Abstract
Exogenous application of gibberellic acid (GA3) was able to reverse the inhibitory effect of salt, oxidative, and heat stresses in the germination and seedling establishment of Arabidopsis (Arabidopsis thaliana), this effect being accompanied by an increase in salicylic acid (SA) levels, a hormone that in recent years has been implicated in plant responses to abiotic stress. Furthermore, this treatment induced an increase in the expression levels of the isochorismate synthase1 and nonexpressor of PR1 genes, involved in SA biosynthesis and action, respectively. In addition, we proved that transgenic plants overexpressing a gibberellin (GA)-responsive gene from beechnut (Fagus sylvatica), coding for a member of the GA3 stimulated in Arabidopsis (GASA) family (FsGASA4), showed a reduced GA dependence for growth and improved responses to salt, oxidative, and heat stress at the level of seed germination and seedling establishment. In 35S:FsGASA4 seeds, the improved behavior under abiotic stress was accompanied by an increase in SA endogenous levels. All these data taken together suggest that this GA-responsive gene and exogenous addition of GAs are able to counteract the inhibitory effects of these adverse environmental conditions in seed germination and seedling growth through modulation of SA biosynthesis. Furthermore, this hypothesis is supported by the fact that sid2 mutants, impaired in SA biosynthesis, are more sensitive to salt stress than wild type and are not affected by exogenous application of GA3.
GAs constitute a group of natural diterpenoids that mediate many developmental processes in higher plants. Genetic studies using Arabidopsis (Arabidopsis thaliana) mutants have demonstrated the role of this phytohormone in several processes, such as seed germination, vegetative growth, flowering induction, or fruit development (Sun and Gubler, 2004).
During the last decade, much progress has been made to understand the mechanism of GA signaling. It is well known that GAs promote plant growth by inducing the degradation of the nuclear family of transcription factors known as DELLA proteins. Thus, DELLA proteins restrain growth, while GAs induce their disappearance (Jiang and Fu, 2007), and allow plant growth, this mechanism being highly conserved between dicots and monocots (Fleet and Sun, 2005).
However, few genes have been reported as targets of GA regulation in Arabidopsis (Raventos et al., 2000). Among these target genes, members of the GA3 stimulated in Arabidopsis (GASA) gene family represent a group of genes that have been characterized in several plant species (Roxrud et al., 2007). Although their functions are not yet clear, it has been reported that some members of this family are involved in flowering, seed development (Roxrud et al., 2007), pathogen defense (Berrocal-Lobo et al., 2002), antioxidant activity (Wigoda et al., 2006), and heat stress response (Ko et al., 2007).
Recently a role for DELLA proteins has been proposed in the responses of plants to adverse environmental signals. Seedlings of a quadruple DELLA mutant exhibit reduced growth inhibition in high-salinity conditions (Achard et al., 2006). In addition it has been suggested that loss-of-function mutations in DELLA proteins improve the resistance of plants to some pathogens through induction of salicylic acid (SA)-dependent defense pathway (Robert-Seilaniantz et al., 2007; Navarro et al., 2008). All these data suggest a role for GAs in plant responses to biotic and abiotic stress conditions.
During recent years there has been increasing evidence on the role of SA in elicitation of plant defense mechanism in several abiotic stress conditions (Horvath et al., 2007), although information about the onset of defense mechanisms mediated by SA at the level of seed germination is very scarce (Rajjou et al., 2006).
In this report we show that the overexpression of a GASA4 gene from beechnut (Fagus sylvatica) in Arabidopsis improves plant tolerance to salt, oxidative, and heat stress, through an increase in SA biosynthesis. In addition, we prove that exogenous application of GA3 is able to reverse the inhibitory effect of different stress conditions in seed germination and seedling establishment and also increases SA biosynthesis, suggesting that GAs are implicated in plant responses to abiotic stress by modulating SA levels.
RESULTS
Isolation and Characterization of a cDNA Clone from Beechnut Seeds Encoding a GASA4 Protein
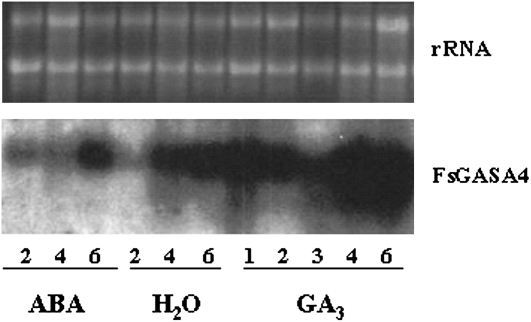
In the last few years we have been investigating the responses of beechnut seeds to abscisic acid (ABA) and GA. By using a differential screening, a GA3-induced cDNA was isolated from a beechnut seed cDNA library (Nicolás et al., 1997). The corresponding full-length clone was registered in the EMBL and GenBank nucleotide sequence databases under accession number AM231807. The isolated cDNA clone was 682 bp long and contained an open reading frame of 321 bp. The deduced protein had 107 amino acids with a predicted molecular mass of 11.95 kD. Comparison of the deduced amino acid sequence with EMBL databases revealed homology with different members of GASA family, mainly with GASA4 from Arabidopsis (data not shown). Thus, this clone was named FsGASA4. The induction of this gene by GA was confirmed by northern-blot assay in beech seeds treated or not with GA3 (Fig. 1).
Figure 1.
Northern-blot analysis of total RNA isolated from beechnut seeds imbibed at 4°C in 100 mm ABA, water, and 100 mm GA3 from 1 to 6 weeks. Ten micrograms of RNA were used per lane and hybridized with a FsGASA4 cDNA probe. Top section: Ethidium bromide-stained gel showing RNAs. The numbers indicate weeks of imbibition.
Generation and Characterization of 35S:GASA4 Transgenic Lines
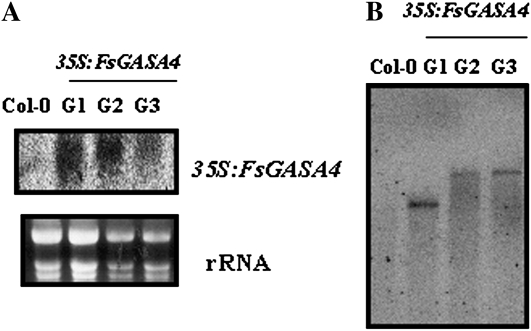
To ascertain the function of FsGASA4, and since transgenic work is not possible in beechnut, we used an overexpression approach in Arabidopsis. Three independent T3 homozygous lines for FsGASA4 (G1 to G3), which showed high levels of expression of the transgene, were selected (Fig. 2A). Southern-blot analysis of these homozygous lines displayed a single insertion of the 35S:FsGASA4 transgene (Fig. 2B).
Figure 2.
Molecular analysis of Arabidopsis wild-type (Col-0) and 35S:FsGASA4 transgenic lines (G1–G3). A, RNA-blot analysis. Total RNA (10 μg) was isolated and hybridized with a specific FsGASA4 probe. Bottom: Ethidium bromide-stained gel showing rRNAs. B, Southern-blot analysis. Genomic DNA was digested with HindIII, blotted onto a nylon membrane, and hybridized with a FsGASA4-specific probe.
Effects of Paclobutrazol on FsGASA4-Overexpressing Lines and gasa4-1 Insertion Lines
Different lots of Arabidopsis seeds were grown on Murashige and Skoog medium supplemented with 10 μm paclobutrazol (PCB), a well-known GA biosynthesis inhibitor. In terms of germination and seedling establishment, seeds from the SALK T-DNA insertion line, gasa4-1, were more sensitive to this compound than those of Columbia-0 (Col-0) ecotype or FsGASA4 transgenic lines. A 10% of gasa4-1 seeds completed germination and developed green cotyledons as compared with 40% for Col-0 and 50% for 35S:FsGASA4 seeds after 6 d of sowing in PCB. When higher concentrations of PCB were used, differences between germination percentages of Col-0 and FsGASA4 transgenic seeds increased.
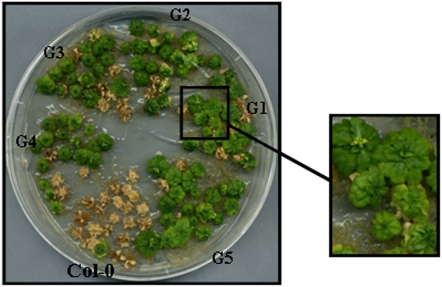
In addition, when Col-0 and FsGASA4 transgenic seeds were grown on Murashige and Skoog media supplemented with 10 μm PCB for 60 d, most of the wild-type plants died whereas FsGASA4 transgenic plants survived (Fig. 3). These results suggest that FsGASA4 overexpression in Arabidopsis reduces the GA dependence of growth.
Figure 3.
Effect of PCB on plant growth. Plant phenotypes after 60 d growth in 10 μm PCB (Col-0, wild-type seeds; G1–G5, FsGASA4 transgenic lines) are shown.
Overexpression of FsGASA4 Improves Plant Resistance to Salt, Oxidative, and Heat Stress
The constitutive expression of FsGASA4 in transgenic plants also resulted in changes in plant responses to salt, oxidative, and heat stress.
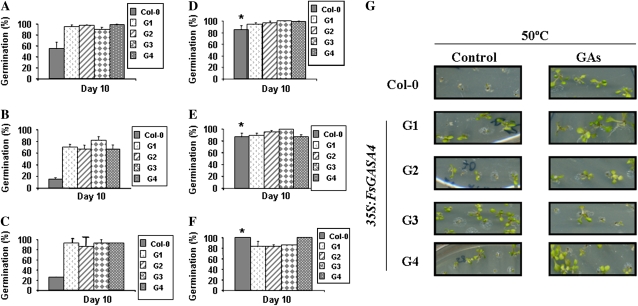
Seed germination and seedling establishment was analyzed in the presence of 150 mm NaCl or 0.5 mm paraquat. After 10 d in the presence of NaCl, over 90% of FsGASA4 seeds were able to complete germination and developed fully expanded green cotyledons compared with the 50% of success observed in wild-type seeds (Fig. 4A). After 10 d of exogenous application of paraquat, the percentage of seeds that completed germination (measured as radicle protrusion since seedling establishment was not observed in any case) was between 60% to 80% in the different transgenic lines whereas less than 20% of the Col-0 seeds germinated (Fig. 4B). The different seed lots were also exposed to 50°C for 3 h and cotyledon emergence was scored after 10 d. FsGASA4 transgenic lines showed a higher heat tolerance than wild-type seeds. Nearly all transgenic lines completed germination and became seedlings with fully expanded green cotyledons whereas in Col-0, only a 20% of the seeds completed this process (Fig. 4C).
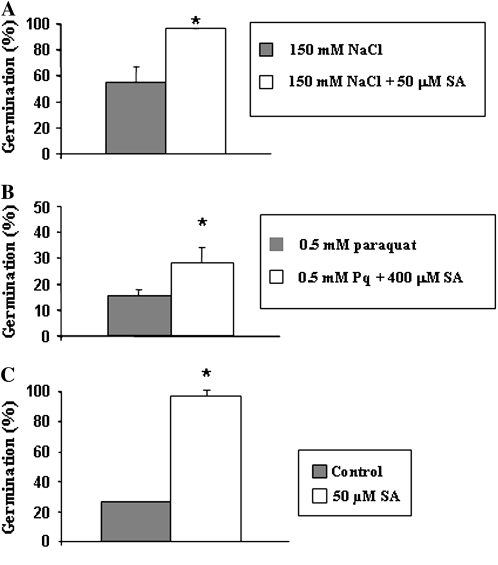
Figure 4.
Percentages of Col-0 seeds that completed germination and developed fully expanded green cotyledons after 10 d under: 150 mm NaCl (A), 0.5 mm paraquat (B), heat treatment (50°C; C), 50 μm GA3 plus 150 mm NaCl (D), 50 μm GA3 plus 0.5 mm paraquat (E), and heat treatment (50°C; F) in the presence of 50 μm GA3. G, Differences in heat tolerance (50°C) in wild-type and transgenic lines treated or not with 50 μm GA3. Approximately 100 seeds of each line were sowed and scored. Data are means ± sd of three independent experiments. Asterisks denote significant differences at P ≤ 0.05 between treated and nontreated seeds.
No significant differences were observed in germination percentages and seedling establishment among wild-type seeds and gasa4-1 mutants (data not shown), probably due to redundancy with other members of the GASA family.
In addition, and since GASA4 is a GA-responsive gene (Herzog et al., 1995), GA3 ability to counteract the inhibitory effect of these abiotic stress conditions in seed germination was analyzed. After exogenous application of 50 μm GA3, seed germination and seedling establishment of Col-0 significantly increased in all cases (Fig. 4, D–G). These data suggest that GAs confer abiotic stress tolerance in germinating seeds.
Seed viability was determined with a tetrazolium staining test (Tesnier et al., 2002). This assay revealed that more than 90% of the seeds were viable. In addition, most of the nongerminated seeds under stress conditions were able to germinate and develop green cotyledons when transferred to normal conditions, indicating that their vigor remained intact. Furthermore, nearly 100% of seed germination was scored when different lots of seeds were sowed under normal conditions.
ABA, Jasmonic Acid, and SA Quantification
Since transgenic plants showed more tolerance to several abiotic stress conditions, the endogenous levels of three hormones involved in plant stress responses were determined in seeds of wild-type, gasa4-1, and FsGASA4 transgenic plants. Slight differences in the levels of ABA (increased concentration in FsGASA4 plants) and jasmonic acid (JA; diminished concentration in FsGASA4 plants) were observed among the different seed lots. Most interestingly, levels of SA increased more than 2-fold in FsGASA4 seeds (Table I), suggesting that the responses of FsGASA4 plants to several types of abiotic stress may be SA dependent.
Table I.
ABA, JA, and SA amount (ng g−1 fresh weight) in Arabidopsis seeds from wild-type, gasa4 mutant, and FsGASA4 transgenic lines (G2 and G3)
Values are means of two replicates ± sd. Statistical significance of Col-0 in the Fisher's exact test (P value < 0.05) is represented by an asterisk.
| Col-0 | gasa4 | G2 | G3 | |
|---|---|---|---|---|
| ABA | 94 ± 1 | 86 ± 2 * | 135 ± 2 * | 119 ± 2 * |
| JA | 59 ± 4 | 44 ± 1 * | 48 ± 9 | 36 ± 2 * |
| SA | 487 ± 17 | 505 ± 11 | 1,276 ± 21 * | 1,332 ± 37 * |
Effects of SA on Plant Responses to Abiotic Stress
Once it had been shown that plant tolerance to abiotic stress was enhanced in FsGASA4-overexpressing lines, we hypothesized that these responses could be due to the increased levels of SA detected in our transgenic plants. Analysis of plant responses to salt, oxidative, and heat stress after exogenous application of SA confirmed that SA counteracts, at least partially, the inhibitory effect of these abiotic stress conditions on seed germination (Fig. 5).
Figure 5.
Percentages of Col-0 seeds (treated or not with SA) that completed germination and developed fully expanded green cotyledons after 10 d under: 150 mm NaCl (A), 0.5 mm paraquat (B), and heat treatment (50°C). Approximately 100 seeds of each line were sowed and scored. Data are means ± sd of three independent experiments. Asterisks denote significant differences at P ≤ 0.05 between treated and nontreated seeds.
Gene Expression in FsGASA4-Overexpressing Lines
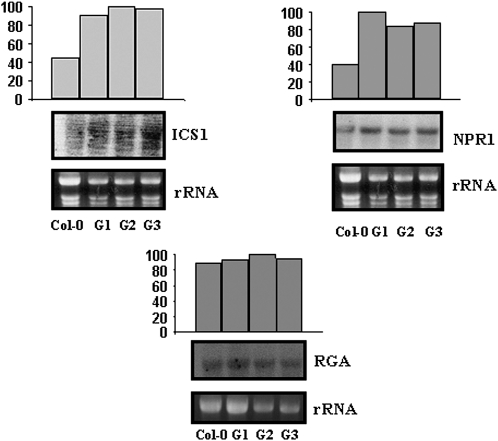
Since FsGASA4 transgenic lines were more resistant to different conditions of abiotic stress in the first stages of postgerminative growth and showed higher levels of SA than those observed in wild-type plants, levels of expression of ics1 gene (isochorismate synthase1, involved in SA biosynthesis) and npr1 gene (nonexpressor of PR1, involved in SA action) were evaluated. Transcript levels corresponding to both genes were clearly higher in FsGASA4 transgenic plants than in wild-type plants (Fig. 6). No differences in the transcript levels of RGA (a DELLA protein) were observed between FsGASA4 transgenic lines and wild-type plants (Fig. 6).
Figure 6.
Expression of the ics1, npr1, and RGA in FsGASA-overexpressing plants (G1–G3) compared to Col-0. mRNA levels of the indicated genes were determined by northern-blot analysis using total RNAs (10 μg/line) isolated from 7-d-old seedlings. Bottom: Ethidium bromide-stained gel showing rRNAs. Top section: Quantification of hybridization signals obtained by using a phosphoimage scanner. Data were normalized to the rRNA value. Blots were repeated twice and yielded similar results.
Effect of FsGASA4 Overexpression, GAs, and SA in Oxidative Damage through Malondialdehyde Measurement
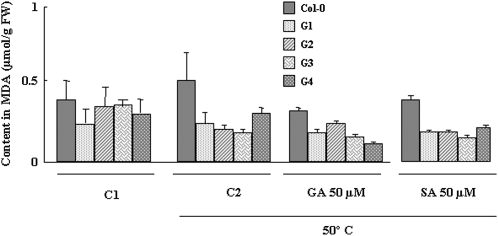
A common response to biotic and abiotic stresses is the generation of reactive oxygen species. To determine the stress-induced oxidative damage, levels of malondialdehyde (MDA), a toxic compound produced in vivo by lipid oxidation (Mene-Saffrane et al., 2007), was examined in transgenic lines and wild-type plants after heat treatment either in the presence or not of GA3 and SA. The highest MDA concentration was observed in wild-type plants after heat treatment at 50°C for 3 h, while a reduction in MDA levels was observed in all FsGASA4 transgenic lines (G1–G4) and in wild-type plants treated with either GA3 or SA (Fig. 7). In addition, MDA content was lower in the different transgenic lines compared with that observed in wild-type plants sowed under normal conditions, although no statistically significant differences were detected.
Figure 7.
MDA content in wild-type and G1 to G4 FsGASA4 transgenic lines under normal conditions (C1), and after heat stress at 50°C for 3 h (C2) treated with 50 mm GA3 and 50 mm SA.
GAs Have a Role in SA Biosynthesis and/or Action
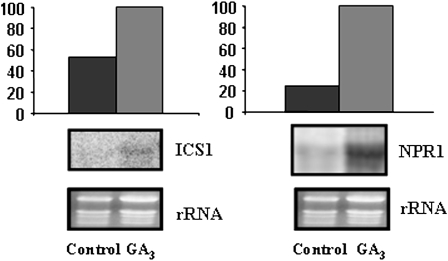
All the results presented in this work indicate that the overexpression of a GA-stimulated gene, FsGASA4, in Arabidopsis plants, enhanced abiotic stress early responses. Exogenous applications of GA3 or SA improved plant responses to these adverse conditions at the level of seed germination and seedling establishment. In addition, FsGASA4 transgenic seeds exhibited considerably higher levels of SA compared with Col-0 seeds. Then, the next question to answer was whether GAs have a role in SA biosynthesis and/or action. Thus, Arabidopsis Col-0 seeds were treated with GA3 and SA levels measured. Additionally ics1 and npr1 gene expression was analyzed. After 24 h, SA content in seeds imbibed in 50 μm GA3 was approximately 2-fold higher than that in seeds imbibed in water (Table II). This result was very similar to that observed in FsGASA4 transgenic seeds, where SA levels are more than 2-fold higher than in wild-type seeds (Table I). Accordingly with this increase in SA content, expression levels of ics1 and npr1 genes were enhanced in Col-0 Arabidopsis plants grown in a medium supplemented with GA3 (Fig. 8).
Table II.
SA amount (ng fresh weight) in Arabidopsis seeds imbibed in water or GA3 for 24 h
Values are means of two replicates ± sd. Statistical significance of water in the Fisher's exact test (P value < 0.05) is represented by an asterisk.
| Water | GA3 | |
|---|---|---|
| SA | 801 ± 13 | 1,559 ± 10 * |
Figure 8.
Expression of the ics1 and npr1 genes in Arabidopsis seedlings. Total RNA was isolated from 7-d-old seedlings, treated or not with 100 μm GA3. Bottom: Ethidium bromide-stained gel showing rRNAs. Top section: Quantification of hybridization signals obtained by using a phosphoimage scanner. Data were normalized to the rRNA value. Blots were repeated twice and yielded similar results.
To verify that GAs may have a role in SA biosynthesis, seed germination and seedling establishment was analyzed in the sid2 mutant, impaired in SA biosynthesis in the presence of 150 mm NaCl and 150 mm NaCl plus 50 μm GA3. After 10 d of treatment, just around 40% of sid2 seeds were able to complete germination and developed fully expanded green cotyledons compared with the 60% observed in wild-type seeds under salt stress conditions. In addition, after exogenous application of GA3, nearly 100% of Col-0 seeds were able to complete germination and become seedlings compared with 50% of success observed in sid2 mutants.
Effect of SA in the Absence of GAs
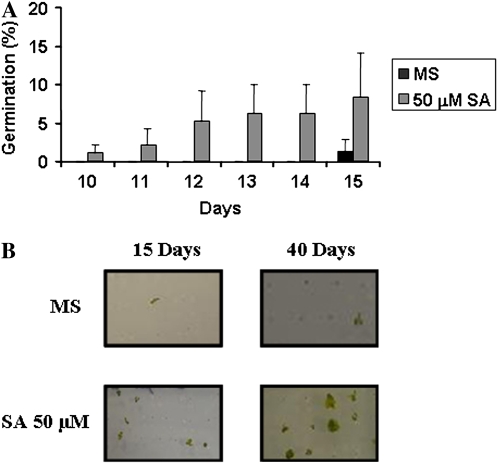
Another important question to answer was if SA could play a role in some of the physiological processes associated with GA. Exogenous application of 50 μm SA was able to both revert the inhibitory effect of PCB on seed germination and improve germination of the GA-deficient mutant ga1-3. After 15 d of growing only 1.4% of ga1-3 seeds were able to complete germination in Murashige and Skoog medium, whereas the addition of SA increased germination percentages up to 9%. After 40 d of growing, germination percentages were around 3% in Murashige and Skoog medium, these percentages being around 49% in the presence of SA (Fig. 9, A and B).
Figure 9.
Effect of SA on ga1 mutant seed germination and seedling growth. A, Percentages of ga1 seeds that completed germination and developed green cotyledons after 15 d in the presence or not of 50 μm SA. B, Phenotypes of ga1 seeds and seedlings after 15 and 40 d in the presence or not of 50 μm SA. Data are means ± sd of three independent experiments.
DISCUSSION
Plants need to integrate external and internal signals to respond to environmental and endogenous cues through a complex network of transduction pathways to produce the correct response and growth. Our knowledge of these signaling cascades has increased during the last years due to the identification of several components involved in these signal transduction pathways by using Arabidopsis mutants impaired in hormone biosynthesis or signaling, as well as transgenic plants. These advances have shown a complex cross-talk among different hormones at both levels biosynthesis and action (Weiss and Ori, 2007). There are many examples of these interactions, but no evidence of a cross talk between GAs and SA has been reported until the last year (Navarro et al., 2008). These authors have shown that the SA content in plants infected with the hemibiotroph Pseudomonas syringae was approximately 2-fold higher in the quadruple DELLA mutant than in the corresponding wild type. As a result, these mutants were more resistant to biotrophs but more susceptible to necrotrophs, concluding that DELLA proteins repress SA biosynthesis and signaling and control plant immune responses by modulating the balance between JA and SA (Navarro et al., 2008). Thus, it seems clear that GAs, by repressing DELLA proteins and changing the SA/JA balance, are able to control plant responses to biotic stress.
In this work, we show evidence that an exogenous treatment with GA3 and also the overexpression of a GA-induced gene from beechnut (FsGASA4) in Arabidopsis are able to control plant responses to abiotic stress through modulation of SA biosynthesis, a hormone that in the last few years has been also involved in the induction of abiotic stress tolerance in plants (Horvath et al., 2007).
GASA-like genes are members of a family of small polypeptides that regulate various aspects of plant development (Roxrud et al., 2007), the tomato (Solanum lycopersicum) gene GAST1 being the first member of this family identified, characterized, and related to GAs (Shi et al., 1992). After that, several other members of this GA-responsive family have been identified in different plant species. In Arabidopsis the GASA gene family consists of 14 genes (Roxrud et al., 2007), GASA1 to GASA4 being the first members identified based on their similarity to tomato GAST1 (Herzog et al., 1995). All 14 Arabidopsis GASA members share common features (Roxrud et al., 2007). This could explain why we do not observe phenotypical differences, except in PCB resistance, in plant responses between wild-type and gasa4-1 T-DNA insertion mutants, probably due to redundancy among GASA genes in the responses to abiotic stress conditions.
Overexpression of FsGASA4 in Arabidopsis confers high tolerance to PCB and reduces the GA dependence for growing (Fig. 3). Similar phenotypes were observed in the GA signaling mutant goe3 obtained by means of a fusion genetic screening of transgenic plants under the control of the GASA1 promoter (Raventos et al., 2000), indicating that GASA genes are not only GA stimulated but also involved in GA action.
As mentioned above, this family of GA-induced genes is involved in several aspects of plant development including plant responses to abiotic stress (Wigoda et al., 2006; Ko et al., 2007). In this work, we have shown that FsGASA4 transgenic lines are more tolerant to salt, oxidative, and heat stress in seed germination, the inhibitory effects of these type of stress in Col-0 seed germination being also reverted by exogenous application of GA3 (Fig. 4). These data are consistent with the observation that reduced GA accumulation, as is the case of PCB-imbibed seeds, causes accumulation of DELLA proteins (King et al., 2001; Silverstone et al., 2001) followed by growth inhibition, while GAs induce their disappearance allowing plant growth. In addition, it has been suggested that abiotic stress inhibits growth by means of the reduction in bioactive GA level, with consequent accumulation of DELLAs (Magome et al., 2004).
In view of FsGASA4-overexpressing lines growing better in the first stages of postgerminative growth under various abiotic stress conditions, the endogenous levels of ABA, JA, and SA were determined in FsGASA4 transgenic seeds. The most interesting data was the increased concentration of SA detected in transgenic seeds (more than 2-fold compared with wild-type seeds; Table I). Then, we confirmed that exogenous application of SA was able to revert, at least partially, the inhibitory effect of salt, oxidative, and heat stress in seed germination (Fig. 5). In the last years there is increasing evidence on the role of SA in plant responses to abiotic stress (Horvath et al., 2007). For example, SA has been implicated in thermotolerance in various plant species, such as mustard (Brassica nigra; Dat et al., 1998), pea (Pisum sativum; Pan et al., 2006), and Arabidopsis (Clarke et al., 2004; Larkindale and Vierling, 2008). Additionally, studies with NahG transgenic plants that are unable to accumulate SA, showed that the role of SA is restricted to basal thermotolerance (Clarke et al., 2004). Besides, the overexpression of a GASA4 in Arabidopsis confers resistance to heat stress (Ko et al., 2007). This result may be explained by an increase in SA levels, as we have found in FsGASA4 transgenic plants. Regarding oxidative stress, it has been proved that SA is an effective compound against oxidative damage, reducing the amount of lipid peroxidation by increasing antioxidant capacity (Strobel and Kuc, 1995; Ananieva et al., 2002, 2004). These results were confirmed in the rice (Oryza sativa) mutant NahG, deficient in SA biosynthesis, which exhibited great sensitivity to paraquat (Yang et al., 2004; Kusumi et al., 2006). Additionally, petunia (Petunia hybrida) plants overexpressing the GIP2 gene, a member of the GASA family, as well as FsGASA4, exhibit increased antioxidant activity (Wigoda et al., 2006).We suggest that this antioxidant activity may be due to increased levels of SA. Furthermore, a proteomic investigation proved that SA induces the accumulation of two superoxide dismutases, suggesting that this hormone increases the antioxidant capacity of Arabidopsis seedlings (Rajjou et al., 2006).
In the same study it was also proved that SA was able to improve seed germination under salt stress conditions (Rajjou et al., 2006). These data confirmed with the results presented in this work, are in agreement with the observation that pathogenesis-related genes contribute to salt regulation of seed germination (Seo et al., 2008).
Heat stress-induced oxidative damage was reduced in transgenic compared with wild-type plants. Exogenous application of either GA3 or SA also reduced this oxidative damage (Fig. 7), confirming that these two hormones may play an important role in plant responses to abiotic stress.
There is not much information on the role of GA on abiotic stress responses, although it has been reported that Kentucky bluegrass plants treated with a GA inhibitor were less heat tolerant than untreated plants (Heckman et al., 2002), pointing out to a role of GAs in thermotolerance. These data are in agreement with the observation that exogenous application of GA3 was able to revert the inhibitory effect of salt, oxidative, and heat stress in Arabidopsis seedlings (Fig. 4).
In view of the results, we were prompted to verify whether GAs were able to increase SA biosynthesis and found that after 24 h, seeds imbibed in GA3 had SA levels 2-fold higher than those imbibed in water (Table II). In addition, expression of ics1 (involved in SA biosynthesis) and npr1 (involved in SA action) genes was enhanced both in FsGASA4 and in Col-0 Arabidopsis seedlings grown in a medium supplemented with GA3 (Figs. 6 and 8), indicating that GA plays a role in SA biosynthesis and action. Additionally, no differences in the gene expression of one DELLA protein, RGA, were observed between FsGASA4 transgenic lines and wild-type plants (Fig. 6). This result was expected, since expression of GASA4 under the 35S promoter control induces a constitutive expression of this gene, but this protein is not supposed to affect the expression of DELLA proteins or other upstream components of GA signaling.
To support our hypothesis that GAs are involved in SA biosynthesis and in early plant stress responses, we sowed seeds of the sid2 mutant, impaired in isochorismate synthase1, the main enzyme involved in SA biosynthesis, under salt stress conditions. In terms of germination and seedling establishment, this mutant was more sensitive to salt stress than Col-0 plants. In addition, just and slight increase in seed germination and seedling establishment was observed in the sid2 mutant after exogenous application of GA3 under salt stress conditions. However, no differences were detected when both types of seeds were sowed under normal conditions. These data suggest that the improved response of wild-type seeds under salt stress conditions after exogenous application of GA3 may be due to the effect of this hormone on SA biosynthesis, confirming that SA improves germination vigor and seedling establishment under stress conditions as previously observed (Rajjou et al., 2006).
All the results presented in this work are in agreement with the high SA content observed in infected quadruple DELLA mutant plants (Navarro et al., 2008), and indicate that GAs not only play a role in plant responses to biotic stress by modulating SA/JA balance but also in plant responses to abiotic stress by modulating SA levels, being GASA4 gene a putative link between these two hormones, which adds a new point in the complex network of hormone cross talks. These complex interactions are, in some cases, reciprocal (Weiss and Ori, 2007). Thus, we analyzed if SA could play a role in some of the physiological processes regulated by GAs, and observed that SA is partially able to rescue ga1-3 germination (Fig. 9), suggesting that some of the effects of GAs in germination may be mediated by SA.
In conclusion, our data support that GA may play a crucial role in early plant responses to adverse environmental conditions by increasing SA biosynthesis. In other words, DELLA proteins and/or the absence of GAs restrain growth by repressing SA biosynthesis. In fact, it has been reported that high salinity inhibits seed germination by repressing GA biosynthesis (Magome et al., 2004; Achard et al., 2006) through inhibition of GA3 oxidase1 gene expression (Kim et al., 2008). Therefore, we suggest that inhibition of SA biosynthesis is a consequence of the stress-induced inhibition of GA biosynthesis and contributes to the failure in germination observed in these studies.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) plants, ecotype Col-0, were used in this research. The gasa4 T-DNA insertion line SALK_042431 (gasa4-1) loss-of-function mutant impaired in GASA4 protein was obtained from the Arabidopsis T-DNA insertion collection of the Salk Institute (http://signal.salk.edu/cgi-bin/tdnaexpress). Selection of homozygous plants for the T-DNA insertion was performed by PCR using the gene-specific primers 5′-CTCCTCTCTCAGTACACTTC-3′ and 5′-AACGAAGGGAGATTTTTTCAAGGG-3′, and checked that gasa4-1 plants produced no detectable GASA4 mRNA (data not shown).
Beechnut (Fagus sylvatica) seeds were obtained from the Danish State Forestry Tree Improvement Station. Seeds were dried to a moisture content of 10% and stored at −4°C in sealed jars.
Growth Conditions
The different lots of Arabidopsis seeds were normally grown in a growth chamber with 40% humidity, at 22°C, under long-day conditions (16 h light/8 h dark; light intensity of 80–100 μmol−2 m−2 s−1) in pots containing a 1:3 vermiculite to soil mixture. For in vitro culture, seeds were surface sterilized in 70% (v/v) ethanol solution containing 1% (v/v) Triton X-100, and washed four times in sterile distilled water. Stratification of seeds was conducted during 3 d at 4°C. Afterward, the seeds were sowed on plates containing Murashige and Skoog basal salts (Murashige and Skoog, 1962) and 1% (w/v) Suc, pH 5.7, solidified with 1% (w/v) agar. Plates were sealed and incubated in a controlled environment growth chamber. Assays were carried out in the presence or absence of different concentrations of SA, PCB, GA3, NaCl, and paraquat.
It has been previously reported that exogenous application of SA above 0.5 mm produced a retardation of growth in Arabidopsis ecotype Landsberg erecta (Rajjou et al., 2006). Thus, we checked different concentrations of SA and found that 50 μm was the best SA concentration under stress conditions that was not harmful for Arabidopsis ecotype Col seed germination and seedling establishment, except in the case of oxidative damage produced by paraquat where 400 μm was the best concentration analyzed.
Seed germination and seedling establishment were scored daily by determining the percentage of seeds that had germinated and became seedlings with fully expanded green cotyledons for 5 to 10 d. Approximately 100 seeds of each line were sowed and scored. At least three replicates of each germination assay were performed.
Transgenic and wild-type seedlings were plated on Murashige and Skoog media containing 10 mm PCB and examined after 60 d to check GA dependence following the procedure described in Raventos et al. (2000).
To check the possible role of GA in SA biosynthesis, the different seed lots were imbibed for 24 h in the presence or not of 50 μm GA3.
In the case of beechnut seeds, the pericarp was manually removed and seeds were sterilized in 1% sodium hypochlorite before imbibition in sterile water or solutions containing 100 μm ABA, 100 μm GA3, or 10 μm PCB, as previously described (Nicolás et al., 1996, 1997). Seeds were maintained in the different media at 4°C in the dark from 1 to 6 weeks.
Tetrazolium Staining Test
Seed viability of the different lots of seeds under stress conditions was analyzed by a tetrazolium staining test, following the procedure described by Tesnier et al. (2002). In brief, seeds were incubated in a 1.75% sodium hypochlorite solution for 15 min, and rinsed several times with distilled water. Seeds were spread upon a double layer of filter papers soaked with a 1% (w/v) tetrazolium solution at 30°C in the dark for 30 h.
Isolation of FsGASA4 cDNA Clone from Beechnut
FsGASA4 was isolated from a cDNA library constructed in the Uni-ZAP XR vector (Stratagene) using poly(A+) RNA from beechnut seeds as a template (Nicolás et al., 1997), by differential screening, carried out by preparing plaque lifts on nylon membranes (Hybond-N, Amersham Pharmacia Biotech), and hybridized with 32P-labeled ss-cDNA probes prepared against poly(A+) RNA obtained from seeds incubated either in GA3 at 4°C for 4 weeks or in ABA for 2 weeks at 4°C.
Vector Construction and Plant Transformation
The coding region of the FsGASA4 cDNA was cloned into the pBIN121 vector, which contains the modified 35S promoter of Cauliflower mosaic virus (Bevan, 1984). 5′-GGATCCATGGCTAAGTTTGTT-3′ (sense) and 5′-CCTAGGTACCGATTCAAACAA-3′ (antisense), containing the BamHI and SacI cloning sites, were used as primers to amplify FsGASA4 cDNA and subcloned in the corresponding sites of the pBIN121 vector. The pBIN121-FsGASA4 construct was introduced into Agrobacterium tumefaciens C58C1 (pGV2260; Deblaere et al., 1985) by heat shock. Col-0 Arabidopsis plants were transformed by the floral-dip method (Clough and Bent, 1998) and transgenic seedlings were selected on kanamycin medium (50 μg mL−1). T2 plants that produced 100% kanamycin-resistant plants in the T3 generation were considered homozygous for the selection marker and used for further studies.
Thermotolerance Assay
To analyze the heat stress response in seed germination, the procedure described by Ko et al. (2007) was followed. In brief, different seed lots were sown on water-saturated filter paper and imbibed at room temperature for 18 h. Then seeds were heat stressed for 3 h at 50°C in the presence or absence of 50 μm GA3 or 50 μm SA. Finally, seeds were grown normally in a growth chamber and cotyledon emergence was determined after 5 d.
Nucleic Acid Analysis
Total RNA was extracted using the RNAwiz kit (Ambion) following the manufacturer's protocol, separated on formaldehyde-agarose gels, and blotted onto a nylon membrane. Blots were hybridized with 32P-labeled specific probes as described in the “Results.” The ics1, npr1, and RGA probes were prepared by reverse transcription (RT)-PCR with the following primers: ICS1sen 5′-GTCTATGAATGGTTGTGATG-3′, antsen 5′-CATAGGCACGAATCAGAGGT-3′; NPR1sen 5′-TTGTCATTCCGATTTTCTAC-3′, antsen 5′-ATTGGCTTATCTTTAGGTCC-3′); and RGAsen 5′-TGGTTCGTCCGGTTTAGCGCCG-3′, antsen 5′-CAGTTCGGTTTAGGTCTTGGTCC-3′.
Genomic DNA was extracted using the Plant DNA isolation kit (Roche Diagnostics) following the manufacturer's recommendations. For Southern-blot analysis, DNA (10 μg) was digested with HindIII, fractionated on a 1% agarose gel, and blotted onto Hybond N nylon membranes (Amersham) according to the manufacturer's instructions. The blot was hybridized with a FsGASA4 probe, labeled at 65°C with 32P using the Random Primed kit (Roche Diagnostics), in 5× sodium chloride/sodium phosphate/EDTA (SSPE; NaCl [0.9 m], NaH2PO4.2H2O [50 mm], EDTA [5 mm], pH 7.7, SDS [0.5%], Denhardt's solution 5×: bovine serum albumin [2%, w/v], Ficoll [2%], polyvinylpyrrolidone [2%]) and 250 mg/mL of DNA from salmon testes DNA. The membranes were washed at 65°C with 3× SSPE, 0.1% SDS for 5 to 10 min; 2× SSPE, 0.1% SDS for 15 to 20 min; and 0.5× SSPE, 0.1% SDS.
Finally, blots from northern- or Southern-blot experiments were exposed and quantified by Phosphorimager analysis (Fujitsu). All RNA gel-blot experiments were repeated at least twice and results from one representative experiment are shown in the figures.
MDA Content Assay
Measurements were performed according to the procedure described by Wen et al. (2008). One gram from Arabidopsis 7-d-old seedlings was frozen, grounded in liquid nitrogen, and extracted with 2 mL of 10% trichloroacetic acid (TCA). After centrifugation at 8,000g the supernatant was mixed with other 2 mL of TCA containing 0.6% thiobarbituric acid (TBA). Samples were then heated at 95°C for 30 min and cooled. After a new centrifugation at 8,000g, the absorbance of the supernatant was measured at 532, 600, and 450 nm. The amount of MDA was calculated according to the formula 6.45 × (A532 − A600) − 0.56 × A450.
ABA, JA, and SA Quantification
Plant hormones were analyzed by HPLC coupled to tandem mass spectrometry as described by Durgbanshi et al. (2005). Plant tissue was extracted in ultrapure water using a tissue homogenizer (Ultra-Turrax, Ika-Werke). Before extraction, samples were spiked with deuterated standards of every compound. After extraction and centrifugation, the pH of the supernatant was adjusted to 3.0 and partitioned twice against diethyl ether. The organic layers were combined and evaporated in a centrifuge vacuum evaporator (Jouan). The dry residue was thereafter resuspended in a water:methanol (9:1) solution, filtered, and injected in a HPLC system (Alliance 2695, Waters Corp.). Hormones were separated in a reversed-phase C18 column using methanol and 0.01% acetic acid in water as solvents. The mass spectrometer, a triple quadrupole (Quattro LC, Micromass Ltd.), was operated in negative ionization electrospray mode and the different plant hormones were detected according to their specific transitions using a multiresidue mass spectrometric method. Further details on the determination procedure are given by Durgbanshi et al. (2005).
Statistical Analyses
The results are presented as mean values ± ses. Comparisons between means were made with the Fisher's exact test at P ≤ 0.05 using SPSS-10 statistical software (SPSS Inc.).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AM231807.
Acknowledgments
We thank Dr. M.A. Blázquez (IBMCP-CSIC, Valencia), and Dr. G. van den Ackerveken (Utrecht University) and Dr. P. García-Agustín (University Jaume I, Castellón) for providing us with Arabidopsis ga1-3 and sid2 mutant seeds, respectively.
This work was supported by the Ministerio de Ciencia y Tecnología (grant no. BFI2006–07622; Spain) and Junta de Castilla y León (grant no. SA073A08 to D.R.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Carlos Nicolás (cnicolas@usal.es).
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van der Straeten D, Peng JR, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311 91–94 [DOI] [PubMed] [Google Scholar]
- Ananieva EA, Alexieva VS, Popova LP (2002) Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J Plant Physiol 159 685–693 [Google Scholar]
- Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161 319–328 [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Segura A, Moreno M, López G, García-Olmedo F, Molina A (2002) Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol 128 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Mur LAJ, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38 432–447 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dat JF, López-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, Degreve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene-transfer to plants. Nucleic Acids Res 13 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gómez-Cadenas A (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J Agric Food Chem 53 8437–8442 [DOI] [PubMed] [Google Scholar]
- Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8 77–85 [DOI] [PubMed] [Google Scholar]
- Heckman NL, Horst GL, Gaussoin RE, Tavener BT (2002) Trinexapac-ethyl influence on cell membrane thermostability of Kentucky bluegrass leaf tissue. Sci Hortic (Amsterdam) 92 183–186 [Google Scholar]
- Herzog M, Dorne AM, Grellet F (1995) GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Mol Biol 27 743–752 [DOI] [PubMed] [Google Scholar]
- Horvath E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26 290–300 [Google Scholar]
- Jiang C, Fu X (2007) GA action: turning on de-DELLA repressing signaling. Curr Opin Plant Biol 10 461–465 [DOI] [PubMed] [Google Scholar]
- Kim SG, Lee AK, Yoon HK, Park CM (2008) A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. Plant J 55 77–88 [DOI] [PubMed] [Google Scholar]
- King KE, Moritz T, Harberd NP (2001) Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CB, Woo YM, Lee DJ, Lee MC, Kim CS (2007) Enhanced tolerance to heat stress in transgenic plants expressing the GASA4 gene. Plant Physiol Biochem 45 722–728 [DOI] [PubMed] [Google Scholar]
- Kusumi K, Yaeno T, Kojo K, Hirayama M, Hirokawa D, Yara A, Iba K (2006) The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol Plant 128 651–661 [Google Scholar]
- Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146 748–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2004) Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J 37 720–729 [DOI] [PubMed] [Google Scholar]
- Mene-Saffrane L, Davoine C, Stolz S, Majcherczyk P, Farmer EE (2007) Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant. J Biol Chem 282 35749–35756 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Navarro L, Bari R, Achard P, Lison P, Nemri A, Harberd NP, Jones JDG (2008) DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 18 650–655 [DOI] [PubMed] [Google Scholar]
- Nicolás C, Nicolás G, Rodríguez D (1996) Antagonistic effects of abscisic acid and gibberellic acid on the breaking of dormancy of Fagus sylvatica seeds. Physiol Plant 96 244–250 [Google Scholar]
- Nicolás C, Rodríguez D, Poulsen F, Eriksen EN, Nicolás G (1997) The expression of an abscisic acid-responsive glycine-rich protein coincides with the level of seed dormancy in Fagus sylvatica. Plant Cell Physiol 38 1303–1310 [DOI] [PubMed] [Google Scholar]
- Pan QH, Zhan JC, Liu HT, Zhang JH, Chen JY, Wen PF, Huang WD (2006) Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci 171 226–233 [Google Scholar]
- Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, Job D (2006) Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141 910–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raventos D, Meier C, Mattsson O, Jensen AB, Mundy J (2000) Fusion genetic analysis of gibberellin signaling mutants. Plant J 22 427–438 [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10 372–379 [DOI] [PubMed] [Google Scholar]
- Roxrud I, Lid SE, Fletcher JC, Schmidt EDL, Opsahl-Sorteberg HG (2007) GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol 48 471–483 [DOI] [PubMed] [Google Scholar]
- Seo PJ, Lee AK, Xiang FN, Park CM (2008) Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol 49 334–344 [DOI] [PubMed] [Google Scholar]
- Shi LF, Gast RT, Gopalraj M, Olszewski NE (1992) Characterization of a shoot specific, GA3 regulated and ABA regulated gene from tomato. Plant J 2 153–159 [PubMed] [Google Scholar]
- Silverstone AL, Jung HS, Dill A, Kawaide H, Kamiya Y, Sun TP (2001) Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13 1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel NE, Kuc A (1995) Chemical and biological inducers of systemic resistance to pathogens protect cucumber and tobacco plants from damage caused by paraquat and cupric chloride. Phytopathology 85 1306–1310 [Google Scholar]
- Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55 197–223 [DOI] [PubMed] [Google Scholar]
- Tesnier K, Strookman-Donkers HM, Van Pijlen JG, Van Der Geest AHM, Bino RJ, Groot SPC (2002) A controlled deterioration test for Arabidopsis thaliana reveals genetic variation in seed quality. Seed Science and Technology 30 149–165 [Google Scholar]
- Weiss D, Ori N (2007) Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol 144 1240–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PF, Chen JY, Wan SB, Kong WF, Zhang P, Wang W, Zhan JC, Pan QH, Huang WD (2008) Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul 55 1–10 [Google Scholar]
- Wigoda N, Ben-Nissan G, Granot D, Schwartz A, Weiss D (2006) The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J 48 796–805 [DOI] [PubMed] [Google Scholar]
- Yang YN, Qi M, Mei CS (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40 909–919 [DOI] [PubMed] [Google Scholar]