Abstract
Most conjugates of plant hormones are inactive, and some function to reduce the active hormone pool. This study characterized the activity of the tryptophan (Trp) conjugate of jasmonic acid (JA-Trp) in Arabidopsis (Arabidopsis thaliana). Unexpectedly, JA-Trp caused agravitropic root growth in seedlings, unlike JA or nine other JA-amino acid conjugates. The response was dose dependent from 1 to100 μm, was independent of the COI1 jasmonate signaling locus, and unlike the jasmonate signal JA-isoleucine, JA-Trp minimally inhibited root growth. The Trp conjugate with indole-3-acetic acid (IAA-Trp) produced a similar response, while Trp alone and conjugates with benzoic and cinnamic acids did not. JA-Trp and IAA-Trp at 25 μm nearly eliminated seedling root inhibition caused by 2 μm IAA. The TIR1 auxin receptor is required for activity because roots of tir1-1 grew only approximately 60% of wild-type length on IAA plus JA-Trp, even though tir1-1 is auxin resistant. However, neither JA-Trp nor IAA-Trp interfered with IAA-dependent interaction between TIR1 and Aux/IAA7 in cell-free assays. Trp conjugates inhibited IAA-stimulated lateral root production and DR5-β-glucuronidase gene expression. JA-deficient mutants were hypersensitive to IAA and a Trp-overaccumulating mutant was less sensitive, suggesting endogenous conjugates affect auxin sensitivity. Conjugates were present at 5.8 pmol g−1 fresh weight or less in roots, seedlings, leaves, and flowers, and the values increased approximately 10-fold in roots incubated in 25 μm Trp and IAA or JA at 2 μm. These results show that JA-Trp and IAA-Trp constitute a previously unrecognized mechanism to regulate auxin action.
Throughout their life, plants use a variety of hormonal signals to adjust growth in response to developmental and external cues. Central among the growth regulating hormones is the auxin indole-3-acetic acid (IAA), which is involved in nearly all aspects of plant development (for review, see Woodward and Bartel, 2005). Optimal growth requires tight control of IAA activity, which is accomplished by diverse mechanisms that include regulating IAA biosynthesis, its transport among tissues, cycling between active and inactive forms of auxin, and hormone degradation through various oxidative pathways (see Ljung et al., 2002; Leyser, 2006; Scheres and Xu, 2006). Regulatory control is also accomplished at the level of IAA interaction with auxin receptors and at numerous steps downstream of this signaling interaction (Mockaitis and Estelle, 2008).
While jasmonates are best known for their role in defense against herbivores and certain pathogens, they also control growth (for recent overviews, see Wasternack, 2007; Howe and Jander, 2008; Browse, 2009). Exogenous jasmonic acid (JA) inhibits growth (Yamane et al., 1980; Dathe et al., 1981; Ueda and Kato, 1982; Staswick et al., 1992), and both JA biosynthesis and jasmonate signaling mutants display growth abnormalities, particularly in reproductive organs and in vegetative tissues during defense responses (Mandaokar et al., 2006; Zavala and Baldwin, 2006; Yan et al., 2007; Zhang and Turner, 2008). It makes sense that jasmonate defense signaling would be tied to growth regulation because under stress plants must adjust their development and reallocate resources toward defense (Herms and Mattson, 1992). Although the full mechanism of jasmonate-mediated growth regulation is unclear, it may involve cell cycle transitions, cell division, and IAA-induced cell elongation (Miyamoto et al., 1997; Swiatek et al., 2002, 2004; Zhang and Turner, 2008).
Plants synthesize conjugated forms of both JA and IAA. Indeed, most IAA in Arabidopsis (Arabidopsis thaliana) is bound through ester or amide linkages to various constituents, including sugars, amino acids, and peptides (Ljung et al., 2002). Conjugates are generally considered inactive metabolites of the hormone. For example, IAA-Asp and IAA-Glu are catabolized, while IAA-Ala and IAA-Leu are stored forms of IAA that can be accessed at a later time by hydrolysis of the amide linkage (Rampey et al., 2004). Early physiological studies suggested that some JA conjugates might have biological activity, but like the IAA conjugates, most were considered inactive or of minor importance compared with JA and its methyl ester (JA-Me; Kramell et al., 1997; Miersch et al., 1999). That perspective changed with the discovery that the Ile conjugate of JA (JA-Ile) is a key jasmonate signal (Staswick and Tiryaki, 2004).
JA-Ile accumulation is strongly but transiently induced in plant defense responses in both Arabidopsis and tobacco (Nicotiana tabacum), and this accumulation is tied to productive defense reactions (Kang et al., 2006; Wang et al., 2007; Suza and Staswick, 2008). Furthermore, JA-Ile promotes interaction between the COI1 F-box protein, the presumed jasmonate receptor, and its ubiquitination targets, the JAZ transcriptional repressors (Chini et al., 2007; Thines et al., 2007; Katsir et al., 2008a; Staswick, 2008). In contrast, JA and JA-Me have essentially no activity in this interaction. Instead, they are precursors that become effective signals when added exogenously to plants because they are converted to JA-Ile by the JAR1 conjugating enzyme (Staswick and Tiryaki, 2004; Tamogami et al., 2008).
JA-Ile signaling closely parallels the mechanism previously established for auxin, although in the latter case, free rather than conjugated IAA is the active signal. IAA binds in a pocket of the TIR1 auxin receptor, which is an F-box component of the E3 ubiquitin ligase complex called SCFTIR1. Auxin binding strengthens the interaction between TIR1 and the Aux/IAA protein targets for ubiquitination (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005; Tan et al., 2007). Degradation of the Aux/IAA transcriptional repressors by the 26 s proteasome then leads to auxin-dependent gene transcription and auxin response. Five TIR1-related proteins, AFB1 through AFB5, have been identified, and they have partially overlapping functions with TIR1 (Dharmasiri et al., 2005b; Mockaitis and Estelle, 2008). In contrast, COI1 is the primary, if not the sole, F-box protein in jasmonate signaling.
Critical tools that have shaped our understanding of auxin activity are auxin response inhibitors of two general classes: those that alter auxin transport and those that perturb auxin signaling. Although a few endogenous auxin inhibitors have been reported, little is known about their in vivo roles. Most studies have employed synthetic inhibitors, such as 1-naphthylphthalamic acid (NPA), p-chlorophenoxyisobutyric acid (PCIB), 4,4,4-trifluoro-3-indole-3-butyric acid, tri-iodobenzoic acid, and 1-naphthoxyacetic acid (MacRae and Bonner, 1953; Jönsson, 1961; Katayama et al., 1995; Tomic et al., 1998; Parry et al., 2001; Rahman et al., 2002). The TIR1 auxin receptor was discovered as a mutant (tir1) resistant to the NPA transport inhibitor, and new antagonists and agonists have been identified in recent chemical genetic screens (Ruegger et al., 1997; Armstrong et al., 2004; Surpin et al., 2005; Yamazoe et al., 2005; Hayashi et al., 2008).
While synthetic auxin inhibitors have been immensely informative, there are potential drawbacks to using these unnatural compounds. They may work in ways that differ from endogenous inhibitors, and potential xenobiotic responses to the foreign compounds can complicate analysis of their mechanism of action (Armstrong et al., 2004). Naturally occurring auxin inhibitors, on the other hand, may refine our understanding of how auxin activity is controlled in planta, and they may illuminate new components of the auxin regulatory pathway. This study was initiated to evaluate possible jasmonate activity for a series of JA-amino acid conjugates. Surprisingly, the Trp conjugates of both JA and IAA are naturally occurring auxin antagonists that interfere with a broad range of IAA-mediated processes. Trp conjugates require TIR1 for full activity, and determining how they work may shed new light on the control of auxin signaling in plants.
RESULTS
JA-Trp Interferes with Root Gravitropic Response
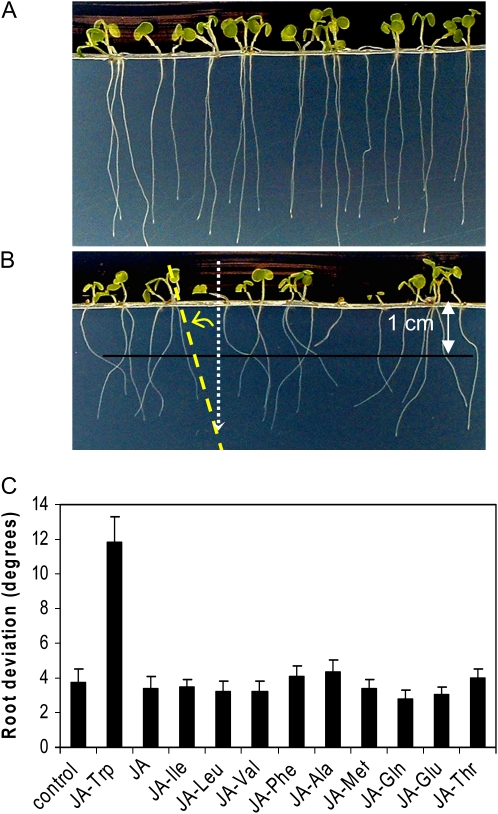
During experiments to examine possible jasmonate-like properties of various JA-amino acid conjugates, it was noted that JA-Trp caused roots to grow agravitropically. Figure 1 illustrates the response, and root deviation from the gravity vector was quantified as described. Although roots were grown within the agar medium and not on the agar surface, only one of the two dimensions of vertical growth was quantified here. On 25 μm JA-Trp, roots had a mean deviation of 11.8° (se = 1.5) compared with 3.8° (se = 0.7) for the control (Fig. 1C). The response was specific to JA-Trp, as results for JA itself and JA conjugates with nine other amino acids did not differ from the control.
Figure 1.
Arabidopsis root response to JA-Trp. A, Growth on MS medium without JA-Trp. B, Growth on a 50 μm mixture of (+)- and (−)-JA-Trp. Agravitropism was quantified by measuring the angle (curved yellow arrow) that roots deviated from the gravity vector (dotted vertical arrow) at the point each root passed through a plane 1 cm from the plane of germination. C, Effect of JA and several JA conjugates (25 μm) on gravitropic response. Vertical axis is root deviation from vertical. Values are the means of 16 to 24 seedlings with se. JA and all of the conjugates synthesized from it were a mixture of the (+) and (−) enantiomers obtained from the commercial source of JA.
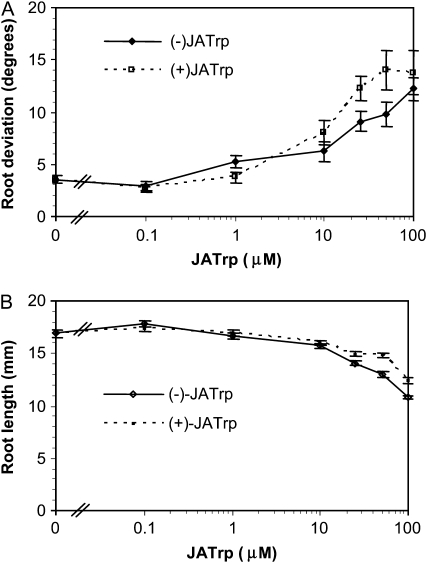
The sensitivity of roots to JA-Trp was determined in a dose-response assay shown in Figure 2A. For this experiment, the naturally occurring (−) and the synthetic (+) enantiomers were first separated by HPLC from JA-Trp synthesized using the commercially available enantiomer mixture of JA. The mean divergence from vertical for (−) JA-Trp was significantly different from the control at as little as 1 μm (t test, one-tail, P = 0.008, n = 27), while that of (+) JA-Trp was significant at 10 μm and higher. (+) JA-Trp was modestly more active than (−) JA-Trp at the higher concentrations. As jasmonates inhibit root growth, the length of roots after 6 d growth on JA-Trp was also determined. Figure 2B shows that this conjugate slowed root growth only modestly. Even at 100 μm, growth inhibition was <50% of the control value. By comparison, typical jasmonates like JA and JA-Ile produce 50% inhibition at concentrations at least two orders of magnitude lower than this (Staswick et al., 1992; Staswick and Tiryaki, 2004). Further experiments showed that growth inhibition by JA-Trp was partially dependent on the JAR1 enzyme that conjugates JA to Ile (Supplemental Fig. S1). This suggests that some JA-Trp is hydrolyzed, releasing JA, which is then conjugated to Ile. Thus, JA-Trp does not appear to be a typical jasmonate signal.
Figure 2.
Root growth in the presence of (+)- and (−)-JA-Trp. A, Root deviation from the gravity vector was measured as described in Figure 1. B, Mean root length after 7 d growth of the same seedlings used for A. Values are the means with se (n = 26–31 seedlings).
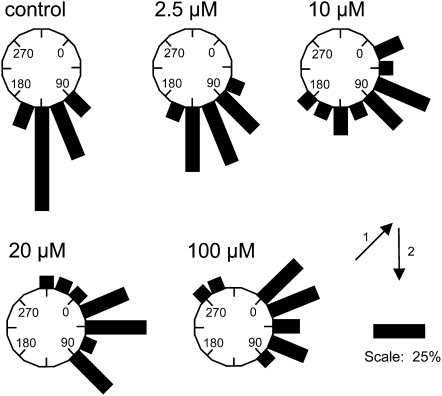
Another measure of agravitropic response is the ability of seedling roots grown on control agar medium to reorient after transfer to test media and then rotating seedlings out of the original gravity vector. Figure 3 summarizes data for seedlings on four different concentrations of (±)-JA-Trp. Bar lengths on the circular histograms represent the proportion of seedlings growing in the indicated direction after 2 d in the dark. Relative to the control, JA-Trp at 2.5 μm interfered with the ability of seedlings to reorient to vertical, and the effect was markedly increased as the concentration of JA-Trp was increased. Together with the near normal root elongation on JA-Trp (Fig. 2B), the evidence suggests that JA-Trp disrupts normal gravitropism and is not merely a toxin avoidance response.
Figure 3.
Effect of JA-Trp on gravitropic response. Seedlings were grown 5 d on the surface of MS agar plates in the absence of JA-Trp and then transferred to plates containing the indicated concentrations of the conjugate. Plates were rotated so that the orientation of seedlings was 135° (arrow 1) from the new gravity vector (arrow 2). Bar lengths represent the percentage of seedlings (n = 13–15 for each treatment) growing at the indicated orientation after 2 d growth in the dark.
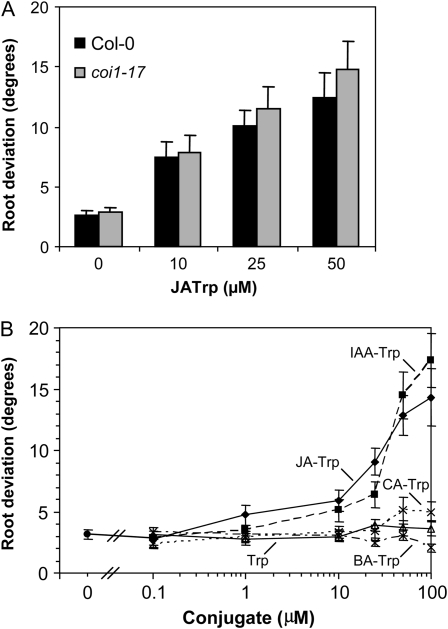
Most jasmonate responses require the COI1 jasmonate locus for activity (Devoto et al., 2005). To determine whether the agravitropic response to JA-Trp was COI1 dependent, the coil-17 allele was tested (Suza and Staswick, 2008). Figure 4A shows that JA-Trp at 10, 25, and 50 μm produced an agravitropic response in the mutant that was equal to or greater than that of wild-type seedlings. This is further evidence that JA-Trp is not a typical jasmonate signal and is consistent with the inability of JA-Trp to function in the molecular interaction between COI1 and JAZ proteins (Katsir et al., 2008b). In contrast, the modest suppression of root elongation by JA-Trp was COI1 dependent, consistent with conversion of small quantities of JA derived from this conjugate to a true jasmonate signal (Supplemental Fig. S1)
Figure 4.
Gravitropic response in coi1-17 and effect of other Trp conjugates. A, Assay of coi1-17 and Col-0 was as described in Figure 1. Bars are the means of 16 to 21 seedlings with se indicated. B, Induction of agravitropism by various Trp conjugates or Trp at indicated concentrations. Values are means (n = 22, se indicated).
To examine whether the agravitropic activity was unique to JA-Trp, the Trp conjugates with IAA, benzoic acid, and trans-cinnamic acid (CA) were also tested. As shown in Figure 4B, Trp alone and BA-Trp had essentially no activity, while trans-CA-Trp produced only a slight increase in root deviation at 50 μm and higher. On the other hand, the response to IAA-Trp was similar to JA-Trp. The inactivity of Trp alone establishes that the agravitropic activity is not simply due to Trp released by conjugate metabolism.
JA-Trp and IAA-Trp Antagonize Auxin-Inhibited Root Growth
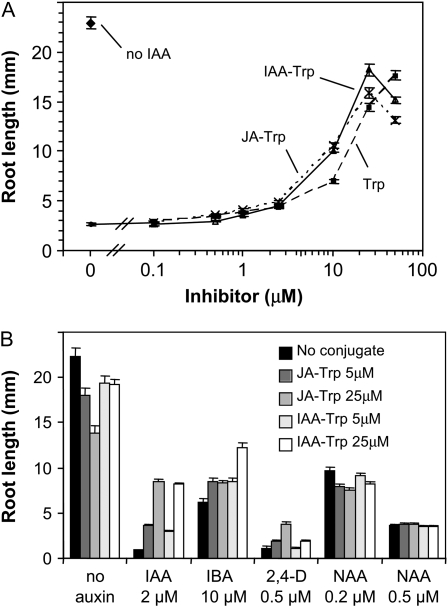
Several synthetic inhibitors of gravitropic response interfere with auxin activity, and some mutants affected in auxin sensitivity are also compromised in gravitropic response (Estelle and Somerville, 1987; Hobbie and Estelle, 1995; Oono et al., 2003; Rojas-Pierce et al., 2007; Hayashi et al., 2008). To test whether JA-Trp and IAA-Trp impair auxin sensitivity, the ability of these conjugates to block IAA-inhibited root growth was evaluated. Figure 5A shows that both conjugates interfered with root inhibition by 2 μm IAA. As little as 0.5 μm of each conjugate significantly increased root growth (t test of means, P < 0.01, n = 19) compared with IAA alone. The maximal effect was seen at 25 μm, where roots on IAA-Trp grew to about 78% of those in the absence of auxin, while the conjugates at 50 μm were slightly less effective. This reduction may result from hydrolysis of the respective conjugates to release small quantities of free JA and IAA, both of which inhibit root growth.
Figure 5.
Suppression of auxin-inhibited root growth by JA-Trp and IAA-Trp. A, Seedling root length was measured after 7 d of growth on MS agar plates with 2 μm IAA plus the indicated amount of conjugate or Trp. Values are the means (n = 19) with se. B, Efficacy of Trp conjugates with other auxins was tested as above with auxins at the indicated concentrations with the indicated conjugates. Root lengths were measured after 8 d of growth, and values are the means (n = 21 seedlings) with se. IBA, Indole-3-butyric acid; 2,4-D, 2,4-dichlorophenoxyacetic acid.
In contrast to the gravitropic response, Trp alone was active in suppressing IAA-inhibited growth (Fig. 5A). At 10 and 25 μm, the effect was modestly less than for the conjugates, but at 50 μm, Trp alone was more effective. Trp did not affect root growth in the absence of exogenous auxin (data not shown), and none of the other 19 protein amino acids diminished the response to IAA (Supplemental Fig. S2).
The ability of JA- and IAA-Trp to antagonize root inhibition by other auxins was examined. Figure 5B shows that in addition to IAA, both conjugates suppressed inhibition by indole-3-butyric acid and 2,4-dichlorophenoxyacetic acid, but they had no effect on root inhibition by 1-naphthalenacetic acid (NAA). In the absence of auxin, both JA-Trp and IAA-Trp modestly inhibited growth (possibly due to hydrolysis of the conjugates), so the antagonistic effect on auxin activity is likely underestimated in this assay.
Trp Conjugates Block IAA-Induced Lateral Root Growth and Gene Expression
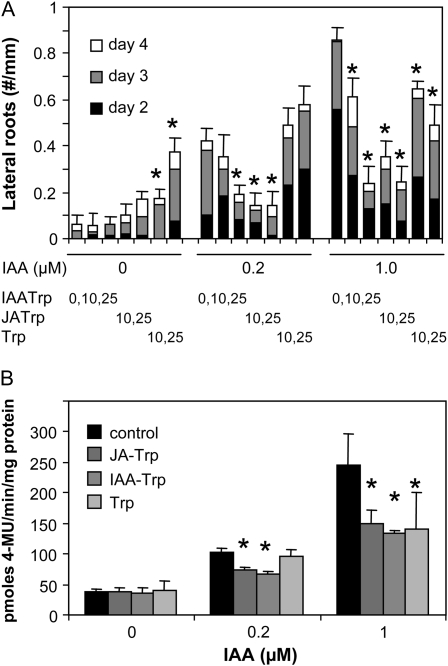
The efficacy of the conjugates to inhibit IAA-stimulated lateral root growth was examined at 2, 3, and 4 d after transferring 4-d-old seedlings to media containing the compounds indicated in Figure 6A. Both conjugates at 25 μm suppressed root production stimulated by IAA at 0.2 and 1.0 μm. Reduction in root number was also seen with conjugates at 10 μm, except that no significant difference occurred for IAA-Trp with 0.2 μm IAA (t test, single-tailed, P < 0.05). Trp also inhibited root production stimulated by 1 μm IAA, albeit less effectively than JA-Trp. When Trp was combined with 0.2 μm IAA, there was no significant effect compared with IAA alone. However, at 25 μm, this amino acid alone actually stimulated root production to about the same level as did 0.2 μm IAA.
Figure 6.
Suppression of IAA-promoted lateral root growth and gene expression by inhibitors. A, Bar segments indicate the incremental increase in lateral roots on days 2, 3, and 4 after transfer of 4-d-old seedlings to agar plates containing the indicated concentrations (μM) of IAA, Trp, and conjugate (means, n = 10–12 seedlings). se bars are for the total root numbers on day 4, and asterisks indicate significant difference from the control after 4 d for each IAA treatment (t test, single-tailed, P < 0.05). B, Five-day-old DR5-GUS reporter line seedlings were transferred to liquid MS medium for 6 h containing indicated concentrations of IAA with Trp, JA-Trp, or IAA-Trp at 25 mm. Values are the means of three replicates of 12 to 15 seedlings each with sd. Asterisks indicate means significantly different from the control for each IAA concentration (t test, single-tailed, P < 0.05).
Conjugates were also tested for their ability to inhibit IAA-induced expression in the Arabidopsis DR5-GUS reporter line (Ulmasov et al., 1997). Conjugates were added at 25 μm along with the indicated concentrations of IAA. Figure 6B shows that after 5 h of induction, modest suppression of IAA-stimulated GUS activity was seen for both the 0.2 and 1.0 μm IAA treatments (t test, single-tailed, P < 0.05). Trp produced no significant effect with 0.2 μm IAA but did reduce the induction seen with 1.0 μm IAA. Altogether, the results establish that exogenous JA-Trp and IAA-Trp interfere with a wide spectrum of auxin-mediated physiological responses.
JA-Trp and IAA-Trp Are Synthesized in Arabidopsis
Addition of Trp to media containing IAA (Fig. 5A) strongly suppressed the root-inhibiting effect of IAA, suggesting that IAA-Trp may be synthesized in these roots. To test this possibility, roots supplied with IAA or JA along with Trp for 16 h were extracted and the conjugates quantified by gas chromatography/mass spectrometry (GC/MS). Table I shows that control roots contained 1.7 pmol g−1 fresh weight (FW) of both JA-Trp and IAA-Trp. Although considerably lower than the amount of free IAA and JA, these values were comparable to the basal level of the jasmonate signal JA-Ile. The level of JA-Trp and IAA-Trp in roots incubated in either JA or IAA along with Trp rose about 10-fold to 17.2 and 23.5 pmol g−1 FW, respectively. Interestingly, IAA-Trp also increased about 5-fold in the JA + Trp treatment, indicating that endogenous IAA was conjugated when additional Trp was available. This confirms that roots contain both conjugates, and they have the capacity to synthesize higher levels when the appropriate substrates are provided.
Table I.
Quantitation of jasmonates and auxins in Arabidopsis tissues
nd, Not detected. Values are the means of three or four independent tissue extractions with se in parentheses.
| Treatmenta | JA-Trp | IAA-Trp | IAA | JA | JA-Ile |
|---|---|---|---|---|---|
| pmol/g FW | |||||
| Root | |||||
| Control | 1.7 (0.3) | 1.7 (0.5) | 46.6 (8.5) | 23.0 (1.5) | 3.7 (1.2) |
| IAA + Trp | 0.8 (0.4) | 23.5 (3.1) | – | 26.8 (3.7) | 2.7 (1.1) |
| JA + Trp | 17.2 (1.5) | 10.5 (2.2) | 60.3 (4.2) | – | 6.9 (0.8) |
| Seedling | |||||
| Light | 1.2 (0.3) | nd | 2.9 (0.3) | 2.9 (0.6) | 5.4 (1.5) |
| Dark | 1.0 (0.3) | nd | 4.7 (0.9) | 4.0 (0.8) | 3.0 (1.5) |
| Leaf | |||||
| Mature | 0.2 (0.02) | nd | 3.8 (1.4) | 78.6 (40.5) | 3.8 (1.4) |
| Mature wound | 0.7 (0.02) | nd | 3.4 (0.5) | 2,064.2 (429) | 348.5 (19.7) |
| Expanding | nd | 5.8 (2.8) | 16.3 (0.9) | 18.3 (3.6) | 7.2 (0.7) |
| Flower | nd | 2.8 (0.6) | 62.1 (2.5) | 47.4 (10.1) | 8.1 (1.8) |
Roots were grown 3 to 4 weeks in MS liquid media followed by 16-h incubation in the same or with IAA or JA at 2 μm and Trp at 25 μm added, as indicated. Seedlings were grown on filter paper with MS liquid media for 5 d in continuous light or continuous dark. Leaves and flowers were from plants grown in soil. Mature leaves were wounded with a hemostat and harvested 1 h later. Expanding leaves were ≤1 cm in length. Detection limit was 0.1 and 0.5 pmol/g FW for JA-Trp and IAA-Trp, respectively.
The amount of Trp conjugates was also examined for several other tissues. Seedlings grown 5 d in either light or dark had low levels of JA-Trp similar to control roots but no detectable IAA-Trp (Table I). In contrast, expanding leaves and flowers had small amounts of IAA-Trp but no detectable JA-Trp. To test whether the amount of JA-Trp might be limited by the availability of endogenous JA, mature leaves were wounded and analyzed after 60 min. As expected, wounding markedly increased both JA and JA-Ile, while JA-Trp increased from only 0.2 to 0.7 pmol g−1 FW. Although this increase was significant (t test, two-tailed, P = 0.0001, n = 4), the low level of JA-Trp even when a large amount of JA is present suggests that JA availability is not the sole limitation to its accumulation in leaves. Analysis of leaves 10 and 240 min after wounding did not yield altered Trp conjugate levels (data not shown).
JA Biosynthesis and Trp-Hyperaccumulating Mutants Have Altered IAA Sensitivity
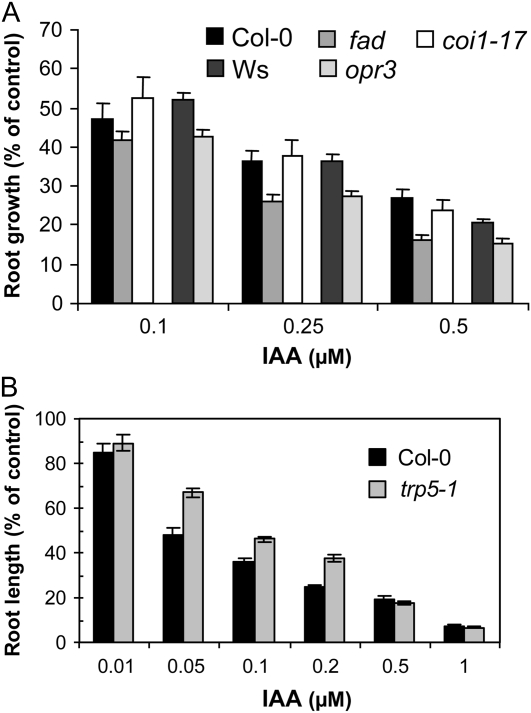
If endogenous JA-Trp helps regulate growth, then mutants defective in the production of JA might be more sensitive to IAA. The JA biosynthesis triple mutant fad3-2 fad7-2 fad8 (McConn and Browse, 1996) and opr3, which is defective in conversion of the JA intermediate 12-oxophytodienoic acid (Stintzi and Browse, 2000), were evaluated for root growth in the presence of 0.1, 0.25, and 0.5 μm IAA. Figure 7A shows that at all three concentrations the fad mutant grew less than its wild-type counterpart, which is Columbia-0 (Col-0). This effect was not dependent on the jasmonate signaling pathway because coi1-17 did not show diminished growth on IAA. The genetic background for opr3 is the Wassilewskija ecotype, and it also displayed decreased growth compared to the corresponding wild type. Although the effect seen here is modest, these results suggest that endogenous root JA-Trp is involved in regulating response to exogenous auxin.
Figure 7.
Sensitivity of mutants to IAA. A, Seedling root length of jasmonate biosynthesis mutants (fad3-2 fad7-2 fad8 and opr3) and signaling mutant (coi1-17) after 7 d of growth on IAA. The genetic background for opr3 is Wassilewskija (Ws) and for the other two mutants is Col-0. Values are expressed as percentage of the length when each genotype was grown on control medium (n = 13–15 seedlings). Error bars represent 95% confidence interval for each ratio of the means. B, Growth of the Trp-overaccumulating mutant trp5 on IAA. Values are expressed as in A and determined after 6 d of growth (n = 25 seedlings).
The ability of increased endogenous Trp to affect auxin resistance was tested in the trp5 mutant, which accumulates soluble Trp about 3-fold above wild-type levels due to altered feedback regulation of anthranilate synthase (Li and Last, 1996). Figure 7B shows that over a range of 0.05 to 0.2 μm, trp5 was more resistant to the root inhibiting effects of IAA than was the wild type, while at higher concentrations, no difference was seen. This result supports the idea that the additional endogenous Trp in the mutant is available for conjugation to IAA, thereby providing increased resistance to auxin.
Trp Conjugate Activity Does Not Require Auxin Transport
The inability of the Trp conjugates to suppress NAA-inhibited root growth (Fig. 5B) might indicate that these conjugates act at the level of auxin transport, since NAA cellular import is independent of an influx carrier (Delbarre et al., 1996). The conjugates were tested for their effect on lateral root development, which is strongly suppressed when the transport inhibitor NPA is applied to the shoot-root junction (Rashotte et al., 2000). Four-day-old seedlings were transferred to new media, and 100 μm JA-Trp, IAA-Trp, or NPA were applied in agar. Table II shows that after 6 d, lateral root production was greatly impaired by NPA, as expected. In contrast, treatment with the Trp conjugates actually enhanced the number of roots modestly. Also consistent with earlier findings (Rashotte et al., 2000), NPA modestly reduced root growth by about 36%, whereas the conjugates had no effect on growth. This evidence indicates that JA-Trp and IAA-Trp do not act by the same mechanism as the auxin transport inhibitor NPA.
Table II.
Effect of Trp conjugates on root growth and lateral root number
Root length is the amount of new growth 4 d after treatment began, and lateral roots were counted 6 d after treatment. Values are the means of 12 plants with se in parentheses.
| Treatmenta | Root Length | Lateral Roots |
|---|---|---|
| mm | no. | |
| Control | 21.8 (1.5) | 4.2 (0.7) |
| NPA | 13.8 (0.7) | 0.3 (0.2) |
| JA-Trp | 21.3 (1.2) | 6.4 (0.5) |
| IAA-Trp | 22.5 (1.1) | 6.4 (0.7) |
Treatments were 100 μm of indicated compound applied in agar to the shoot-root junction of 4-d-old seedlings.
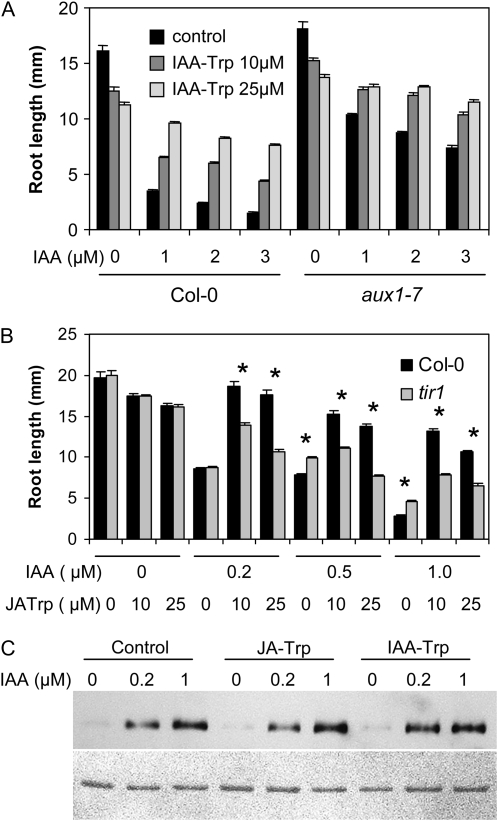
AUX1 is required for auxin transport, and aux1 mutants are resistant to exogenous auxin and strongly agravitropic (Marchant et al., 1999). To further examine whether the Trp conjugates act at the level of auxin transport, the sensitivity of aux1-7 to IAA-Trp was examined. JA-Trp was not evaluated here because it inhibits growth to a greater extent than IAA-Trp (Fig. 5B), thereby masking the suppression of IAA inhibition. Figure 8A shows that even though the mutant root growth is strongly resistant to IAA, IAA-Trp at 10 and 25 μm still reduces inhibition caused by up to 3 μm IAA. Although the effect of IAA-Trp was not as striking as for the wild type, 25 μm IAA-Trp completely abolished IAA inhibition for aux1-7 roots grown on 1 and 2 μm IAA. This suggests that IAA-Trp does not act at the level of AUX1-mediated auxin transport.
Figure 8.
Role of AUX1 and the TIR1 in mediating Trp conjugate activity. A and B, Root lengths for aux1 and tir1 were determined 5 and 7 d, respectively, after growth in medium containing the indicated amounts of IAA and Trp conjugates. Values are the mean of 17 and 19 seedlings for experiments with aux1 and tir1, respectively, with se indicated. Asterisks in B indicate means that differed significantly between the two genotypes for a given treatment (t test, P < 0.01). C, Pull down of TIR1-myc with GST-Aux/IAA7. IAA was added to the interaction mix at the concentrations indicated, and JA-Trp or IAA-Trp was added at 40 mm. The bottom panel is a loading control for Aux/IAA7 stained with Ponceau Red.
JA-Trp Requires TIR1 for IAA Inhibition
TIR1 is a critical auxin receptor, so its role in the activity of JA-Trp was evaluated in tir1-1. Figure 8B shows that in the absence of IAA root length of tir1-1 did not differ from Col-0 when grown on 0, 10, or 25 μm JA-Trp. This mutant is modestly resistant to IAA, and yet tir1-1 roots were only 56% to 72% of the wild-type length when JA-Trp at 10 or 25 μm was included along with 0.2 to 1 μm IAA. The decreased efficacy of the conjugate in tir1-1 indicates that TIR1 signaling is required for maximal JA-Trp activity.
One way that JA-Trp and IAA-Trp might act is to compete directly with IAA for binding of TIR1. This was tested in an in vitro pull-down assay that uses a glutathione S-transferase (GST)-Aux/IAA7 fusion protein to select SCFTIR1 from cell extracts of transgenic plants that carry a myc-tagged TIR1 (Dharmasiri et al., 2003). As seen in Figure 8C, 0.2 and 1 μm IAA effectively stimulated the interaction. However, adding either conjugate to the reaction at 40 μm had no effect on the ability of IAA to promote interaction between TIR1 and Aux/IAA7.
DISCUSSION
With the exception of the well-established role of JA-Ile as a hormonal signal, amino acid conjugates of both JA and IAA have been regarded as inactive metabolites of the hormones. This study identifies an unexpected function for JA-Trp and IAA-Trp as endogenous inhibitors of several physiological responses to auxin. The activity of IAA-Trp is particularly interesting because synthesis of this conjugate not only removes free IAA from the active auxin pool, as for IAA conjugation to other amino acids, but also converts it to an antagonist of any remaining IAA. Thus, IAA-Trp is a kind of “super inactivator” of IAA.
JA-Trp was first identified in Vicia faba, particularly in flowers and fruits, and it was reportedly elevated in asparagus (Asparagus officinalis) shoots following harvest and during senescence (Brückner et al., 1988; Gapper et al., 2002). JA-Trp had little or no ability to induce genes that were activated by JA or JA-Ile in barley (Hordeum vulgare), suggesting it was not a jasmonate signal (Kramell et al., 1997; Miersch et al., 1999). That JA-Trp is not a typical jasmonate signal is supported here because it only minimally inhibits root growth compared with JA-Ile. Conversely, JA and JA-Ile do not produce an agravitropic root response like the Trp conjugate, and agravitropism was COI1 independent, contrary to most characterized jasmonate responses. These results also agree with recent biochemical studies showing that unlike JA-Ile, JA-Trp does not promote SCFCOI1 interaction with JAZ proteins (Thines et al., 2007). On the other hand, some JA-responsive Arabidopsis genes are regulated independently of COI1 (Devoto et al., 2005). The mechanism is unknown, but it seems plausible that conjugation of JA to Trp might be involved, which could then alter gene expression through an auxin signaled pathway.
Endogenous JA-Trp and IAA-Trp
Although JA-Trp and IAA-Trp were detected at low levels or not at all in some Arabidopsis tissues, they may still have a significant role. Auxin activity is highly regulated by a complex network of interacting mechanisms, and endogenous auxin antagonists might be expected to remain low in most cells under conditions of normal growth. Higher concentrations might occur in response to specific environmental stimuli or in localized tissues. For this study, conjugates were extracted from whole roots, seedlings, and leaves, which would dilute higher concentrations that might occur at more restricted sites of accumulation.
The increase in auxin sensitivity in JA synthesis mutants (Fig. 7A) supports, although does not prove, that endogenous JA-Trp is functional. The defect in these mutants was modest, but the loss might be partially compensated for by IAA-Trp or by other endogenous auxin inhibitors. Interestingly, a role for JA in gravitropic response was previously found in rice (Oryza sativa) coleoptiles, which accumulated a gradient of JA that was opposite the IAA gradient that formed under gravistimulation (Gutjahr et al., 2005). Flooding coleoptiles with JA delayed the onset of gravitropic bending, and the jasmonate-deficient hebiba mutant responded more slowly to gravity than did wild-type rice coleoptiles. It would be interesting to know whether JA-Trp also accumulates asymmetrically in these rice coleoptiles, as this seems a plausible hypothesis to explain how JA functions in the observed gravity response.
Substrate Availability May Limit Conjugate Synthesis
Arabidopsis roots synthesized excess IAA-Trp and JA-Trp when the appropriate substrates were provided to roots, indicating that substrate availability limits conjugate production under normal conditions. Trp appears limiting because addition of this amino acid to media containing IAA decreased root sensitivity to auxin essentially as effectively as did IAA-Trp itself. Furthermore, roots incubated in only JA and Trp produced IAA-Trp in addition to JA-Trp, indicating that endogenous IAA was available for conjugation. The Trp-overaccumulating mutant trp5 was also more resistant to IAA than the wild type, possibly due to increased IAA-Trp production. On the other hand, wounding leaves to markedly increase endogenous JA only minimally increased JA-Trp, consistent with a limitation in Trp availability. Previous evidence that amino acid availability influences the IAA conjugate spectrum comes from the Arabidopsis Gln-overaccumulating mutant gluS, which had elevated levels of IAA-Gln in place of IAA-Asp (Barratt et al., 1999). Synthesizing IAA-Trp under cellular conditions where Trp is abnormally high might be a particularly effective strategy since as a precursor for IAA synthesis, Trp overabundance might also lead to excess auxin.
Presently, we do not know which enzymes are involved in Trp conjugate synthesis. Members of the GH3 family seem likely candidates because in vitro assays indicate they conjugate JA and IAA to several amino acids, including Trp (Staswick and Tiryaki, 2004; Staswick et al., 2005). Preliminary phenotypic analysis of several GH3 mutants did not identify obvious candidates for the Trp conjugating enzymes that function in vivo (data not shown), but redundancy among the IAA-conjugating family members may require the evaluation of multigene mutants. Functional redundancy between IAA-Trp and JA-Trp could also complicate the genetic identification of the conjugating enzymes that are involved.
Trp Conjugate Activity
Trp appears to be a structural requirement for an effective conjugate because nine other amino acid conjugates of JA were ineffective promoters of agravitropism and no amino acid other than Trp suppressed the root inhibiting activity of IAA. On the other hand, JA-Trp and IAA-Trp produced similar physiological responses, even though JA and IAA are structurally quite diverse. BA-Trp and CA-Trp were essentially inactive, suggesting that there is structural specificity for this component, although it is possible that uptake of these conjugates was ineffective or that they were inactivated in vivo by a mechanism that does not affect the active Trp conjugates. Because two diverse Trp conjugates were active, it would not be surprising if other related compounds are also auxin antagonists. Initial results indicate that dihydro JA-Trp is at least as active as JA-Trp in the agravitropic response (data not shown). Certain hydroxylated jasmonates are abundant in plants, and they also might be conjugated to form active Trp conjugates (Miersch et al., 2008). Trp conjugates of other compounds related to IAA could also exist and may contribute to regulation of auxin activity.
The amount of exogenous Trp conjugates necessary for activity is considerably higher than the activity range for exogenous IAA. For example, IAA is strongly inhibitory to root growth at 1 μm, while 10- to 50-fold more of the Trp conjugates was required to markedly counter this auxin activity. However, the observed activity is generally consistent with other auxin inhibitors that have been investigated (Oono et al., 2003; Armstrong et al., 2004; Yamazoe et al., 2005; Rojas-Pierce et al., 2007; Hayashi et al., 2008). Low activity could also result if JA-Trp and IAA-Trp were metabolized after uptake by plants. Enzymatic hydrolysis of IAA amide conjugates is known to release free IAA (Rampey et al., 2004). However, the characterized Arabidopsis auxin conjugate hydrolases have relatively low activity on IAA-Trp (LeClere et al., 2002), and the fact that IAA-Trp only weakly inhibited root growth (Fig. 5B) suggests that little free IAA was derived from this conjugate. It would not be surprising if plants had other mechanisms to limit excess accumulation of the Trp conjugates, such as the oxidative catabolism of IAA-Glu (Ljung et al., 2002).
This study suggests that JA is released from JA-Trp because root inhibition by this conjugate was dependent on both JAR1 and COI1. The higher agravitropic activity of (+)-JA-Trp and its lower root inhibiting activity relative to (−)-JA-Trp could arise if the naturally occurring conjugate was a better substrate for enzymatic cleavage. Specific hydrolases active on JA amide conjugates have not been reported, although the auxin conjugate hydrolase IAR3 also apparently acts on JA conjugates (LeClere et al., 2002). As for auxin conjugates, other inactivating modifications of JA-Trp are also possible.
Mechanism of Action
The mechanism for Trp conjugate activity is still unclear. The greater IAA sensitivity of tir1-1 roots when grown with JA-Trp (even though this mutant is resistant to IAA; Fig. 8B), indicates that the TIR1 signaling pathway is required. However, the conjugates did not interfere with IAA in the pull-down assays even at a 200-fold molar excess over IAA. Inactivity in a similar assay was also found for terfestatin A, an auxin inhibitor from Streptomyces sp. F40 (Yamazoe et al., 2005). On the other hand, alkyl-substituted IAAs antagonized the activity of IAA in the interaction assay (Hayashi et al., 2008). It is possible that the Trp conjugates interfere with TIR1 activity in plant cells. Only one of 29 Arabidopsis Aux/ IAA proteins was examined here, and the conjugates might affect TIR1 interaction with other Aux/IAAs differently. Conjugates may also require interaction with other cellular components for activity, and these may be too dilute in the extracts used for pull-down experiments. Alternatively, the antagonism might occur elsewhere in the TIR1 signaling pathway. The conjugates do not appear to affect the auxin transport mechanisms involving AUX1 or NPA-sensitive transport. We are currently analyzing mutants with diminished response to JA-Trp to elucidate the mechanism for Trp conjugate activity.
Auxin antagonists vary markedly in how they affect plant growth. For example, a synthetic auxin inhibitor having an alkyl substitution at the α-position of IAA stimulated root growth markedly, which was attributed to the inhibition of endogenous auxin (Hayashi et al., 2008). In contrast, PCIB suppressed root growth, and this was dependent on TIR1 and Aux/IAA7, suggesting it was not a xenobiotic effect (Oono et al., 2003). PCIB action was hypothesized to be the result of either a direct interference with IAA binding of TIR or a disruption of auxin homeostasis leading to excess IAA production (Biswas et al., 2007). In this study, JA-Trp and IAA-Trp did not promote root growth at any concentration tested, and the weak inhibitory activity observed was likely due largely to JA and IAA activity, as described earlier.
Coordination among hormone signaling paths is a common theme in plants, and shared functions among jasmonate and auxin signaling components are well known (Schwechheimer et al., 2002; Tiryaki and Staswick, 2002; Gray et al., 2003). Jasmonates are integral to normal flower development, and both JA production in flowers and flower development require the ARF6 and ARF8 transcription factors. JA methylester also alters flux through the Trp pathway and increases the level of IAA in Arabidopsis (Nagpal et al., 2005; Dombrecht et al., 2007). The antagonistic activity of JA-Trp on IAA adds to this complex list of mechanisms by which the JA and IAA pathways intersect to coordinate growth, development, and stress response.
MATERIALS AND METHODS
Plant Materials, Growth, and Biochemical Assays
Arabidopsis (Arabidopsis thaliana), ecotype Col-0, was used in all experiments except as noted. The mutants trp5 (Li and Last, 1996) and tir1-1 (Ruegger et al., 1997) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH), coi1-17 and jar1-1 were previously described (Staswick et al., 1992; Suza and Staswick, 2008), and the triple mutant fad3-2 fad7-2 fad8 (McConn and Browse, 1996) and opr3 (Stintzi and Browse, 2000) were from J. Browse. The DR5-GUS reporter line was provided by T. Guilfoyle (Ulmasov et al., 1997). Seedlings were sown on agar media containing the compounds indicated using surface-sterilized seeds essentially as previously described (Staswick and Tiryaki, 2004). Growing roots within the agar by germinating seeds on slots cut into the Murashige and Skoog (MS) media (half-strength MS, pH 6.0, 0.5% Suc, and 1% agar [w/v]) was found to produce roots that grew straightest in the absence of added hormones. Hormones or conjugates were added from 1,000-fold stock solutions in ethanol immediately before pouring plates. Plates containing seeds were incubated at 4°C for 2 to 4 d and then placed on edge in a Percival plant culture chamber and grown at 22°C under 16-h-fluorescent-light/8-h-dark cycles. Root lengths were measured from the point of germination to the root tip. When root length of genotypes differed on control plates, the treatment values were expressed as a percentage of the control length for each genotype, and confidence intervals (95%) were calculated using the delta method. Reorientation of roots to the new gravity vector (Fig. 3) was tested as described by Oono et al. (2003) except that MS medium was used. DR5-GUS experiments were carried out as described by Oono et al. (2003), and fluorimetric determination of enzyme activity followed the methods of Jefferson (1987). Transport inhibitor studies were done according to Rashotte et al. (2000) as described in Table II. Each experiment was done at least three times.
Tissue for hormone extraction was grown as indicated in Table I. Mature plants were grown in Redi Earth (W.R. Grace) in plastic pots in a Conviron growth chamber under the light (approximately 100 μE m−2 s−2) and temperature conditions described above. Harvested tissue was quickly frozen in liquid N and then ground to a powder and stored at −80°C.
Pull-down assays were performed essentially as described by Dharmasiri et al. (2003). Reactions with 800 μg total protein extracted from TIR1-myc seedlings were reacted with glutathione agarose-bound GST-Aux/IAA7 protein in 500 μL of homogenization buffer (50 mm Tris-HCl, pH 7.2,100 mm NaCl, 0.1% Tween 20 [v/v], 10 μm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 10 μm MG132, and miniprotease inhibitor cocktail [Roche]). After rotating 30 min at 4°C, agarose beads were washed three times for 4 min in 1 mL of homogenization buffer minus protease inhibitors. Agarose-bound proteins were dissolved in SDS-PAGE sample buffer, electrophoresed, and transferred to nitrocellulose as described earlier (Staswick, 1989). Immunodetection of TIR1-myc was with an anti-c-myc antibody (Roche) using a Supersignal West Pico chemiluminescent detection kit as described by the manufacturer (Thermo Scientific).
Synthesis of Amino Acid Conjugates
Hormones and other chemicals were from Sigma-Aldrich. Amino acid conjugates were prepared by mixed anhydride condensation reactions essentially as outlined earlier, except that acetonitrile replaced tetrahydrofuran in the reaction (Kramell et al., 1988, 1999; Staswick and Tiryaki, 2004). Conjugates were separated from precursors and side products by silica gel chromatography (1.5 × 50 cm) in chloroform:ethyl acetate:acetic acid (14:6:1 or 3:5:1 [v/v]). Organic solvents were evaporated under a stream of nitrogen from the appropriate column fractions identified by thin-layer chromatography. Conjugates were further purified on reverse phase C18 solid phase extraction columns (Burdick and Jackson; 500 mg, 8 mL) as described earlier (Staswick and Tiryaki, 2004). The two enantiomers of JA-Trp were separated on a semipreparative HPLC column (Luna 5μ C18, 250 mm × 15 mm; Phenomenex) using isocratic 75% methanol (v/v) at 3 mL min−1. Purity and structure of each conjugate was verified by gas GC/MS following derivatization with diazomethane. The spectra for JA-Trp was consistent with that reported previously (Kramell et al., 1988), and that of IAA-Trp agreed with a standard provided by B. Bartel (LeClere et al., 2002). BA-Trp and CA-Trp produced molecular masses on GC/MS that agreed with the expected values for the derivatized compounds. JA conjugates with other amino acids were either described previously (Staswick and Tiryaki, 2004) or synthesized here by similar methods. Internal stable isotope standards for conjugate quantitation by GC/MS were synthesized by scaling down the above protocol and using [13C6]IAA for IAA-Trp or [D6]Trp for JA-Trp (Cambridge Isotope Laboratories). Products gave the expected modified mass spectra compared to the nonisotopic compounds. The other internal standards used were previously described (Staswick and Tiryaki, 2004; Staswick et al., 2005).
Extraction and Quantitation of Jasmonates from Tissue
Solvents were HPLC grade, and chloroform contained 1% ethanol (v/v) as stabilizer. The general methods of Kramell et al. (2000) were followed with modifications as described. Weighed frozen tissue (0.2–0.4 g) was added to 7 mL of 85% (v/v) methanol with butylated hydroxytoluene (100 ng mL−1) and appropriate quantities of internal standards. Tissue was ground 1 min on high speed with an Omni tissue homogenizer (Omni International) and then centrifuged 3 min at 6,000 rpm. Diethylaminoethyl Sephadex A-25 (Sigma-Aldrich) was equilibrated in 0.5 m sodium acetate in methanol and then washed thoroughly with methanol. Ion exchange was performed with a 2-mL bed volume of DEAE resin in a 10-mL disposable syringe with a filter paper frit to retain the resin. After sample loading, columns were washed with 12 mL of methanol and then eluted with 14 mL of 12% formic acid (v/v) in methanol. The eluate was dried in a stream of nitrogen at 48°C. Residue was dissolved in 800 μL of methanol, dried, dissolved in 300 μL of 5% HCl (v/v), and extracted with an equal volume of chloroform. The extract was dried and derivatized in 50 μL of acetone with 1 μL of 1-ethylpiperidine and 5 μL of 2,3,4,5,6-pentafluorobenzyl bromide for 30 min at 55°C as described (Epstein and Cohen, 1981). After drying, the residue was dissolved in 150 μL of chloroform and applied to a mini column with 2 mL of bed volume of silica equilibrated in chloroform. The column was washed with 2 mL of hexane and then eluted with 2 mL of ethyl acetate. After drying, the sample was resuspended in 100 μL of ethylacetate for analysis by GC/MS using negative chemical ionization. A Finnigan Trace GC with an Rtx 5MS column (15 m × 0.25 mm, 0.1 μm) from Restec coupled to a DSQ mass spectrometer was used for the analysis. The injector port was at 280°C, and the gradient for column temperature changes was 25°C min−1. The start temperature of 150°C was held for 1 min, 195°C for 4 min, 245°C for 5 min, and 320°C for 8 min. Rt for pentafluorobenzyl esters of JA-Trp and IAA-Trp were 18.36 and 19.55 min, respectively. The reagent gas was methane with a source temperature of 200°C, and the instrument was operated in selected ion monitoring mode. Frequent cleaning of the ion volume, lenses, and injector liner was necessary for maximal sensitivity of Trp conjugates. Quantitative data were obtained by comparing the integrated peak areas for the respective molecular ion of each compound and the internal standards.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of JA-Trp on root growth of coi1-17 and jar1-1.
Supplemental Figure S2. Growth of Arabidopsis roots on IAA in presence of amino acids.
Supplementary Material
Acknowledgments
I thank M. Estelle for providing the TIR1-myc Arabidopsis line and the Aux/IAA7 GST construct in Escherichia coli for the pull-down assays, J. Browse for JA synthesis mutants, and T. Guilfoyle for the DR5-GUS line. The technical assistance of M. Rowe is gratefully acknowledged.
This research is a contribution of the University of Nebraska Agricultural Research Division, supported in part by funds from the Hatch Act. Additional support was provided by the National Science Foundation (Award IOS–0744758).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Paul E. Staswick (pstaswick1@unl.edu).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Armstrong JI, Yuan S, Dale JM, Tanner VN, Theologis A (2004) Identification of inhibitors of auxin transcriptional activation by means of chemical genetics in Arabidopsis. Proc Natl Acad Sci USA 101 14978–14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt NM, Dong W, Gage DA, Magnus V, Town CD (1999) Metabolism of exogenous auxin by Arabidopsis thaliana: identification of the conjugate Nα-(indol-3-ylacetyl)-glutamine and initiation of a mutant screen. Physiol Plant 105 207–217 [Google Scholar]
- Biswas KK, Ooura C, Higuchi K, Miyazaki Y, Van Nguyen V, Rahman A, Uchimiya H, Kiyosue T, Koshiba T, Tanaka A, et al (2007) Genetic characterization of mutants resistant to the antiauxin p-chlorophenoxyisobutyric acid reveals that AAR3, a gene encoding a DCN1-like protein, regulates responses to the synthetic auxin 2,4-dichlorophenoxyacetic acid in Arabidopsis roots. Plant Physiol 145 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60 183–205 [DOI] [PubMed] [Google Scholar]
- Brückner C, Kramell R, Schneider G, Schmidt J, Preiss A, Sembdner G, Schreiber K (1988) N-[(-)-Jasmonoyl]-s-tryptophan and a related tryptophan conjugate from Vicia faba. Phytochemistry 27 275–276 [Google Scholar]
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, et al (2007) The JAZ family of repressors is the missing link in jasmonate signaling. Nature 448 666–671 [DOI] [PubMed] [Google Scholar]
- Dathe W, Ronsch H, Preiss A, Schade W, Sembdner G (1981) Endogenous plant hormones of the broad bean, Vicia faba L (-)-jasmonic acid, a plant-growth inhibitor in pericarp. Planta 153 530–535 [DOI] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxyacetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198 532–541 [DOI] [PubMed] [Google Scholar]
- Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T, Turner JG (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58 497–513 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005. a) The F-box protein TIR1 is an auxin receptor. Nature 453 441–445 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Jones AM, Estelle M (2003) Auxin action in a cell-free system. Curr Biol 13 1418–1422 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jurgens G, Estelle M (2005. b) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9 109–119 [DOI] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19 2225–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Cohen JD (1981) Microscale preparation of pentafluorbenzyl esters. Electron-capture gas chromatographic detection of indole-3-acetic acid from plants. J Chromatogr 209 413–420 [Google Scholar]
- Estelle MA, Somerville CR (1987) Auxin-resistant mutants of Arabidopsis with an altered morphology. Mol Gen Genet 206 200–206 [Google Scholar]
- Gapper NE, Norris GE, Clarke SF, Lill RE, Jameson PE (2002) Novel jasmonate amino acid conjugates in Asparagus officinalis during harvest-induced and natural foliar senescence. Physiol Plant 114 116–124 [DOI] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE (2003) Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Riemann M, Müller A, Düchting P, Weiler EW, Nick P (2005) Cholodny-Went revisited: a role for jasmonate in gravitropism of rice coleoptiles. Planta 222 575–585 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H (2008) Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc Natl Acad Sci USA 105 5632–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67 283–335 [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7 211–220 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G (2008) Plant immunity to plant herbivores. Annu Rev Plant Biol 59 41–66 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1987) Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep 5 387–405 [Google Scholar]
- Jönsson A (1961) Chemical structure and growth activity of auxin and antiauxins. In W Ruhland, ed, Encyclopedia of Plant Physiology, Vol 14. Springer, Berlin, pp 959–1006
- Kang J, Wang L, Giri A, Baldwin IT (2006) Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell 18 3303–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama M, Kato K, Kimoto H, Fujii S (1995) (S)-(+)-4,4,4-trifuluoro-3-(indole-3)butyric acid, a novel fluorinated plant growth regulator. Experientica 51 721–724 [Google Scholar]
- Katsir L, Chung HS, Koo AJ, Howe GA (2008. a) Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol 11 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008. b) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435 446–451 [DOI] [PubMed] [Google Scholar]
- Kramell R, Miersch O, Atzorn R, Parthier B, Wasternack C (2000) Octadecanoid-derived alteration of gene expression and the “oxylipin signature” in stressed barley leaves. Implications for different signaling pathways. Plant Physiol 123 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramell R, Miersch O, Hause B, Ortel B, Parthier B, Wasternack C (1997) Amino acid conjugates of jasmonic acid induce jasmonate-responsive gene expression in barley (Hordeum vulgare L.) leaves. FEBS Lett 414 197–202 [DOI] [PubMed] [Google Scholar]
- Kramell R, Miersch O, Schneider G, Wasternack C (1999) Liquid chromatography of jasmonic acid amine conjugates. Chromatographia 49 42–46 [Google Scholar]
- Kramell R, Schmidt J, Schneider G, Sembdner G, Schreiber K (1988) Synthesis of N-(jasmonyl)amino acid conjugates. Tetrahedron 44 5791–5807 [Google Scholar]
- LeClere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B (2002) Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277 20446–20452 [DOI] [PubMed] [Google Scholar]
- Leyser O (2006) Dynamic integration of auxin transport and signaling. Curr Biol 16 R424–R433 [DOI] [PubMed] [Google Scholar]
- Li J, Last RL (1996) The Arabidopsis thaliana trp5 mutant has a feedback-resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol 110 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G (2002) Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 50 309–332 [DOI] [PubMed] [Google Scholar]
- MacRae DH, Bonner J (1953) Chemical structure and antiauxin activity. Physiol Plant 6 485–510 [Google Scholar]
- Mandaokar A, Thines B, Shin B, Lange BM, Choi G, Koo YJ, Yoo YJ, Choi YD, Browse J (2006) Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J 46 984–1008 [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Kramell R, Parthier B, Wasternack C (1999) Structure–activity relations of substituted, deleted or stereospecifically altered jasmonic acid in gene expression of barley leaves. Phytochemistry 50 353–361 [Google Scholar]
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 177 114–127 [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24 55–80 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Oka M, Ueda J (1997) Update on the possible mode of action of the jasmonates: focus on the metabolism of cell wall polysaccharides in relation to growth and development. Physiol Plant 100 631–638 [Google Scholar]
- Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, et al (2005) Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132 4107–4118 [DOI] [PubMed] [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimiya H (2003) p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis roots. Plant Physiol 133 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ (2001) Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. Plant J 25 399–406 [DOI] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130 1908–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey A, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B (2004) A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol 135 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Pierce M, Titapiwatanakun B, Sohn EJ, Fang F, Larive CK, Blakeslee J, Cheng Y, Cutler SR, Peer WA, Murphy AS, et al (2007) Arabidopsis P-glycoprotein19 participates in the inhibition of gravitropism by gravacin. Chem Biol 14 1366–1376 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Xu J (2006) Polar auxin transport and patterning: grow with the flow. Genes Dev 20 922–926 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Deng XW (2002) Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 14 2553–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE (1989) Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol 89 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE (2008) JAZing up jasmonate signaling. Trends Plant Sci 13 66–71 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate biosynthesis. Proc Natl Acad Sci USA 97 10625–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M, Rojas-Pierce M, Carter C, Hicks GR, Vasquez J, Raikhel NV (2005) The power of chemical genomics to study the link between endomembrane system components and the gravitropic response. Proc Natl Acad Sci USA 102 4902–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suza W, Staswick P (2008) The role of JAR1 in jasmonoyl-L-isoleucine production in Arabidopsis wound response. Planta 227 1221–1232 [DOI] [PubMed] [Google Scholar]
- Swiatek A, Lenjou M, Van Bockstaele D, Inze D, Van Onckelen H (2002) Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol 128 201–211 [PMC free article] [PubMed] [Google Scholar]
- Swiatek A, Van Dongen W, Esmans EL, Van Onckelen H (2004) Metabolic fate of jasmonates in tobacco bright yellow-2 cells1. Plant Physiol 135 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamogami S, Rakwal R, Agrawal GK (2008) Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Biophys Res Commun 376 723–727 [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446 640–645 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448 661–665 [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Staswick PE (2002) An Arabidopsis thaliana mutant defective in jasmonate response is allelic to the auxin signaling mutant axr1. Plant Physiol 130 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic S, Gabdoulline RR, Kojic-Prodic B, Wade RC (1998) Classification of auxin plant hormones by interaction property similarity indices. J Comput Aided Mol Des 12 63–79 [DOI] [PubMed] [Google Scholar]
- Ueda J, Kato J (1982) Inhibition of cytokinin-induced plant-growth by jasmonic acid and its methyl-ester. Physiol Plant 54 249–252 [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT (2007) Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 226 159–167 [DOI] [PubMed] [Google Scholar]
- Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Sugawara J, Susuki Y, Shimamura E, Takahashi N (1980) Syntheses of jasmonic acid related compounds and their structure-activity relationship on the growth of rice seedlings. Agric Biol Chem 44 2857–2864 [Google Scholar]
- Yamazoe A, Hayashi K, Kepinski S, Leyser O, Nozaki H (2005) Characterization of terfestatin A, a new specific inhibitor for auxin signaling. Plant Physiol 139 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT (2006) Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant Cell Environ 29 1751–1760 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Turner JG (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3 e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.