curves of qL, a measure for the redox state of the PSII acceptor site. C, Light-response curves of nonphotochemical quenching (qN). PAR, Photosynthetic active radiation.
The reduced amount of chlorophyll per leaf area in GGD-containing dgd1 and dgd1dgd2 lines presumably is caused by the presence of chloroplasts with a lower number of thylakoid membranes, as observed by electron microscopy (Fig. 4). The additional decrease of DGD in the double mutant dgd1dgd2 (white triangles in Fig. 5) as compared with dgd1 (white circles) results in a further decrease in PSII quantum efficiency (Fig. 5A; Table II; Kelly et al., 2003). The accumulation of GGD in line dgd1dgd2GlcT (black squares) leads to a partial complementation of PSII quantum efficiency to a level significantly above that of dgd1dgd2 (white triangles) and only slightly lower as compared with dgd1GlcT (black inverted triangles).
The reduced photochemical efficiency of PSII in the DGD-deficient mutants indicates chronic PSII photoinhibition at normal light intensities. This might be due to higher rates of photodamage in the plants, either as a consequence of impaired photoprotective mechanisms such as thermal dissipation of excess excitation energy in the PSII antenna bed (qE) or as a consequence of higher intrinsic rates of PSII (e.g. D1 protein) photodamage. Furthermore, the PSII repair cycle might also be compromised in the mutants. To discriminate between these three scenarios, we first measured light-response curves of qL and qN, chlorophyll a fluorescence parameters indicative of the redox state of the PSII acceptor site and of the capacity of total nonphotochemical quenching, respectively (Baker et al., 2007). Plants were dark adapted for 15 min prior to the measurements, so that the rapidly reversible qE component of nonphotochemical quenching was relaxed completely (see below).
PSII photoinhibition is often triggered by an overreduction of the PSII acceptor site due to insufficient reoxidation rates of QA and of the mobile redox carrier plastoquinol (QB) by linear electron flux. Overreduction of the PSII acceptor site increases charge recombination within PSII, giving rise to the production of singlet oxygen and resulting in oxidative destruction, especially of the D1 reaction center protein but also of other PSII subunits (Aro et al., 2005; Krieger-Liszkay, 2005). The light-response curves of qL (Fig. 5B) clearly argue against a scenario that a massive impairment of the oxidation of the PSII acceptor site by linear electron transport is causal for the increased PSII photoinhibition, as only minor differences between the wild type and galactolipid mutants were observed. Interestingly, nonphotochemical dissipation rates are increased in all transformants at all light intensities, relative to the wild type (Fig. 5C). qN is the sum of the rapidly reversible qE component of thermal excitation dissipation in the PSII antenna bed, which completely decays within 15 min in darkness, and the slowly reversible qI component associated with PSII photoinhibition and its repair, which decays with a half-time of more than 40 min (Krause and Weis, 1989). Therefore, to determine the individual components of qN, qE, and qI, we monitored the decay kinetics of qN during 15 min after different high-light treatments (Fig. 6). A third component contributing to qN, qT, can be neglected here, as it is related to “state transitions” only occurring under low-light conditions (Lemeille et al., 2009). Plants were subjected to high-light stress (1,000 μmol m−2 s−1) for 10 and 60 min. After a 10-min exposure to high light, total qN was clearly higher in all mutants than in the wild type (0.74 in the wild type to 0.82 or higher in the mutants; Fig. 6A). Furthermore, the contribution of qI to total qN was increased in all mutants already after 10 min in high light. This effect was most pronounced in the dgd1dgd2 double mutant. As the slow PSII repair cycle with a half-time of 40 min does not play a role in the partitioning of qN into qI and qE during this short-term high-light experiment, we conclude that the rate of photodamage to PSII is increased in the mutants.
Figure 6.
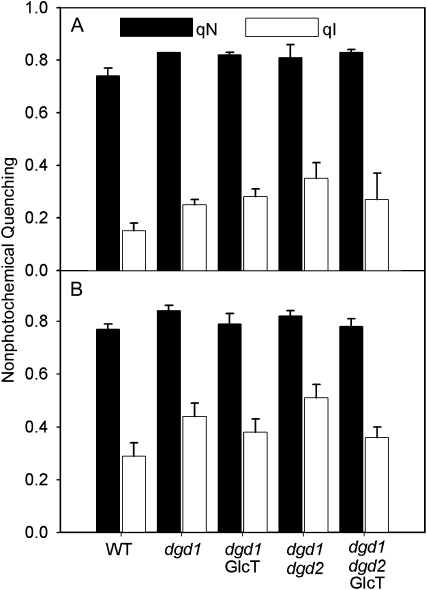
Contribution of photoinhibition-related nonphotochemical quenching (qI) to total nonphotochemical quenching (qN) after high-light stress. Plants were grown under normal light conditions (120 μmol m−2 s−1) and dark adapted for 15 min prior to the experiment. The plants were then exposed to the high-light stress (1,000 μmol m−2 s−1) for 10 min (A) or 60 min (B). After the end of high-light stress, total qN was measured (black bars). After an additional dark exposure of 15 min, the rapidly reversible qE-type component of qN relaxes completely, so that the residual proportion of qN remaining after 15 min in darkness is attributable to the PSII photoinhibition qI (white bars). WT, Wild type.
Next, we increased the duration of the high-light exposure to 60 min (Fig. 6B). After 60 min of high-light treatment, total qN is not significantly different from the values after 10 min of light stress. However, the contribution of qI to total qN is further increased in all plants. All mutants have significantly higher contributions of qI to total qN than the wild type. Furthermore, qI is slightly higher in dgd1 than in dgd1GlcT and much higher in dgd1dgd2 than in dgd1dgd2GlcT, clearly showing that complementation of the DGD-deficient plants with GGD partly alleviates PSII photoinhibition. This observation is also in line with the more pronounced reduction in maximal PSII quantum efficiency in the dgd1dgd2 double mutant as compared with dgd1dgd2GlcT (Table II; Fig. 5A).
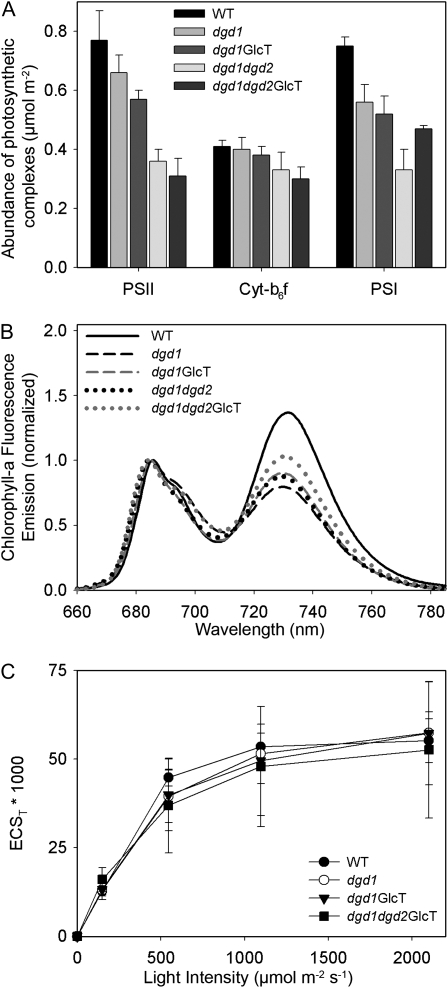
To study the effects of the increased sensitivity of PSII to photodamage on photosynthetic complex accumulation, we isolated thylakoids from wild-type and mutant plants after growth at normal light conditions (Fig. 7A). The contents of photosynthetic complexes were determined spectroscopically on a chlorophyll basis and then recalculated on a leaf area basis. In line with the increased sensitivity of PSII to light stress and with the reduced maximal PSII efficiency, the accumulation of PSII is reduced in all mutants. Interestingly, while the GlcT-complemented lines were less sensitive to photoinhibition, the PSII contents per leaf area were more reduced in these plants than in the dgd1 and dgd1dgd2 mutants. This is most likely a consequence of improved plant growth and increased leaf area in the GGD-accumulating mutants, resulting in a dilution effect on PSII content per leaf area. The accumulation of the cytochrome b6f complex is very similar in dgd1 and dgd1GlcT. Only the double knockout and the dgd1dgd2GlcT-complemented lines display slightly reduced amounts of cytochrome b6f. Similar to PSII, PSI accumulation per leaf area is affected in all mutants, suggesting that PSI biogenesis or its stability requires DGD. The loss of the amounts of photosystems per leaf area is more pronounced than the total loss in chlorophyll per leaf area, especially in dgd1dgd2 and dgd1dgd2GlcT, suggesting that LHC abundance should be increased, relative to the reaction centers. The observed reductions in the chlorophyll a/b ratios in all mutants are well in line with this conclusion (Table II), as the LHC proteins contain considerably more chlorophyll b than the reaction centers.
Figure 7.
Composition of the components of the photosynthetic electron transport chain (A), antenna organization (B), and thylakoid membrane energization (C) in Arabidopsis wild type (WT) and DGD-deficient mutants. Plants were grown under normal light conditions (120 μmol m−2 s−1). A, The contents of PSII, the cytochrome b6f complex, and PSI were determined by difference absorption spectroscopy in isolated thylakoids and then normalized to the leaf area. B, 77K chlorophyll a fluorescence emission spectra were normalized to the emission maximum of PSII-LHCII. The shifts in the emission maxima of PSII-LHCII (around 685-nm wavelength) as well as of the emission maximum of PSI-LHCI (around 730-nm wavelength) are indicative for the presence of uncoupled light-harvesting complexes in the DGD-deficient mutants. C, The generation of the proton motive force across the thylakoid membrane, measured as the amplitude of the total electrochromic absorption shift (ECST), is unaffected by DGD deficiency.
Furthermore, 77K chlorophyll a fluorescence emission measurements show that the LHC proteins are partly uncoupled from the two reaction centers (Fig. 7B): the spectra were normalized to the fluorescence emission maximum of PSII-LHCII. The emission maximum in the wild type was at 686 nm, while in the mutants, it was slightly shifted toward 684 nm. This shift is indicative for the presence of a small amount of LHCII complexes excitonically uncoupled from PSII, well in line with the strong reduction of PSII reaction centers. For the PSI emission maximum, a similar shift is visible: while the wild-type signal peaks at 732 nm, the emission maximum of the mutants is shifted to lower wavelengths (730–729 nm), indicating the presence of LHCI proteins that are either weakly or not at all coupled to PSI reaction centers, an effect that is usually observed in plants suffering from a decrease in PSI reaction centers (Stöckel et al., 2006) that has previously been reported for the dgd1 mutant as well (Ivanov et al., 2006).
Finally, we studied whether the decrease in DGD has an impact on thylakoid membrane energization. This can be measured by means of the electrochromic absorption shift, which is strictly proportional to the electrochemical component of the proton motive force across the thylakoid membrane (Fig. 7C). By measuring the dark-interval relaxation of the proton motive force, during which the ΔpH component is also transiently converted into an inverted electrochemical field across the membrane, the total amplitude of the proton motive force can be determined. It had previously been shown that MGD deficiency in the mgd1 mutant strongly reduces thylakoid membrane energization, especially at saturating light (Aronsson et al., 2008). Due to its small leaf size, we could not measure the electrochromic absorption shift signal on the dgd1dgd2 double mutant. However, unlike the situation in the mgd1 mutant, we did not observe any significant differences between the wild type and the DGD-deficient mutants, indicating that DGD is not required for the tight electrochemical coupling of the thylakoid membrane and that it can be replaced with GGD.
Photosynthesis and Growth at High Light
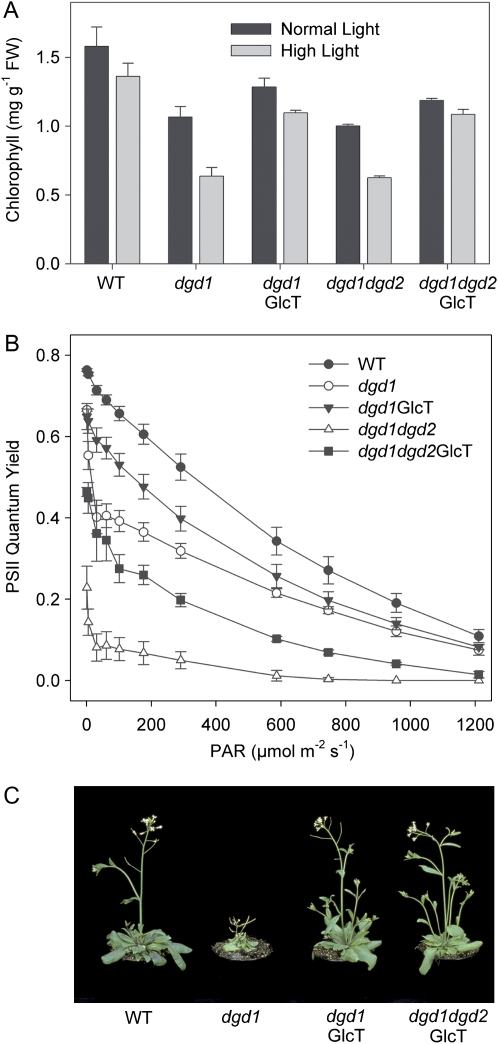
As short-term high-light stress resulted in a strong increase in photodamage in the mutants, we investigated the effect of long-term high-light exposure on plant growth and photosynthesis. When plants were grown at high light (600 μmol m−2 s−1) for 4 d, the differences in chlorophyll content between the wild type and the galactolipid-deficient lines became more pronounced. Introduction of GGD into galactolipid mutants (lines dgd1GlcT and dgd1dgd2GlcT) resulted in partial complementation of chlorophyll content at high light (Fig. 8A). Chlorophyll fluorescence measurements of high-light-grown plants revealed that the DGD-free mutants dgd1dgd2 (white triangles) and dgd1dgd2GlcT (black squares) were much more sensitive to high light and suffered from a severe decrease in PSII quantum efficiency (Fig. 8B). Thus, the partial compensatory effect of GGD observed in dgd1dgd2GlcT (black squares) grown under normal light (Fig. 5A) was strongly compromised when the mutants were shifted to high light, with dgd1dgd2GlcT performing worse than the dgd1 single mutant. To study the effect of long-term high-light exposure on growth, plants were grown at normal light for 2 weeks after germination, followed by high-light exposure (600 μmol m−2 s−1) for another 3 weeks. After 3 weeks at high light, growth of the GlcT-complemented lines was similar as compared with the wild type (Fig. 8C). Therefore, complementation of growth in GlcT-expressing lines was independent of light intensity. Despite its better photosynthetic performance relative to dgd1dgd2GlcT, the growth of dgd1 was much more compromised than that of the two GlcT-complemented lines, indicating that PSII efficiency is not limiting for growth of the DGD-deficient mutants dgd1 and dgd1dgd2 (Fig. 8A). This suggests that growth retardation of dgd1 is not primarily caused by defects in photosynthesis.
Figure 8.
Long-term high-light exposure does not impair growth while photosynthesis is affected in GlcT-complemented transformants. A, Chlorophyll a + b content of mutant plants exposed to high light (600 μmol m−2 s−1) for 4 d is reduced. B, PSII quantum efficiency is strongly decreased in DGD-deficient plants exposed to high light (600 μmol m−2 s−1) for 4 d. C, Growth at high light (3 weeks at 600 μmol m−2 s−1) of the wild type and DGD-deficient lines complemented with the GlcT gene. FW, Fresh weight; PAR, photosynthetic active radiation; WT, wild type.
DISCUSSION
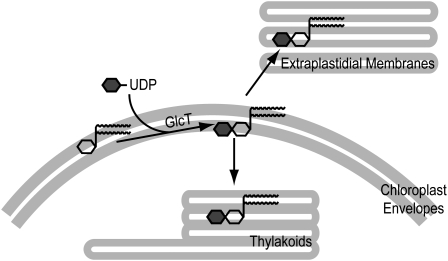
Phosphate deprivation results in the most severe alterations of membrane lipid composition in plant cells with an increase of the two glycolipids sulfoquinovosyldiacylglycerol (SQD) and DGD, accompanied by a decrease in the proportions of phospholipids (Essigmann et al., 1998; Härtel et al., 2000). The accumulation of GGD in transgenic plants during phosphate limitation suggests that the bacterial lipid can functionally replace DGD as a substitute for phospholipids. Expression of the Chloroflexus GlcT gene in transgenic plants was under the control of the strong constitutive cauliflower mosaic virus promoter. Therefore, GlcT expression is constitutive but does not increase during phosphate deprivation. In contrast, DGD1 and DGD2 are expressed under the control of endogenous, phosphate-dependent promoters (Kelly et al., 2003; Hölzl et al., 2006). Therefore, due to the high GlcT expression level in transgenic plants, GlcT activity is not limiting for the production of GGD during phosphate limitation. The regulation of GGD content in GlcT lines, and of DGD in the wild type, seems to be subject to the same control mechanisms that result in maintaining a constant amount of diglycosyl lipids in all lines in the range of 15 to 20 mol % under normal conditions and of 25 to 30 mol % under phosphate-deficient conditions. It is interesting that overexpression of the DGD synthase cDNAs DGD1 and DGD2 (cauliflower mosaic virus 35S promoter) in Arabidopsis did not result in any further DGD increase (data not shown). GGD synthesis in transgenic lines is localized to the chloroplast envelope membranes, similar to DGD synthesis in the wild type (Hölzl et al., 2006). During phosphate limitation, GGD and DGD are transported from the envelope to the thylakoids and extraplastidial membranes (Fig. 9). Therefore, the transport systems do not discriminate between the two diglycosyl lipids.
Figure 9.
Diglycosyldiacylglycerol synthesis and transport in DGD-deficient and GlcT-expressing lines. Synthesis of DGD and GGD in the wild type and GlcT-expressing lines, respectively, is localized to the envelope membranes. During phosphate deprivation, DGD and GGD accumulate in the chloroplast membranes. Furthermore, DGD and GGD are exported to extraplastidial membranes. Glc and Gal are depicted as gray and white hexagons, respectively, and the diacylglycerol moiety of galactolipids is depicted as a vertical line with two wavy horizontal lines.
While growth was fully complemented after introduction of the GGD lipid into DGD-deficient lines, the deficiency in photosynthetic efficiency was only partially rescued. Replacement of DGD with GGD in transgenic GlcT plants did not result in full complementation of maximal PSII quantum efficiency, indicating that DGD is required for optimal PSII function (Fig. 5A). It also did not result in recovery of PSII accumulation (Fig. 7A). X-ray analysis of crystallized pigment-protein complexes revealed that DGD is an intrinsic component of two complexes, LHCII and PSII (Liu et al., 2004; Loll et al., 2005). The dimeric cyanobacterial PSII complex contains 14 modeled lipids per monomer (i.e. six MGD, four DGD, three SQD, and one phosphatidylglycerol). Four of these lipids (two DGD, one MGD, and one SQD) contribute to the formation of a hydrophobic channel that leads to the QB binding site (Jones, 2007). The core of PSII (D1/D2 proteins) is surrounded by a belt of lipids, including four molecules of DGD (Loll et al., 2005). It was speculated that these lipids around the D1/D2 proteins are required as a “lubricant” for the replacement of D1 with newly synthesized D1 protein after damage by radiation. Thus, deficiency of DGD in the lipid belt was suggested to affect the D1 replacement mechanism. In line with this expectation, the mutants are much more sensitive to PSII photoinhibition after 1 h of high-light stress than the wild type (Fig. 6B). However, a second mechanism besides an impairment of the PSII repair cycle must exist that contributes to the increase in PSII photoinhibition in the mutants. A much higher accumulation of photodamaged PSII was observed in the mutants already after short-term high-light exposure (10 min; Fig. 6A), which cannot be explained by an impairment in PSII repair cycle activity (requiring at least 40 min). Because the redox state of the PSII acceptor site is similar in mutants and the wild type (as deduced from qL light-response curves; Fig. 5B), the increased sensitivity of PSII to photodamage must be attributed to differences in the rate of photodamage versus photochemistry in the PSII reaction centers of the mutants. This explanation is in line with the increase in recombination reactions between P680+ and QA− in the dgd1 mutant (Steffen et al., 2005), which might give rise to singlet oxygen production. As also outlined by Steffen et al. (2005), the negative correlation between the increase in recombination reactions and the decrease in DGD content is nonlinear, because the effect is only observed in plants with a strong DGD reduction, most likely because PSII shows a high affinity to residual amounts of DGD. Therefore, the increased susceptibility of the dgd1dgd2 mutants to 10 min of high-light treatment is well in line with a role of DGD during the stable charge separation within PSII.
LHCII trimers were previously shown to bind three molecules of DGD (Liu et al., 2004). DGD deficiency in the dgd1 mutant results in the destabilization of LCHII trimers as observed in green gels (Dörmann et al., 1995). Nevertheless, LHCII accumulation seems to be less sensitive to DGD deprivation than PSII accumulation, as suggested by the following observations: the loss of PSI and PSII is more pronounced than that of total chlorophyll, the chlorophyll a/b ratios are reduced in all mutants relative to the wild type, and the 77K chlorophyll a fluorescence signals clearly demonstrate the presence of small amounts of LHCII and LHCI proteins uncoupled from the two photosystems. In line with this, Härtel et al. (1997) determined a reduction in the D1-LHCII ratio as measured by immunoblots.
It might seem surprising that the compromised photosynthetic efficiency of the DGD-deficient mutants does not necessarily result in impaired plant growth: the dwarf mutant growth observed for dgd1 was originally believed to be due to its impaired photosynthetic performance. However, while plant growth can be fully rescued by GlcT complementation under normal and high-light conditions, photosynthetic performance is not enhanced by GGD accumulation to a similar extent. This is most obvious when plants are grown under high light. Although the quantum efficiency of dgd1dgd2GlcT is lower than that of dgd1, growth of the GlcT-complemented line is very similar to that of the wild type. Under low light, the accumulation of PSII per leaf area is most strongly reduced in dgd1dgd2GlcT (similarly, dgd1GlcT has a lower PSII content than dgd1), while the two GGD-accumulating mutants grow much better than dgd1 and dgd1dgd2. Obviously, neither PSII accumulation nor PSII efficiency is limiting for plant growth. At first sight, this is well in line with data showing that photosynthetic electron transport capacity is mainly controlled at the level of the cytochrome b6f complex, while considerable surplus capacities of the two photosystems seem to exist, relative to the capacity of the intersystem electron transport chain (Anderson, 1992; Price et al., 1995; Schöttler et al., 2004). However, cytochrome b6f complex accumulation is not much affected in any of the mutants and thus cannot explain the differences in growth, strongly indicating that photosynthesis is not causal for the growth retardation of the dgd1 and dgd1dgd2 mutants. In line with this, the decreased efficiency of photosynthetic light reactions did not affect the rate of photosynthetic oxygen evolution per leaf area in dgd1, and the amounts of carbohydrates (starch and Suc) measured on a fresh weight basis were not altered in dgd1 as compared with the wild type (Härtel et al., 1997, 1998). Only when calculated per plant was the total carbohydrate content of dgd1 reduced.
Light microscopic analyses revealed that leaves of dgd1 are thinner by only 10% as compared with wild-type leaves (118 ± 12 μm and 106 ± 5 μm for the wild type and dgd1; n = 15). The sizes of mesophyll cells were very similar in the wild type, dgd1, and dgd1dgd2 (data not shown). Furthermore, the number of chloroplasts per cell cross-section in dgd1 was reduced by only 10% as compared with the wild type (Dörmann et al., 1995). Taken together, these data indicate that the reduced growth of dgd1 plants is primarily caused by a decrease in cell number per leaf, while the cell size and the number of chloroplasts per cell are only slightly decreased. DGD as an abundant chloroplast lipid represents one of the major building blocks for thylakoid membrane assembly. Thus, the deficiency in DGD synthesis presumably affects thylakoid and chloroplast proliferation and thereby slows down cell division processes and plant growth. There is tentative evidence that a tight control of the proportion of the volume of all chloroplasts relative to total cell volume is a major determinant for mesophyll cell differentiation by means of anterograde and retrograde signals, although the actual signals involved are not understood (Lopez-Juez and Pyke, 2005). A retardation of chloroplast development impairs palisade cell expansion and thereby formation of the palisade parenchyma in several plastid developmental mutants such as the dcl mutant of tomato (Solanum lycopersicum; Keddie et al., 1996), the var3 mutant of Arabidopsis (Naested et al., 2004), and the dag mutant of Antirrhinum majus (Chatterjee et al., 1996). Chloroplast division mutants grow normally if the reduced chloroplast number is compensated by an increased chloroplast size. However, when total chloroplast volume is reduced, this leads to severe developmental effects on the leaf level (Pyke and Leech, 1992, 1994; Pyke et al., 1994; Austin and Webber, 2005). In line with these results, our data suggest that a decrease in the capacity to form chloroplast membranes originating from galactolipid deficiency per se, and not the reduced photosynthetic efficiency, is the primary cause for the strong growth retardation of DGD-deficient lines.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) was grown at 21°C and 60% humidity with 16 h of light per day (120 μmol m−2 s−1) or at high-light conditions of 600 μmol m−2 s−1 where indicated. Phosphate limitation experiments were done with plants growing on petri dishes on synthetic medium (Härtel et al., 2000). The Arabidopsis mutants dgd1 (ecotype Columbia-2) and dgd2 (dgd2-1 allele) and the double mutant dgd1dgd2 were described previously (Dörmann et al., 1995; Kelly et al., 2003).
Isolation of the GlcT open reading frame chlo02003783 (GenBank accession no. ZP_00356752) from Chloroflexus aurantiacus was described previously (Hölzl et al., 2006). The GlcT gene was ligated into binary vectors in fusion with an N-terminal signal sequence for targeting to the chloroplast envelope membranes and employed for transformation of dgd1 (Hölzl et al., 2006). A line expressing the Chloroflexus GlcT gene in the dgd1dgd2 double mutant background was isolated via crossing a dgd1GlcT line with dgd2-1. The double homozygous, GlcT-expressing plants were screened by thin-layer chromatography (TLC) of leaf lipids, and the presence of the dgd2-1 mutation (T-DNA insertion) was confirmed by PCR.
Lipid and Chlorophyll Measurements
Lipids were isolated from leaves, separated by TLC, and stained with iodine vapor or α-naphthol (Dörmann et al., 1995; Hölzl et al., 2005). Individual lipids isolated from TLC plates were used to prepare fatty acid methyl esters, which were quantified by gas chromatography (GC) with pentadecanoic acid (15:0) as an internal standard (Browse et al., 1986a). Subcellular fractionation of plastidial and extraplastidial membranes from leaf cells and extraction of lipids were described previously (Härtel et al., 2000). Determination of chlorophyll content in leaves was done photometrically (Porra et al., 1989).
Photosynthesis Measurements
The photosynthetic complexes were quantified by difference absorption spectroscopy in thylakoids isolated according to Schöttler et al. (2004) and then recalculated to a leaf area basis. The contents of PSII and of the cytochrome b6f complex were determined from difference absorption spectra of cytochrome b559 (PSII) and of the cytochromes b6 and f (cytochrome b6f complex). For this purpose, thylakoid membranes equivalent to 50 μg chlorophyll mL−1 were destacked in a low-salt medium (Kirchhoff et al., 2002). All cytochromes were oxidized by addition of 1 mm ferricyanide and subsequently reduced by the addition of 10 mm sodium ascorbate (reduction of cytochrome f and of the high-potential form of cytochrome b559) and 10 mm sodium dithionite (reduction of cytochrome b6 and of the low-potential form of cytochrome b559). At each redox potential, absorption spectra were measured between 575- and 540-nm wavelength using a V-550 photometer equipped with a head-on photomultiplier (Jasco). Ten spectra were averaged for each redox condition. The monochromator slit width was set to 1 nm. After calculating the difference absorption spectra by subtraction of the ferricyanide signal from the ascorbate signal and of the ascorbate signal from the dithionite signal, the contributions of the different cytochromes were deconvoluted using reference spectra and difference extinction coefficients as described (Kirchhoff et al., 2002).
PSI was quantified from the difference absorption signal of the reaction center chlorophyll a dimer P700, measured in the far-red range at 830- to 870-nm wavelength using the Dual-PAM instrument (Heinz Walz). Thylakoids equivalent to 50 μg chlorophyll mL−1 were incubated with 100 μm methylviologen as electron acceptor and 10 mm sodium ascorbate as electron donor. P700 was photooxidized by a pulse of red light (650 nm, 2,000 μmol m−2 s−1 light intensity, 200-ms duration). A difference extinction coefficient of 6.0 μmol cm−2 was used.
The proton motive force across the thylakoid membrane was determined from the dark relaxation kinetic of the electrochromic absorption shift as described by Aronsson et al. (2008) using the KLAS-1000 LED-array spectrophotometer (Heinz Walz). In vivo chlorophyll fluorescence was recorded using the fiber optics version of the Dual-PAM-100 instrument (Heinz Walz). 77K chlorophyll a fluorescence emission spectra between 660 and 800 nm were measured on isolated thylakoids equivalent to 10 μg chlorophyll mL−1 using the F-6500 fluorometer (Jasco). Fluorescence was excited at 430 nm (10-nm bandwidth), and the emission spectra were recorded with a slit width of 1 nm.
Transmission Electron Microscopy
For the primary fixation, leaf sections (1 mm2) were incubated at room temperature for 4 h in 50 mm cacodylate buffer (pH 7.2) containing 0.5% (v/v) glutaraldehyde and 2.0% (v/v) formaldehyde, followed by washing with buffer (once) and with destined water (twice). For the secondary fixation, samples were transferred to a solution of 1% (w/v) OsO4. After 1 h, the samples were washed three times with distilled water. Dehydration was done by stepwise increasing the concentration of ethanol: 30% (v/v), 50% (v/v), 60% (v/v), 75% (v/v), 90% (v/v), and two times 100% (v/v) ethanol for 1 h each. After 1 h of dehydration with propylene oxide, the samples were infiltrated using a series of solutions with Agar Low Viscosity resin (Plano) with increasing concentrations as follows: 33% (v/v), 50% (v/v), and 66% (v/v) resin in propylene oxide for 4 h each and then 100% (v/v) resin overnight. Samples were transferred into embedding molds, incubated for 6 h in fresh resin, and polymerized at 60°C for 24 h. Ultra-thin sections approximately 70 nm thick were cut with a Leica Ultramicrotome UCT (Leica Microsystems) and a diamond knife for electron microscopic analysis using a FEI Tecnai Sphera G2 transmission electron microscope at 120 kV. Prior to examination, sections were contrasted with a saturated methanolic solution of uranyl acetate and lead citrate.
Acknowledgments
We thank Wolfram Thiele (Max Planck Institute, Potsdam, Germany) for excellent technical assistance with thylakoid isolations and photosynthetic complex quantifications.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ho 3870/1–1 to G.H. and grant no. SFB 429–B6 to P.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter Dörmann (doermann@uni-bonn.de).
Open Access articles can be viewed online without a subscription.
References
- Anderson JM (1992) Cytochrome-b6f-complex, dynamic molecular organization, function and acclimation. Photosynth Res 34 341–357 [DOI] [PubMed] [Google Scholar]
- Andersson MX, Larsson KE, Tjellström H, Liljenberg C, Sandelius AS (2005) Phosphate-limited oat: the plasma membrane and the tonoplast as major targets for phospholipid-to-glycolipid replacement and stimulation of phospholipases in the plasma membrane. J Biol Chem 280 27578–27586 [DOI] [PubMed] [Google Scholar]
- Aro E, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, Saleem A, Battchikova N, Rintamäki E (2005) Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J Exp Bot 56 347–356 [DOI] [PubMed] [Google Scholar]
- Aronsson H, Schöttler MA, Kelly AA, Sundqvist C, Dörmann P, Karim S, Jarvis P (2008) Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiol 148 580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J, Webber A (2005) Photosynthesis in Arabidopsis thaliana mutants with reduced chloroplast number. Photosynth Res 85 373–384 [DOI] [PubMed] [Google Scholar]
- Baker N, Harbinson J, Kramer D (2007) Determining the limitations and regulation of photosynthetic energy production in leaves. Plant Cell Environ 30 1107–1125 [DOI] [PubMed] [Google Scholar]
- Benson AA (1971) Lipids of chloroplasts. In M Gibbs, ed, Structure and Function of Chloroplasts. Springer Verlag, Berlin, pp 129–148
- Browse J, McCourt PJ, Somerville CR (1986. a) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152 141–145 [DOI] [PubMed] [Google Scholar]
- Browse J, Warwick N, Somerville CR, Slack CR (1986. b) Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem J 235 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Sparvoli S, Edmunds C, Garosi P, Findlay K, Martin C (1996) DAG, a gene required for chloroplast differentiation and palisade development in Antirrhinum majus. EMBO J 15 4194–4207 [PMC free article] [PubMed] [Google Scholar]
- Dörmann P, Benning C (2002) Galactolipids rule in seed plants. Trends Plant Sci 7 112–118 [DOI] [PubMed] [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann B, Güler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 95 1950–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zhang Z, Bi Y, Yang W, Xu Y, Zhang L (2005) Decreased stability of photosystem I in dgd1 mutant of Arabidopsis thaliana. FEBS Lett 579 3619–3624 [DOI] [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Grimm B, Benning C (1997) Changes in the composition of the photosynthetic apparatus in the galactolipid-deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol 115 1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Trethewey RN, Benning C (1998) Photosynthetic light utilization and xanthophyll cycle activity in the galactolipid deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol Biochem 36 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E, Roughan PG (1983) Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol 72 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzl G, Witt S, Kelly AA, Zähringer U, Warnecke D, Dörmann P, Heinz E (2006) Functional differences between galactolipids and glucolipids revealed in photosynthesis of higher plants. Proc Natl Acad Sci USA 103 7512–7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzl G, Zähringer U, Warnecke D, Heinz E (2005) Glycoengineering of cyanobacterial thylakoid membranes for future studies on the role of glycolipids in photosynthesis. Plant Cell Physiol 46 1766–1778 [DOI] [PubMed] [Google Scholar]
- Ivanov A, Hendrickson L, Krol M, Selstam E, Öquist G, Hurry V, Huner N (2006) Digalactosyl-diacylglycerol deficiency impairs the capacity for photosynthetic intersystem electron transport and state transitions in Arabidopsis thaliana due to photosystem I acceptor side limitations. Plant Cell Environ 47 1146–1157 [DOI] [PubMed] [Google Scholar]
- Jones MR (2007) Lipids in photosynthetic reaction centres: structural roles and functional holes. Prog Lipid Res 46 56–87 [DOI] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauß N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411 909–917 [DOI] [PubMed] [Google Scholar]
- Jouhet J, Maréchal E, Baldan B, Bligny R, Joyard J, Block MA (2004) Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria. J Cell Biol 167 863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J, Maréchal E, Miège C, Block MA, Dorne A-J, Douce R (1998) Structure, distribution and biosynthesis of glycerolipids from higher plant chloroplasts. In PA Siegenthaler, N Murata, eds, Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 21–52
- Keddie J, Carroll B, Jones J, Gruissem W (1996) The DCL gene of tomato is required for chloroplast development and palisade cell morphogenesis in leaves. EMBO J 15 4208–4217 [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, Dörmann P (2002) DGD2, an Arabidopsis gene encoding a UDP-galactose-dependent digalactosyldiacylglycerol synthase is expressed during growth under phosphate-limiting conditions. J Biol Chem 277 1166–1173 [DOI] [PubMed] [Google Scholar]
- Kelly AA, Froehlich JE, Dörmann P (2003) Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell 15 2694–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H, Mukherjee U, Galla H (2002) Molecular architecture of the thylakoid membrane: lipid diffusion space for plastoquinone. Biochemistry 41 4872–4882 [DOI] [PubMed] [Google Scholar]
- Krause G, Weis E (1989) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42 313–349 [Google Scholar]
- Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56 337–346 [DOI] [PubMed] [Google Scholar]
- Lemeille S, Willig A, Depège-Fargeix N, Delessert C, Bassi R, Rochaix JD (2009) Analysis of chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7 e45. [DOI] [PMC free article] [PubMed]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428 287–292 [DOI] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438 1040–1044 [DOI] [PubMed] [Google Scholar]
- Lopez-Juez E, Pyke K (2005) Plastids unleashed: their development and their integration into plant development. Int J Dev Biol 49 557–577 [DOI] [PubMed] [Google Scholar]
- Naested H, Holm A, Jenkins T, Nielsen H, Harris C, Beale M, Anderson M, Mant A, Scheller H, Camara B, et al (2004) Arabidopsis VARIEGATED3 encodes a chloroplast-targeted, zinc-finger protein required for chloroplast and palisade cell development. J Cell Sci 117 4807–4818 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Price G, Yu J, von Caemmerer S, Evans J, Chow W, Anderson J, Hurry V, Badger M (1995) Cytochrome-b6/f and ATP synthase complexes in tobacco: transformation with antisense RNA against nuclear-encoded transcripts for the Rieske FeS and ATP-delta polypeptides. Aust J Plant Physiol 22 285–297 [Google Scholar]
- Pyke K, Leech R (1992) Chloroplast division and expansion is radically altered by nuclear mutations in Arabidopsis thaliana. Plant Physiol 99 1005–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K, Leech R (1994) A genetic analysis of chloroplast division and expansion in Arabidopsis thaliana. Plant Physiol 104 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K, Rutherford S, Robertson E, Leech R (1994) Arc6, a fertile Arabidopsis mutant with only two mesophyll cell chloroplasts. Plant Physiol 106 169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifarth F, Christen G, Seeliger AG, Dörmann P, Benning C, Renger G (1997) Modification of the water oxidizing complex in leaves of the dgd1 mutant of Arabidopsis thaliana deficient in the galactolipid digalactosyldiacylglycerol. Biochemistry 36 11769–11776 [DOI] [PubMed] [Google Scholar]
- Schöttler M, Kirchhoff H, Weis E (2004) The role of plastocyanin in the adjustment of the photosynthetic electron transport to the carbon metabolism in tobacco. Plant Physiol 136 4265–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen R, Kelly AA, Huyer J, Dörmann P, Renger G (2005) Investigations on the reaction pattern of photosystem II in leaves from Arabidopsis thaliana wild type plants and mutants with genetically modified lipid content. Biochemistry 44 3134–3142 [DOI] [PubMed] [Google Scholar]
- Stöckel J, Bennewitz S, Hein P, Oelmüller R (2006) The evolutionarily conserved tetratrico peptide repeat protein Pale Yellow Green7 is required for photosystem I accumulation in Arabidopsis and copurifies with the complex. Plant Physiol 141 870–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, Choquet Y, Popot JL, Picot D (2003) An atypical haem in the cytochrome b6f complex. Nature 426 413–418 [DOI] [PubMed] [Google Scholar]
- Xu C, Fan J, Riekhof W, Froehlich JE, Benning C (2003) A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J 22 2370–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]