Abstract
In the Solanaceae, biotic and abiotic elicitors induce de novo synthesis of sesquiterpenoid stress metabolites known as phytoalexins. Because plant hormones play critical roles in the induction of defense-responsive genes, we have explored the effect of abscisic acid (ABA) on the synthesis of capsidiol, the major wild tobacco (Nicotiana plumbaginifolia) sesquiterpenoid phytoalexin, using wild-type plants versus nonallelic mutants Npaba2 and Npaba1 that are deficient in ABA synthesis. Npaba2 and Npaba1 mutants exhibited a 2-fold higher synthesis of capsidiol than wild-type plants when elicited with either cellulase or arachidonic acid or when infected by Botrytis cinerea. The same trend was observed for the expression of the capsidiol biosynthetic genes 5-epi-aristolochene synthase and 5-epi-aristolochene hydroxylase. Treatment of wild-type plants with fluridone, an inhibitor of the upstream ABA pathway, recapitulated the behavior of Npaba2 and Npaba1 mutants, while the application of exogenous ABA reversed the enhanced synthesis of capsidiol in Npaba2 and Npaba1 mutants. Concomitant with the production of capsidiol, we observed the induction of ABA 8′-hydroxylase in elicited plants. In wild-type plants, the induction of ABA 8′-hydroxylase coincided with a decrease in ABA content and with the accumulation of ABA catabolic products such as phaseic acid and dihydrophaseic acid, suggesting a negative regulation exerted by ABA on capsidiol synthesis. Collectively, our data indicate that ABA is not required per se for the induction of capsidiol synthesis but is essentially implicated in a stress-response checkpoint to fine-tune the amplification of capsidiol synthesis in challenged plants.
The induction of the synthesis of secondary stress metabolites known as phytoalexins represents part of the metabolic responses induced in plants following the action of abiotic and biotic elicitors (Kuc, 1995). As a consequence of this stress-induced metabolism, sesquiterpenoid phytoalexins are synthesized in solanaceous plants (Kuc, 1982). In pepper (Capsicum annuum) and tobacco (Nicotiana tabacum), the bicyclic sesquiterpene capsidiol represents the main type of phytoalexin. Capsidiol is synthesized from farnesyl diphosphate via a two-step process catalyzed by 5-epi-aristolochene synthase (EAS; Facchini and Chappell, 1992) and 5-epi-aristolochene hydroxylase (EAH; Ralston et al., 2001). Despite continuing efforts, our understanding of the mechanisms that regulate the amplification of capsidiol synthesis in challenged plants is poorly understood, compared with what we know about camalexin in Arabidopsis (Arabidopsis thaliana; Ren et al., 2008). Reactive oxygen species have been implicated in the expression of EAS in tobacco (Rusterucci et al., 1996; Yin et al., 1997) and in pepper (Maldonado-Bonilla et al., 2008) and in the accumulation of capsidiol in tobacco (Perrone et al., 2003) and in pepper (Arreola-Cortés et al., 2007). In addition, it has been suggested that Ca2+, calmodulin, Ca2+-dependent protein kinases, and phosphoinositide signaling are involved in the regulation of capsidiol synthesis in tobacco (Vogeli et al., 1992; Preisig and Moreau, 1994; Tavernier et al., 1995) and in pepper (Ma, 2008).
Recent investigations have highlighted an essential role of plant hormones in the induction of plant defense responses (Jones and Dangl, 2006; Asselbergh et al., 2008b; Lopez et al., 2008; Spoel and Dong, 2008). In tomato (Solanum lycopersicum), which produces rishitin as the main sesquiterpene phytoalexin, the susceptibility to the phytopathogen fungus Phytophthora parasitica is decreased by salt and water stresses, which elevate the concentration of abscisic acid (ABA; Ristaino and Duniway, 1989). Studies examining loss-of-function mutations in the ABA pathway reveal that ABA deficiency enhances the resistance of tomato to infection by the necrotrophic fungus Botrytis cinerea (Audenaert et al., 2002) and to the bacterial pathogen Erwinia chrysanthemi (Asselbergh et al., 2008a). This trend is reinforced by the fact that tobacco plants treated with ABA become more susceptible to Tobacco mosaic virus (Balazs et al., 1973) and to the blue mold pathogen Peronospora tabacina (Salt et al., 1986). Interestingly, the transcription of genes encoding β-1,3-glucanase isoforms known to be involved in defense responses is down-regulated in tobacco cell cultures treated with exogenous ABA (Rezzonico et al., 1998). With respect to phytoalexin, it has been reported that application of exogenous ABA reduces the accumulation of rishitin and lubimin, two sesquiterpenoid stress phytoalexins produced in potato (Solanum tuberosum) tuber slices infected by incompatible races of Phytophthora infestans but not by the compatible races (Henfling et al., 1980). However, the reduction of rishitin and lubimin synthesis by ABA could not be observed if potato tubers were not previously stored at 4°C (Bostock et al., 1983). Analysis of the role of plant hormones on capsidiol synthesis is limited to the fact that jasmonic acid is not a direct regulator of the capsidiol pathway in tobacco (Mandujano-Chavez et al., 2000) and in pepper (Ma, 2008).
In this study, we utilized wild-type Nicotiana plumbaginifolia and the nonallelic mutants Npaba2 (Marin et al., 1996) and Npaba1 (Kraepiel et al., 1994), which are impaired in the biosynthesis of ABA, in order to directly investigate if ABA regulates the synthesis of capsidiol following elicitation with cellulase and arachidonic acid (AA) and infection with Botrytis cinerea and to explore the potential mechanisms by which this regulation is achieved. We provide evidence that ABA is not required for capsidiol synthesis but plays a key role in the adjustment of capsidiol production level in plants. We show that ABA down-regulates the transcription of capsidiol biosynthetic genes and subsequently the level of capsidiol production. We also show that in wild-type plants, the biosynthesis of capsidiol is accompanied by decreased bioactive ABA and the induction of ABA 8′-hydroxylase (ABAH) involved in ABA catabolism, consistent with a negative regulation of ABA in capsidiol synthesis. Collectively, our data suggest that ABA plays an essential role in fine-tuning the amplification of capsidiol synthesis in challenged plants.
RESULTS
ABA-Deficient Mutants Exhibit Excessive Capsidiol Synthesis
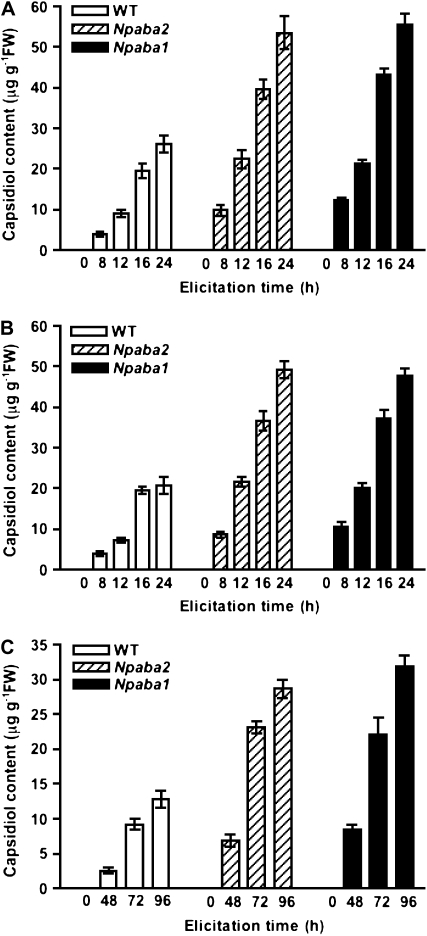
The capacity of wild-type N. plumbaginifolia plants to produce capsidiol was first tested using cellulase and AA as elicitors and following infection by B. cinerea. The accumulation of total capsidiol was analyzed and measured over time from the leaf discs and the aqueous leaf disc diffusates. The synthesis of capsidiol was induced following cellulase and AA treatments and in response to B. cinerea infection, as shown by the typical gas chromatography-mass spectrometry (GC-MS) profile (Supplemental Fig. S1). The synthesis of capsidiol was strongly elicited both by cellulase and AA treatments, with maximum values around 35 ± 3 and 28 ± 2 μg g−1 fresh weight at 30 h for cellulase and AA, respectively. A significant period of time was required for B. cinerea to elicit the synthesis of capsidiol, and the response was less intense (15 ± 2 μg g−1 fresh weight at 120 h) compared with cellulase and AA, whatever the incubation time. Capsidiol was not produced in measurable amounts in nonelicited leaf discs.
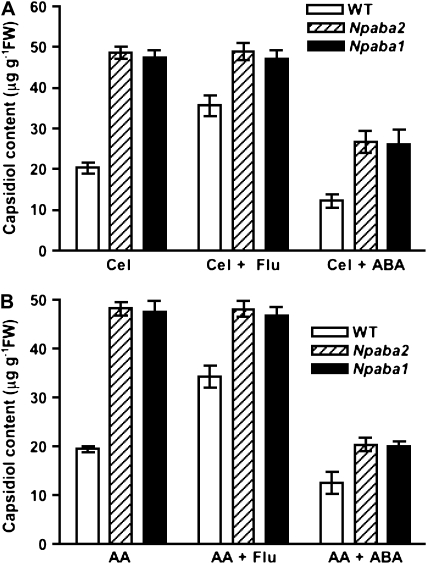
To determine if capsidiol synthesis was dependent upon ABA levels, we used wild-type N. plumbaginifolia and two ABA-deficient mutants, Npaba2, impaired in zeaxanthin epoxidase (ZEP; Marin et al., 1996), and Npaba1 (Kraepiel et al., 1994), deficient in abscisic aldehyde oxidase, which catalyzes later steps of ABA synthesis. Equivalent quantities of leaf discs excised from wild-type plants, Npaba2, and Npaba1 were treated with cellulase or AA or infected by B. cinerea. The accumulation of capsidiol was analyzed at designated time intervals over a 24-h period for cellulase and AA and at 96 h for plants infected by B. cinerea. Total capsidiol produced by Npaba2 and Npaba1 mutants nearly doubled that observed for wild-type plants (Fig. 1), even in the case of plants infected with B. cinerea, known to produce ABA (Inomata et al., 2004). Based on the magnitude of the quantitative increase of capsidiol, ABA may be a key negative modulator of enhanced capsidiol synthesis in challenged plants. To further test this hypothesis, we made use of the phytoene desaturase inhibitor fluridone (Bartels and Watson, 1978) to block the upstream branch of the ABA pathway. Previous studies have shown that fluridone blocks de novo ABA biosynthesis in pathogen-challenged plants (Koga et al., 2004). When discs were cotreated with cellulase and fluridone (10 μm), the accumulation of capsidiol in leaf discs was strongly enhanced in wild-type plants, suggesting an ABA-dependent effect in leaf tissue (Fig. 2A). In contrast, the level of capsidiol in Npaba2 and Npaba1 mutants remained unchanged under these conditions (Fig. 2A). Very similar results were obtained with AA (Fig. 2B). Thus, the fluridone-induced increase of capsidiol in elicited wild-type plants recapitulates the effect observed with Npaba2 and Npaba1 mutants treated with cellulase or AA alone and, hence, is consistent with negative regulation exerted by ABA. This prominent role of ABA is further supported by the fact that cotreatment of wild-type, Npaba2, and Npaba1 plants with ABA (25 μm) and cellulase or AA markedly reduced the induced synthesis of capsidiol in wild-type plants and in Npaba2 and Npaba1 mutants (Fig. 2). ABA and fluridone in the absence of cellulase or AA did not induce capsidiol synthesis (data not shown).
Figure 1.
Capsidiol synthesis in N. plumbaginifolia wild-type (WT), Npaba2, and Npaba1 plants in response to elicitors and infection with B. cinerea. Leaf discs were incubated with cellulase (0.5%; A) or AA (1 mm; B) or infected by B. cinerea (C) for the indicated times before capsidiol analysis. Data represent means ± se of triplicate samples. FW, Fresh weight.
Figure 2.
Effects of fluridone and ABA on elicitor-induced capsidiol synthesis in N. plumbaginifolia wild-type (WT), Npaba2, and Npaba1 plants. A, Leaf discs were treated with cellulase (Cel; 0.5%) alone, cellulase (0.5%) plus fluridone (Flu; 10 μm), or cellulase (0.5%) plus ABA (25 μm). B, Leaf discs were treated with AA (1 mm) alone, AA (1 mm) plus fluridone (10 μm), or AA (1 mm) plus ABA (25 μm). After 22 h of incubation, capsidiol synthesis was determined. Data represent means ± se of triplicate samples. FW, Fresh weight.
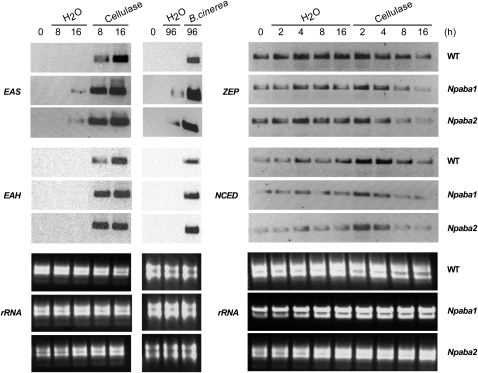
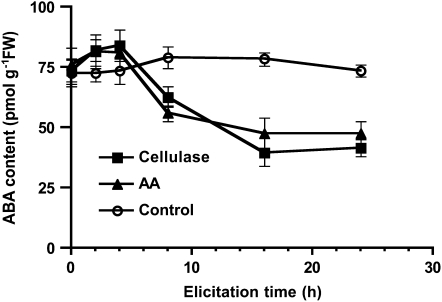
To further analyze the mechanism by which ABA modulates capsidiol synthesis in cellulase-elicited plants, we determined the expression of EAS and EAH, which encode the enzymes of the later steps of capsidiol synthesis, in addition to ZEP and 9-cis-epoxy-carotenoid dioxygenase (NCED), which encode enzymes of the upstream pathway of ABA synthesis. We observed that EAS and EAH transcript accumulation reflected the induced synthesis of capsidiol, in good agreement with previous data obtained from tobacco (Facchini and Chappell, 1992; Yin et al., 1997) and pepper (Hugueney et al., 1996; Fig. 3). However, the transcript levels of EAS and EAH were higher in Npaba2 and Npaba1 compared with wild-type plants (Fig. 3). The expression of the N. plumbaginifolia pathogenesis-related protein gene PR-1a (Payne et al., 1988), monitored by reverse transcription (RT)-PCR, displayed an expression pattern similar to that of EAS and EAH (data not shown) and is in agreement with the fact that exogenous ABA down-regulated the accumulation of β-1,3-glucanase (Rezzonico et al., 1998). The accumulation of ZEP and NCED mRNAs was observed in unelicited discs and during the initial period of elicitation (Fig. 3). During the period of capsidiol accumulation, ZEP and NCED mRNAs decreased (Fig. 3). Although we used a northern-blot hybridization procedure to better judge the quality of the mRNAs, we confirmed the expression patterns by two independent semiquantitative RT-PCR analyses (data not shown). Consistent with these findings, the level of ABA in wild-type plants was slightly increased during the first period following exposure to cellulase and AA and decreased thereafter significantly concomitant with capsidiol accumulation (Fig. 4). Considered together, these data suggest that ABA negatively regulates the amplification of capsidiol biosynthesis in challenged plants.
Figure 3.
Northern-blot analysis of ABA and capsidiol biosynthetic genes in N. plumbaginifolia wild-type (WT), Npaba2, and Npaba1 plants elicited with cellulase or infected by B. cinerea. Leaf discs were incubated with cellulase (0.5%), infected by B. cinerea, or treated with water for the indicated times before RNA analysis. The ethidium bromide-stained gel displaying ribosomal RNA (rRNA) is shown as a loading control.
Figure 4.
Changes in the ABA content of N. plumbaginifolia wild-type plants elicited with cellulase or AA. Leaf discs from wild-type plants were elicited with cellulase (0.5%) or AA (1 mm) or incubated with water (Control) for the indicated times, and ABA was extracted and quantified using an indirect ELISA method as described in “Materials and Methods.” Data represent means ± se of triplicate samples. FW, Fresh weight.
Induction of ABA Catabolism during Elicited Synthesis of Capsidiol
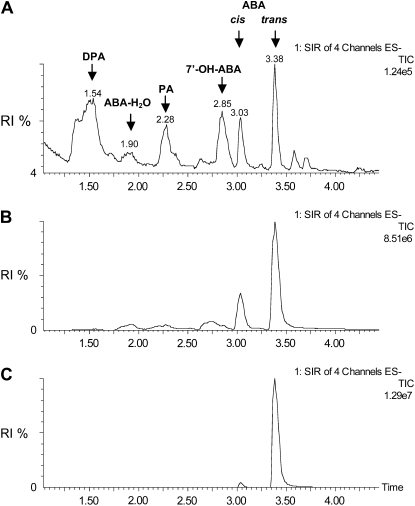
The above data indicate that the synthesis of capsidiol in elicited plants is up-regulated in Npaba2 and Npaba1 ABA-deficient mutants and in wild-type plants treated with fluridone. These changes were paralleled by a decrease in ABA concentration during the phase of capsidiol accumulation. Based on this evidence, one could suggest that the amplification of capsidiol synthesis requires at least decreased availability of free ABA. How might this occur? One may predict that pathways leading to ABA catabolism could be activated concomitant to the induced synthesis of capsidiol. The main ABA catabolic pathway is mediated by ABAH to yield the unstable 8′-hydroxy-ABA, which spontaneously rearranges to phaseic acid (PA), which produces dihydrophaseic acid (DPA) after reduction (Cutler and Krochko, 1999). We observed by exploiting available genome-wide array analysis that in Arabidopsis challenged with Pseudomonas syringae, not only ABAH but also gibberellin 2-oxidase (GA-2Ox), which encodes a gibberellin catabolic oxidase, were induced (de Torres-Zabala et al., 2007). GA-2Ox catalyzes the 2β-hydroxylation of gibberellin to generate biologically inactive gibberellins (Thomas et al., 1999). Similarly, mRNA differential display analysis of genes expressed in tobacco infected by the phytopathogen Rhodococcus fascians revealed the expression of putative ABAH and GA-2Ox genes (Simon-Mateo et al., 2006). Thus, we further analyzed the possible impact of ABAH during the elicited synthesis of capsidiol. We cloned the putative N. plumbaginifolia cDNA ABAH (NpABAH) and analyzed its activity using an in vivo transient expression procedure. Leaves from wild-type plants were transfected with the expression plasmid (NpABAH) or the vector alone (NpΔABAH). At 72 h after transfection, leaf discs expressing NpABAH and NpΔABAH were incubated with exogenous ABA (25 μm) for 20 h prior to the analysis of ABA derivatives. The presence of ABA and ABA catabolites was determined based on the retention time (tR) of each chromatographic peak and by tandem mass spectrometry (MS/MS) using ultra-performance liquid chromatography coupled with electrospray ionization-mass spectrometry (UPLC-ESI-MS/MS). The examination of the chromatograms obtained in both full-scan MS mode (data not shown) and single ion recording-MS mode revealed that for ABA and ABA derivatives, the best ionization mode was the negative mode, as reported elsewhere (Chiwocha et al., 2003). The unraveling of the fragmentation patterns obtained by daughter scan monitoring MS/MS analysis for each of the ABA derivatives permitted the determination of the most intense daughter fragments, which have been subsequently used as diagnostic fragments for multiple reaction monitoring (MRM) analysis. As a result of the MRM analysis, key evidence was obtained to confirm the structure of ABA and ABA derivatives. MRM analysis of NpABAH leaf extracts allowed the identification of four compounds corresponding to DPA (tR 1.54 min, mass-to-charge ratio [m/z] 281, daughter ion m/z 171), PA (tR 2.28 min, m/z 279, daughter ion m/z 139), 7′-OH-ABA (tR 2.85 min, m/z 279, daughter ion m/z 151), cis-ABA (tR 3.03 min, m/z 263, daughter ion m/z 153), and trans-ABA (tR 3.38 min, m/z 263, daughter ion m/z 153; Fig. 5; Supplemental Fig. S2). Although exogenous ABA could induce its own catabolism (Windsor and Zeevaart, 1997; Saito et al., 2004), we observed basal levels or undetectable ABA catabolites both in leaves transiently overexpressing the control vector (NpΔABAH; Fig. 5) and in untransformed leaves (data not shown). Collectively, these data demonstrate that NpABAH is functional in vivo.
Figure 5.
Functional analysis of NpABAH and UPLC-MS/MS analysis of reaction products. A and B, UPLC-MS/MS analysis of extracts from wild-type plants overexpressing NpABAH (A) or the empty vector control (NpΔABAH; B) incubated with exogenous ABA. C, Chromatogram of the ABA standard. NpABAH and NpΔABAH were expressed in leaves of wild-type N. plumbaginifolia using an Agrobacterium transient expression system. At 72 h after transfection, 30 discs punched out from leaves overexpressing NpABAH or NpΔABAH were incubated with exogenous ABA (25 μm) for 20 h before chromatographic analysis. UPLC-MS/MS analysis was conducted using a selected ion recording (SIR)-MS mode. The selected ion recording-MS chromatograms were obtained by monitoring the of the parent mass: m/z 263 for ABA, m/z 279 for PA, m/z 279 for 7′-OH-ABA, and m/z 281 for DPA. RI, Relative intensity; TIC, total ion chromatogram.
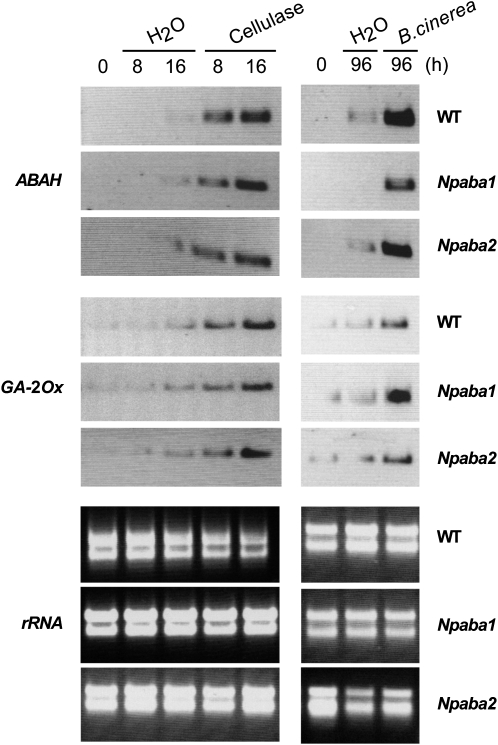
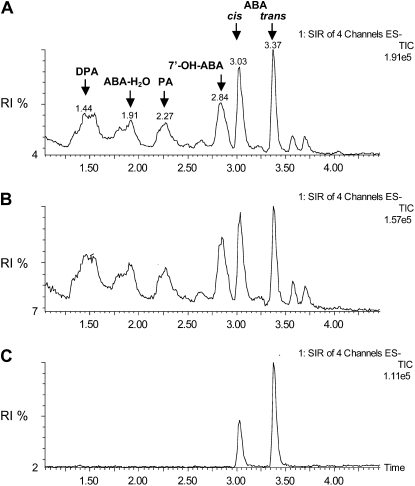
To further investigate the implication of ABAH in the elicited/induced response, we analyzed the expression of ABAH following elicitation by cellulase and B. cinerea infection. Expression of ABAH was induced in wild-type plants and in Npaba2 and Npaba1 mutants after cellulase treatment at time points corresponding to the expression of EAS and EAH and capsidiol synthesis (Fig. 6). Along with the induction of ABAH after cellulase treatment, expression of GA-2Ox was also induced (Fig. 6; see also Figs. 1 and 3) concomitant with the accumulation of EAS and EAH transcripts and the enhanced synthesis of capsidiol (Figs. 1 and 3). Consistent with these findings, ABA catabolites (PA, DPA, and 7′-OH-ABA) could be detected in wild-type plants following elicitation by cellulase or AA (data not shown) or after infection by B. cinerea relative to controls (Fig. 7A). Overall, the observations herein support the implication of ABA and possibly GA in the synthesis of capsidiol.
Figure 6.
Northern-blot analysis of ABAH and GA-2Ox transcript levels in N. plumbaginifolia wild-type (WT), Npaba2, and Npaba1 plants elicited with cellulase or infected by B. cinerea. Leaf discs were incubated with cellulase or infected by B. cinerea for the indicated times and processed as described in Figure 3. rRNA, Ribosomal RNA.
Figure 7.
UPLC-MS/MS analysis of ABA catabolites in wild-type N. plumbaginifolia elicited with cellulase or infected by B. cinerea. Leaf discs were elicited with cellulase for 20 h (A), infected with B. cinerea for 96 h (B), or incubated in water (control) for 96 h (C) before extraction and analysis of ABA catabolites. The selected ion recording (SIR)-MS chromatograms were obtained as in Figure 5. RI, Relative intensity; TIC, total ion chromatogram.
DISCUSSION
Role of ABA in Phytoalexin Production
Phytoalexins are produced in plants in response to pathogen infection or to biotic and abiotic elicitors. Mutations leading to phytoalexin deficiency can increase plant susceptibility (Thomma et al., 1999). A number of studies have documented the role played by hormones in the control of plant stresses and diseases. Exogenous ABA enhances the resistance or the susceptibility of various plants to pathogens (for reviews, see Mauch-Mani and Mauch, 2005; Robert-Seilaniantz et al., 2007; Asselbergh et al., 2008b; Lopez et al., 2008). In some extreme cases, as observed in Arabidopsis infected by Ralstonia solanacearum, which leads to xylem occlusion, 40% of the up-regulated genes are linked or belong to the ABA signaling or biosynthetic pathway (Hu et al., 2008). The above-mentioned implication of ABA in biotic stresses prompted us to analyze its effect on the biosynthesis of capsidiol, the main sesquiterpenoid phytoalexin produced in tobacco (Kuc, 1982).
In this report, we took advantage of the wild type and Npaba2 and Npaba1 lines of N. plumbaginifolia to explore the effect of ABA on the regulation of capsidiol synthesis. First, we determined the capacity of different elicitors, including cellulase, AA, and B. cinerea, to induce the synthesis of capsidiol in challenged N. plumbaginifolia. Although the synthesis of capsidiol was induced following cellulase and AA treatments and in response to infection by B. cinerea, the amount of caspidiol nearly doubled in Npaba2 and Npaba1 mutant lines compared with wild-type plants. Thus, ABA negatively impacted the amplification of capsidiol synthesis. If the effect was due to ABA itself, similar results should be obtained by cotreatment with cellulase or AA and fluridone, a carotenoid desaturase inhibitor that affects the upstream branch of the ABA pathway. When wild-type, Npaba2, and Npaba1 plants were cotreated with fluridone and cellulase or AA and fluridone, the accumulation of capsidiol nearly doubled in wild-type plants and remained unchanged in Npaba2 and Npaba1 mutant plants (Fig. 2). Interestingly, a significant decrease in ABA content occurred during capsidiol synthesis in wild-type plants (Fig. 4). In a similar vein, ABA level was reduced in soybean (Glycine max) infected by Phytophthora megasperma (Cahill and Ward, 1989). Consistent with this trend, in sugar beet (Beta vulgaris) infected by Cercospora beticola, the early stage of the infection is characterized by an increased production of ABA, probably synthesized by the pathogen or the plant itself through fungal stimulation. Following this initial stage, the concentration of ABA is decreased (Schmidt et al., 2008). The change in the ABA content that we observed was reflected by the expression pattern of NCED and ZEP and is consistent with previous studies demonstrating that the geranylgeranyl diphosphate pathway leading to carotenoids is down-regulated or unaffected following elicitation or pathogen infection (Hugueney et al., 1996; Truman et al., 2006). Collectively, our results suggest that ABA represses the amplification of capsidiol synthesis. Several studies suggest that ABA may have a role in the regulation of phytoalexins in legumes. In peanut (Arachis hypogaea), drought stress is associated with increased susceptibility to Aspergillus flavus and to reduced endogenous phytoalexin (Wotton and Strange, 1987). The synthesis of the two bean (Phaseolus vulgaris) phytoalexins, phaseolin and kievitone, is differently regulated following mercuric chloride elicitation. ABA down-regulates mercuric chloride-induced kievitone synthesis, while phaseolin synthesis is unaffected (Goossens and Vendrig, 1982). In soybean, following infection by P. megasperma (Ward et al., 1989) or Phytophthora sojae (Mohr and Cahill, 2001), the synthesis of glyceollin is reduced in an ABA-dependent manner (Ward et al., 1989). In Solanaceae, the reduction of rishitin and lubimin sesquiterpenoid phytoalexins by application of exogenous ABA has been shown in potato tubers infected by P. infestans (Henfling et al., 1980; Bostock et al., 1983). However, the reduction of rishitin and lubimin could only be demonstrated after a cold pretreatment at 4°C (Bostock et al., 1983). The role of the cold treatment in the measured responses is not understood at present.
The mechanism whereby ABA mediates its effects on capsidiol synthesis requires further study. Because exogenous ABA down-regulates the expression of defense genes induced by jasmonic acid, ethylene, and salicylic acid (Anderson et al., 2004; Thaler and Bostock, 2004; Yasuda et al., 2008), the antagonism between ABA and these hormones has usually been highlighted. However, the situation is probably more complex, because the hypersensitivity of the Arabidopsis enhanced disease resistance1 mutant to ABA is associated with enhanced expression of PR-1 protein (Frye and Innes, 1998; Wawrzynska et al., 2008) and salicylate-enhanced resistance to powdery mildew (Frye et al., 2001). Alternatively, it has been suggested that ABA acts via ethylene response factors, which regulate GCC box-containing defense genes (Zhou et al., 2008). According to this hypothesis, ABA affects the interaction between ethylene response factors and GCC boxes. High ABA content destabilizes ethylene response factors and GCC box interactions and impairs the expression of defense genes, while low ABA favors the interaction and their transcription (Zhou et al., 2008). However, although GCC boxes are indeed present in several pathogenesis-related proteins, neither tobacco EAS (Yin et al., 1997) nor pepper EAS has GCC boxes. Furthermore, the ethylene biosynthetic inhibitor aminoethoxyvinylglycine does not inhibit the accumulation of capsidiol in tobacco as elicited by Phytophthora nicotianae (Nemestothy and Guest, 1990). Alternatively, ABA may play a role via cADP-Rib, an intracellular calcium mobilizer (Wu et al., 1997). Consistent with this hypothesis, the synthesis of sesquiterpenoid phytoalexin rishitin in potato (Zook et al., 1987) and capsidiol in tobacco (Vogeli et al., 1992; Preisig and Moreau, 1994; Tavernier et al., 1995) and in pepper (Ma, 2008) is dependent upon Ca2+. Interestingly, through the use of the calcium sensor aequorin, Ca2+ mobilization could be observed during elicitation in tobacco (Knight et al., 1991).
Induction of Hormone Catabolic Pathways during Defense Responses
The expression of ABAH was induced during capsidiol accumulation (Fig. 6). Because ABAH is a key enzyme of the ABA catabolic pathway, we reasoned that ABAH might affect ABA homeostasis by reducing the level of free ABA concomitant with capsidiol accumulation. We first assessed the functionality of NpABAH. Transient overexpression of ABAH in wild-type N. plumbaginifolia leaf incubated with exogenous ABA resulted in the accumulation of the typical ABA catabolic products PA, DPA, and 7′-OH-ABA (Fig. 5). In wild-type plants, the implication of ABAH was tested in vivo following elicitation by cellulase and infection by B. cinerea. As an indicator of ABAH activity, one may expect that the accumulation pattern of ABAH mRNAs could be correlated to the decrease of free ABA (Fig. 4) and to the accumulation of typical ABA catabolic products (Fig. 7). In addition, we observed that the hormone catabolic pathway is not limited to influence by ABA but probably is affected by the gibberellins as well. GA-2Ox was induced in parallel with the accumulation of capsidiol and EAS and EAH (Fig. 6). GA-2Ox serves as a marker for the catabolism of GA, and its activity is in general low under normal conditions (Thomas et al., 1999). Interestingly, it has been shown that two known inhibitors of GA biosynthesis, prohexadione-calcium and trinexapac-ethyl, enhance the resistance of apple (Malus domestica) trees to Rewinia amylovora concomitant with an increased expression of genes encoding pathogenesis-related proteins (Maxson and Jones, 2002). In addition, in rice (Oryza sativa), overexpression of the elongated uppermost internode gene, which deactivates GA, leads to disease resistance (Yang et al., 2008). Thus, the expression pattern we observe for ABAH and GA-2Ox suggests a regulatory role of ABA and possibly GA on capsidiol synthesis exerted via ABA and GA catabolic pathways. This hypothesis is supported by data mining of several transcriptomic analyses. For instance, At4g19230 and At3g19270, which encode two functionally characterized ABAHs and GA-2Ox, are induced in Arabidopsis infected by P. syringae (de Torres-Zabala et al., 2007). Similarly, transcriptomic analysis of Arabidopsis infected by R. solanacearum also revealed induction of GA-2Ox and ABAH (Hu et al., 2008). An mRNA differential display analysis of N. tabacum infected by R. fascians shows overexpression of ABAH and GA-2Ox (Simon-Mateo et al., 2006). Likewise, decreased expression of the kaurenoic acid oxidase is reported in sitiens, an ABA-deficient tomato mutant infected by B. cinerea (Asselbergh et al., 2007). Interestingly, changes in expression of several auxin- and gibberellin-modifying enzymes have also been observed in microarray experiments using Arabidopsis and the salicylic analog benzothiadiazole S-methylester (Wang et al., 2006, 2007). Finally, it has been shown that rice plants overexpressing GH3, which is implicated in the conjugation of plant hormones to amino acids, display enhanced resistance to fungal pathogens (Domingo et al., 2009). Although these catabolic pathways are implicated in the negative regulation exerted by ABA and GA in plants subjected to elicitors and pathogens, alternative possibilities may exist. For example, it has been reported that in Arabidopsis infected by the pathogen Blumeria graminis, a NAC transcription factor designated ATAF1 down-regulates the accumulation of ABA according to an unknown mechanism while enhancing the resistance to the pathogen (Jensen et al., 2008).
Implication of the Role of ABA in Biotic Interactions
Finally, we show that the induction of capsidiol synthesis per se is not dependent upon ABA itself, but the amplification of capsidiol synthesis is regulated by ABA. ABA attenuates excessive synthesis of capsidiol following elicitation or pathogen invasion. This may account for the elevated level of capsidiol in ABA-deficient plants. We can imagine the in vivo scenario to include the negative regulation exerted by ABA ensuring that an adequate level of capsidiol is synthesized in challenged plants. Once the challenge is mastered, ABA down-regulates the synthesis of capsidiol to restrict its overaccumulation, which otherwise may lead to tissue injury in the host (Polian et al., 1997) or trigger exhaustive draining of carbon precursors from primary metabolism, as observed during the synthesis of amino acid-derived phytoalexins (Schaaf et al., 1995; Zhao and Last, 1996; Batz et al., 1998; Logemann et al., 2000; van der Fits and Memelink, 2000; Ren et al., 2008). ABA could also represent a plant signature sensed by pathogens. Replication and propagation require signals at the pathogen level to adjust a pathogen's sexual cycle and virulence. This nonclassic signaling role of ABA is supported by the fact that in the apicomplexan parasite Toxoplasma gondii, ABA dictates the transition between the dormant cyst stage and the lytic growth (Nagamune et al., 2008). Because 82% of fungi live in association with terrestrial plants (Schmit and Mueller, 2007), plant-derived signals may play key roles in the modulation and regulation of nonplant organism development. In this context, Cryptococcus species are lethal fungal pathogens of humans and animals that can complete their life cycle on plant surfaces due to the presence of myoinositol exudate from Arabidopsis and Eucalyptus leaves (Xue et al., 2007). Interestingly, auxin acts synergistically with myoinositol to stimulate the mating of Cryptococcus species (Xue et al., 2007). It is interesting that although ABA does not directly affect fungal growth (Henfling et al., 1980), it could perturb fungal reproduction. For example, the sexual reproduction of Hyaloperonospora parasitica is abolished in the Arabidopsis ABA-deficient mutant aba1-1 (Mauch-Mani and Mauch, 2005). Thus, it is not surprising that some pathogens “hijack” the ABA biosynthetic or signaling pathway to adapt their level of virulence (de Torres-Zabala et al., 2007; Zhou et al., 2008). In the context of symbiosis, it has been shown that ABA (Ding et al., 2008) and GA (Maekawa et al., 2009) are negative regulators of legume nodulation, suggesting a wider implication of ABA and probably GA in the fine-tuning of biotic interactions through a negative regulatory mode.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Elicitation Procedure
Nicotiana plumbaginifolia wild-type seeds and Npaba2 and Npaba1 mutant seeds were kindly provided by Dr. A. Marion-Poll (INRA). Npaba2 of N. plumbaginifolia is impaired in the epoxidation of zeaxanthin catalyzed by ZEP (Marin et al., 1996), and Npaba1 in the last step of ABA biosynthesis (i.e. the oxidation of ABA-aldehyde into ABA, catalyzed by abscisic aldehyde oxidase; Kraepiel et al., 1994). Plants were grown under greenhouse conditions or in growth chambers saturated at 80% relative humidity for Npaba2. Leaf discs (1.75 cm diameter) were excised with a cork borer from leaves of 75-d-old plants. Leaf discs were floated (adaxial surface up) on a solution of cellulase (0.5%) from Trichoderma viride (Sigma-Aldrich; Hugueney et al., 1996) or 1 mm AA (Sigma-Aldrich; Bloch et al., 1984) to induce the synthesis of capsidiol. A conidial suspension of Botrytis cinerea (isolate Flo-07) kindly provided by Drs. S. Wiedmann-Merdinoglu and P. Hugueney (INRA and Université de Strasbourg) was used to infect leaf discs as described previously (Asselbergh et al., 2007). Triplicate samples of 10 leaf discs were used for each treatment. ABA (Sigma-Aldrich) and fluridone (Duchefa) solubilized in ethanol:water (90:10, v/v) were added to the elicitation medium at 25 and 10 μm concentrations, respectively. The incubation was carried out at 25°C for the indicated periods of time.
Capsidiol Analysis
Capsidiol was extracted from elicited leaf discs as described previously (Hugueney et al., 1996) using dichloromethane:methanol (2:1, v/v), and the dried extract was applied to a Pasteur pipette half-filled with silica gel preconditioned with cyclohexane. Apolar compounds were eluted with cyclohexane:ethyl acetate (80:20, v/v), and the capsidiol fraction was eluted with cyclohexane:ethyl acetate (50:50, v/v). The dried sample was analyzed by GC-MS as described previously (Hugueney et al., 1996). Alternatively, an HPLC procedure was used as described previously (Moreau et al., 1992).
Extraction of ABA Catabolites and Mass Spectrometry Analysis
Freeze-dried N. plumbaginifolia leaf discs were ground in liquid nitrogen and extracted three times with 1-propanol:water (80:40, v/v; acidified with 80 μL of concentrated HCl; Pan et al., 2008). The resulting extract was treated with 2 volumes of dichloromethane and centrifuged at 10,000g for 10 min. The dichloromethane extract was subjected to prepurification by thin-layer chromatography on a silica gel plate developed with dichloromethane:methanol:water (75:22:3, v/v). The area on the silica (60 F-254) gel plate between RF 0.05 and 0.6 was scraped from the plate and eluted with 1-propanol:water (80:40, v/v; acidified with 80 μL of concentrated HCl) before liquid chromatography analysis. Characterization of ABA, PA, DPA, and 7′-OH-ABA from leaf disc extracts was performed by comparing tR, MS transitions, and MS/MS analysis using UPLC-MS/MS. All analyses were performed using a Waters Quattro Premier XE equipped with an ESI source and coupled to an Acquity UPLC system (Waters). Chromatographic separation was achieved using an Acquity UPLC BEH C18 column (100 × 2.1 mm, 1.7 μm; Waters) coupled to an Acquity UPLC BEH C18 precolumn (2.1 × 5 mm, 1.7 μm; Waters). The mobile phase consisted of the following solvents: A, methanol:water (30:70, v/v; acidified with 0.1% formic acid); B, methanol:water (55:45, v/v; 0.1% formic acid); C, methanol:water (90:10, v/v; 0.1% formic acid); and D, 100% methanol acidified with 0.1% formic acid. The following gradient was used: solvent A (1 min), linear gradients A to B (2 min), B to C (5 min), and C to D (2 min) before isocratic elution using D (2 min) and linear gradient to A (0.8 min), followed by an isocratic run using A (3.2 min) to return to initial conditions. The total run time was 15 min. The column was operated at 48°C with a flow rate of 0.4 mL min−1 (sample injection volume of 3 μL). Nitrogen generated from pressurized air in a N2G nitrogen generator (Mistral; Schmidlin-dbs-AG) was used as the drying and nebulizing gas. The nebulizer gas flow was set to approximately 50 L h−1 and the desolvation gas flow to 900 L h−1. The interface temperature was set at 400°C and the source temperature at 135°C. The capillary voltage was set at 3 kV, and the cone voltage and the ionization mode (positive and negative) were optimized for each molecule. The selected ion recording-MS mode was used to determine parent mass transitions of ABA (m/z 263), PA (m/z 279), DPA (m/z 281), and 7′-OH-ABA (m/z 279). Fragmentation was performed by collision-induced dissociation with argon at 1.0 × 10−4 mbar. The collision energy was optimized for each compound using daughter scan monitoring and MRM. Mass spectrometry conditions for ABA and ABA catabolites were set after optimization as follows (polarity, ES−; capillary, 3 kV; cone, 25 V) for ABA, PA, DPA, and 7′-OH-ABA. Low and high mass resolution were 13 for both mass analyzers, ion energies 1 and 2 were 0.5 V, entrance and exit potential were 2 and 1 V, and detector (multiplier) gain was 650 V. Collision-induced dissociation of deprotonated parent ions was accomplished with a collision energy of 10 V for ABA, PA, and DPA and 20 V for 7′-OH-ABA. For ABA and ABA catabolites (PA, DPA, and 7′-OH-ABA), daughter scan monitoring and MRM permitted the identification for each compound of the transition from deprotonated parent ion to the predominant daughter fragment ion. The combination of chromatographic tR, parent mass, and unique fragment ion analysis was used to selectively monitor ABA (263 > 153), PA (279 > 139), DPA (281 > 189 > 171), and 7′-OH-ABA (279 > 151) Data acquisition and analysis were performed with MassLynx software (version 4.1) running under Windows XP Professional on a Pentium personal computer.
Quantitative Analysis of ABA
ABA was extracted as described above using dl-cis,trans-[G-3H]ABA (55 Ci mmol−1; GE Healthcare) to evaluate the recovery and quantified as described previously (Xiong et al., 2001) using an ELISA kit (Phytodetek ABA; AGDIA) according to the manufacturer's protocol.
Cloning and Functional Characterization of ABAH
NpABAH cDNA was amplified by RT-PCR as described previously (Bouvier et al., 2006) using forward (5′-ATGACTAATTTTGACTTATTTTTC-3′) and reverse (5′-GGTTGAAGTGGTAGATTCTTTCCAAAATC-3′) primers and Phusion High-Fidelity DNA Polymerase (Finnzymes) according to the manufacturer's instructions. NpABAH cDNA was cloned into the XbaI site of pKYLX71-35S2 vector (Maiti et al., 1993; Bouvier et al., 2006). Following sequence verification, the resulting plasmid designated pKNpABAH and an empty control vector designated pK(NpΔABAH) were transformed into Agrobacterium tumefaciens strain GV3101. Before plant infiltration, Agrobacterium harboring pKNpABAH and pK(NpΔABAH) was diluted to an optical density at 600 nm of 0.2 using the infiltration buffer (10 mm MES, pH 5.6, 1 mm sodium phosphate, 200 μm acetosyringone, 0.5% Glc, and 2 mm MgCl2) and injected through the stomata on the lower epidermal surface of leaves of 75-d-old wild-type N. plumbaginifolia plants using a 1-mL plastic syringe without a needle (Batoko et al., 2000). Expression of the transgene was monitored by RT-PCR using the forward primer (5′-CACTATCCTTCGCAAGACCCTTC-3′) specific for the vector NpABAH and the reverse primer (5′-GATATGAGTGATTTGGTTACGAATG-3′) specific for NpABAH to amplify an expected 585-bp band. Ethidium bromide staining of ribosomal RNAs was used as a loading control. At 3 d after infiltration, transfected plant leaves were incubated with 25 μm ABA for 20 h before extraction and analysis of ABA catabolites.
RNA Gel-Blot Analysis
Total RNA from leaf discs was prepared using the NucleoSpin RNA plant kit from Macherey-Nagel. Following agarose gel electrophoresis and transfer onto nylon membranes, filters were hybridized overnight with EAS, EAH, ZEP, NCED, ABAH, and GA-2Ox cDNA probes and processed as described previously (Bouvier et al., 2006). The hybridization signals were visualized using a Fujifilm-FLA-7000 phosphorimager. Expression levels obtained by northern blots were confirmed by two independent semiquantitative RT-PCR analyses (Bouvier et al., 2006). The primers used were the following: EAS, 5′-CACATGTAAGGACTCATGCTGAC-3′ and 5′-CTTTGTATGGCATCTGTGTATGC-3′; EAH, 5′-CTTTCTGTTGAGGAAATGGAAGAAC-3′ and 5′-CTACACGTCATGGAGCTTGCAAACC-3′, ZEP, 5′-CTGGCTAGAATGGCTGCAATC-3′ and 5′-GCGAGTAGGGAAGTTTGGAGATG-3′; NCED, 5′-GATTTGGTGTTTTGGACAAATATG-3′ and 5′-CCATTCTTGCTCATTGTGTAC-3′; ABAH, 5′-CCTAATATTTTCTTCATTAACAGGC-3′ and 5′-GCTTGAAGAGCCTTTCTATAAGG-3′; GA-2Ox, 5′-GGCTATGGAAACAAGAAGATTGG-3′ and 5′-GCCTTACACTCTTAAATCTCCCG-3′. The expression of N. plumbaginifolia PR1 protein (Payne et al., 1988) was assessed by semiquantitative PCR as described previously (Bouvier et al., 2006) using the forward (5′-TGCCTTCATTTCTTCTTGTC-3′) and reverse (5′-CAAACCACCTGAGTATAGTGTCC-3′) primers.
The sequences reported in this paper have been deposited in the GenBank/EMBL data libraries under the following accession numbers: FM244692 for NpABAH, FM244696 for NpEAH, FM244695 for NpEAS, FM244693 for NpGA-2Ox, and FM244694 for NpNCED.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. GC-MS analysis of capsidiol produced by N. plumbaginifolia following elicitor treatments or infection with B. cinerea.
Supplemental Figure S2. UPLC-MS/MS analysis using MRM of leaf disc extracts from wild-type N. plumbaginifolia overexpressing NpABAH.
Supplementary Material
Acknowledgments
We thank Dr. A. Marion-Poll for the kind gift of seeds of wild-type and ABA-deficient mutants of N. plumbaginifolia and Drs. S. Wiedmann-Merdinoglu and P. Hugueney for B. cinerea cultures. We thank A. Bailly, M. Kerneis, S. Staerck, and R. Wagner for taking care of the plants and M. Alioua for DNA sequencing. The UPLC-MS/MS apparatus was cofinanced by the CNRS, the Université Louis Pasteur, the Région Alsace, the INRA, and the Tepral Company.
This work was supported by the Agence Nationale de la Recherche (grant no. ANR NT05–3_44792) and by a doctoral fellowship from the République du Congo Brazzaville to A.S.M.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Bilal Camara (bilal.camara@ibmp-ulp.u-strasbg.fr).
The online version of this article contains Web-only data.
References
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola-Cortés A, Castro-Mercado E, Lozoya-Gloria E, García-Pineda E (2007) Capsidiol production in pepper fruits (Capsicum annuum L.) induced by arachidonic acid is dependent of an oxidative burst. Physiol Mol Plant Pathol 70 69–76 [Google Scholar]
- Asselbergh B, Achuo AE, Höfte M, van Breusegem F (2008. a) Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol Plant Pathol 9 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh B, Curvers K, Franca SC, Audenaert K, Vuylsteke M, Van Breusegem F, Hofte M (2007) Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol 144 1863–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh B, De Vleesschauwer D, Hofte M (2008. b) Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21 709–719 [DOI] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Hofte MM (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs E, Gaborjanyi R, Kiraly Z (1973) Leaf senescence and increased virus susceptibility in tobacco: the effect of abscisic acid. Physiol Mol Plant Pathol 3 341–346 [Google Scholar]
- Bartels PG, Watson CW (1978) Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci 26 198–203 [Google Scholar]
- Batoko H, Zheng HQ, Hawes C, Moore I (2000) A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz O, Logemann E, Reinold S, Hahlbrock K (1998) Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. Biol Chem 379 1127–1135 [DOI] [PubMed] [Google Scholar]
- Bloch CV, De Wit PJGM, Kuc JA (1984) Elicitation of phytoalexins by arachidonic and eicosapentanoic acids: a host survey. Physiol Plant Pathol 25 199–208 [Google Scholar]
- Bostock RM, Nuckles E, Henfling JWDM, Kuc JA (1983) Effects of potato tuber age and storage on sesquiterpenoid stress metabolite accumulation, steroid glycoalkaloid accumulation, and response to abscisic and arachidonic acids. Phytopathology 73 435–438 [Google Scholar]
- Bouvier F, Linka N, Isner JC, Mutterer J, Weber AP, Camara B (2006) Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development. Plant Cell 18 3088–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill D, Ward EWB (1989) Rapid localized changes in abscisic acid concentrations in soybean in interactions with Phytophthora megasperma f. sp. glycinea or after treatment with elicitors. Physiol Mol Plant Pathol 35 483–493 [Google Scholar]
- Chiwocha SD, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross AR, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35 405–417 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4 472–478 [DOI] [PubMed] [Google Scholar]
- de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bogre L, Grant M (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 26 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, Harris JM, Oldroyd GE (2008) Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 20 2681–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C, Andrés F, Tharreau D, Iglesias DJ, Talon M (2009) Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol Plant Microbe Interact 22 201–220 [DOI] [PubMed] [Google Scholar]
- Facchini PJ, Chappell J (1992) Gene family for an elicitor-induced sesquiterpene cyclase in tobacco. Proc Natl Acad Sci USA 89 11088–11092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Innes RW (1998) An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell 10 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Tang D, Innes RW (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA 98 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens JFV, Vendrig JC (1982) Effects of abscisic acid, cytokinins, and light on isoflavonoid phytoalexin accumulation in Phaseolus vulgaris L. Planta 154 441–446 [DOI] [PubMed] [Google Scholar]
- Henfling JWDM, Bostock RM, Kuc J (1980) Effect of abscisic acid on rishitin and lubimin accumulation and resistance to Phytophthora infestans and Cladosporium cucumerinum in potato tuber tissue slices. Phytopathology 70 1074–1078 [Google Scholar]
- Hu J, Barlet X, Deslandes L, Hirsch J, Feng DX, Somssich I, Marco Y (2008) Transcriptional responses of Arabidopsis thaliana during wilt disease caused by the soil-borne phytopathogenic bacterium, Ralstonia solanacearum. PLoS One 3 e2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugueney P, Bouvier F, Badillo A, Quennemet J, d'Harlingue A, Camara B (1996) Developmental and stress regulation of gene expression for plastid and cytosolic isoprenoid pathways in pepper fruits. Plant Physiol 111 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M, Hirai N, Yoshida R, Ohigashi H (2004) The biosynthetic pathway to abscisic acid via ionylideneethane in the fungus Botrytis cinerea. Phytochemistry 65 2667–2678 [DOI] [PubMed] [Google Scholar]
- Jensen MK, Hagedorn PH, de Torres-Zabala M, Grant MR, Rung JH, Collinge DB, Lyngkjaer MF (2008) Transcriptional regulation by an NAC (NAM-ATAF1,2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J 56 867–880 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526 [DOI] [PubMed] [Google Scholar]
- Koga H, Dohi K, Mori M (2004) Abscisic acid and low temperatures suppress the whole plant-specific resistance reaction of rice plants to infection of Magnaporthe grisea. Physiol Mol Plant Pathol 65 3–9 [Google Scholar]
- Kraepiel Y, Rousselin P, Sotta B, Kerhoas L, Einhorn J, Caboche M, Miginiac E (1994) Analysis of phytochrome- and ABA-deficient mutants suggests that ABA degradation is controlled by light in Nicotiana plumbaginifolia. Plant J 6 665–672 [Google Scholar]
- Kuc J (1982) Phytoalexins from the Solanaceae. In JA Bailey, JW Mansfield, eds, Phytoalexins. Blackie and Son, Glasgow, UK, pp 81–133
- Kuc J (1995) Phytoalexins, stress metabolism, and disease resistance in plants. Annu Rev Phytopathol 33 275–297 [DOI] [PubMed] [Google Scholar]
- Logemann E, Tavernaro A, Schulz W, Somssich IE, Hahlbrock K (2000) UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc Natl Acad Sci USA 97 1903–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MA, Bannenberg G, Castresana C (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11 420–427 [DOI] [PubMed] [Google Scholar]
- Ma CJ (2008) Cellulase elicitor induced accumulation of capsidiol in Capsicum annuum L. suspension cultures. Biotechnol Lett 30 961–965 [DOI] [PubMed] [Google Scholar]
- Maekawa T, Maekawa-Yoshikawa M, Takeda N, Imaizumi-Anraku H, Murooka Y, Hayashi M (2009) Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J 58 183–194 [DOI] [PubMed] [Google Scholar]
- Maiti IB, Murphy JF, Shaw JG, Hunt AG (1993) Plants that express a polyvirus VPg-proteinase gene are resistant to virus infection. Proc Natl Acad Sci USA 90 6110–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Bonilla LD, Betancourt-Jiménez M, Lozoya-Gloria E (2008) Local and systemic gene expression of sesquiterpene phytoalexin biosynthetic enzymes in plant leaves. Eur J Plant Pathol 121 439–449 [Google Scholar]
- Mandujano-Chavez A, Schoenbeck MA, Ralston LF, Lozoya-Gloria E, Chappell J (2000) Differential induction of sesquiterpene metabolism in tobacco cell suspension cultures by methyl jasmonate and fungal elicitor. Arch Biochem Biophys 381 285–294 [DOI] [PubMed] [Google Scholar]
- Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A (1996) Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J 15 2331–2342 [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8 409–414 [DOI] [PubMed] [Google Scholar]
- Maxson KL, Jones AL (2002) Management of fire blight with gibberellin inhibitors and SAR inducers. Acta Hortic 590 217–223 [Google Scholar]
- Mohr P, Cahill D (2001) Relative roles of glyceollin, lignin and the hypersensitive response and the influence of ABA in compatible and incompatible interactions of soybeans with Phytophthora sojae. Physiol Mol Plant Pathol 58 31–41 [Google Scholar]
- Moreau RA, Preisig CL, Osman SF (1992) A rapid quantitative method for the analysis of sesquiterpene phytoalexins by high performance liquid chromatography. Phytochem Anal 3 125–128 [Google Scholar]
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD (2008) Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 451 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemestothy GS, Guest DI (1990) Phytoalexin accumulation, phenylalanine ammonia lyase activity and ethylene biosynthesis in fosetyl-Al treated resistant and susceptible tobacco cultivars infected with Phytophthora nicotianae var. nicotianae. Physiol Mol Plant Pathol 37 207–219 [Google Scholar]
- Pan X, Welti R, Wang X (2008) Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69 1773–1781 [DOI] [PubMed] [Google Scholar]
- Payne G, Parks TD, Burkhart W, Dincher S, Ahl P, Metraux JP, Ryals J (1988) Isolation of the genome clone for pathogenesis-related protein-1a from Nicotiana tabacum cv Xanthi-Nc. Plant Mol Biol 11 89–94 [DOI] [PubMed] [Google Scholar]
- Perrone ST, McDonald KL, Sutherland MW, Guest DI (2003) Superoxide release is necessary for phytoalexin accumulation in Nicotiana tabacum cells during the expression of cultivar-race and non-host resistance towards Phytophthora spp. Physiol Mol Plant Pathol 62 127–135 [Google Scholar]
- Polian C, Coulomb PJ, Lizzi Y, Coulomb C (1997) Membrane alteration of capsidiol-treated pepper leaf protoplasts. Comptes Rendus de l'Académie des Sciences 320 721–727 [Google Scholar]
- Preisig CL, Moreau RA (1994) Effects of potential signal transduction antagonists on phytoalexin accumulation in tobacco. Phytochemistry 36 857–863 [Google Scholar]
- Ralston L, Kwon ST, Schoenbeck M, Ralston J, Schenk DJ, Coates RM, Chappell J (2001) Cloning, heterologous expression, and functional characterization of 5-epi-aristolochene-1,3-dihydroxylase from tobacco (Nicotiana tabacum). Arch Biochem Biophys 393 222–235 [DOI] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzonico E, Flury N, Meins F Jr, Beffa R (1998) Transcriptional down-regulation by abscisic acid of pathogenesis-related beta-1,3-glucanase genes in tobacco cell cultures. Plant Physiol 117 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristaino JB, Duniway JM (1989) Effect of preinoculation and postinoculation water-stress on the severity of Phytophthora root-rot in processing tomatoes. Plant Dis 73 349–352 [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10 372–379 [DOI] [PubMed] [Google Scholar]
- Rusterucci C, Stallaert V, Milat ML, Pugin A, Ricci P, Blein JP (1996) Relationship between active oxygen species, lipid peroxidation, necrosis, and phytoalexin production induced by elicitins in Nicotiana. Plant Physiol 111 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M (2004) Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt SD, Tuzun S, Kuc J (1986) Effect of β-ionone and abscisic acid on the growth of tobacco and resistance to blue mold: mimicry of effects of stem infection by Peronospora tabacina Adam. Physiol Mol Plant Pathol 28 287–297 [Google Scholar]
- Schaaf J, Walter MH, Hess D (1995) Primary metabolism in plant defense (regulation of a bean malic enzyme gene promoter in transgenic tobacco by developmental and environmental cues). Plant Physiol 108 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Pflugmacher M, Klages S, Maser A, Mock A, Stahl DJ (2008) Accumulation of the hormone abscisic acid (ABA) at the infection site of the fungus Cercospora beticola supports the role of ABA as a repressor of plant defence in sugar beet. Mol Plant Pathol 9 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit JP, Mueller GM (2007) An estimate of the lower limit of global fungal diversity. Biodivers Conserv 16 99–111 [Google Scholar]
- Simon-Mateo C, Depuydt S, De Oliveira Manes CL, Cnudde F, Holsters M, Goethals K, Vereecke D (2006) The phytopathogen Rhodococcus fascians breaks apical dominance and activates axillary meristems by inducing plant genes involved in hormone metabolism. Mol Plant Pathol 7 103–112 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3 348–351 [DOI] [PubMed] [Google Scholar]
- Tavernier E, Wendehenne D, Blein JP, Pugin A (1995) Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol 109 1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Bostock RM (2004) Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85 48–58 [Google Scholar]
- Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 96 4698–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Nelissen I, Eggermont K, Broekaert WF (1999) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19 163–171 [DOI] [PubMed] [Google Scholar]
- Truman W, de Zabala MT, Grant M (2006) Type III effectors orchestrate a complex interplay between transcriptional networks to modify basal defence responses during pathogenesis and resistance. Plant J 46 14–33 [DOI] [PubMed] [Google Scholar]
- van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289 295–297 [DOI] [PubMed] [Google Scholar]
- Vogeli U, Vogeli-Lange R, Chappell J (1992) Inhibition of phytoalexin biosynthesis in elicitor-treated tobacco cell-suspension cultures by calcium/calmodulin antagonists. Plant Physiol 100 1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17 1784–1790 [DOI] [PubMed] [Google Scholar]
- Ward EW, Cahill DM, Bhattacharyya MK (1989) Abscisic acid suppression of phenylalanine ammonia-lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f.sp. glycinea. Plant Physiol 91 23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzynska A, Christiansen KM, Lan Y, Rodibaugh NL, Innes RW (2008) Powdery mildew resistance conferred by loss of the ENHANCED DISEASE RESISTANCE1 protein kinase is suppressed by a missense mutation in KEEP ON GOING, a regulator of ABA signaling. Plant Physiol 148 1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor ML, Zeevaart JA (1997) Induction of ABA 8′-hydroxylase by (+)-S-, (−)-R- and 8′-8′-8′-trifluoro-S-abscisic acid in suspension cultures of potato and Arabidopsis. Phytochemistry 45 931–934 [DOI] [PubMed] [Google Scholar]
- Wotton HR, Strange RN (1987) Increased susceptibility and reduced phytoalexin accumulation in drought-stressed peanut kernels challenged with Aspergillus flavus. Appl Environ Microbiol 53 270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua NH (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278 2126–2130 [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Tada Y, Dong X, Heitman J (2007) The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 1 263–273 [DOI] [PubMed] [Google Scholar]
- Yang DL, Li Q, Deng YW, Lou YG, Wang MY, Zhou GX, Zhang YY, He ZH (2008) Altered disease development in the eui mutants and Eui overexpressors indicates that gibberellins negatively regulate rice basal disease resistance. Mol Plant 1 528–537 [DOI] [PubMed] [Google Scholar]
- Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, Maruyama-Nakashita A, Kudo T, Shinozaki K, Yoshida S, et al (2008) Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 20 1678–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Mei L, Newman J, Back K, Chappell J (1997) Regulation of sesquiterpene cyclase gene expression: characterization of an elicitor- and pathogen-inducible promoter. Plant Physiol 115 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Last RL (1996) Coordinate regulation of the tryptophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell 8 2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Yang Y, Zhang Z, Zhang H, Hu X, Chen J, Wang XC, Huang R (2008) Abscisic acid regulates TSRF1-mediated resistance to Ralstonia solanacearum by modifying the expression of GCC box-containing genes in tobacco. J Exp Bot 59 645–652 [DOI] [PubMed] [Google Scholar]
- Zook MN, Rush JS, Kuc JA (1987) A role for Ca2+ in the elicitation of rishitin and lubimin accumulation in potato tuber tissue. Plant Physiol 84 520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.