Abstract
Circadian rhythms have been described for numerous transmitter synthesizing enzymes in the brain but rarely in spinal cord. We measured spinal tyrosine-hydroxylase (TH) and nitric oxide synthase (NOS) levels in the thoracic intermediolateral nucleus, the location of sympathetic preganglionic neurons, in male wild type (WT) and dopamine D3 receptor knockout mice (D3KO). TH and NOS levels both displayed circadian patterns in WT and D3KO animals with overall reduced TH and increased NOS expression in the D3KO mice. The circadian pattern of NOS expression was similar in WT and D3KO mice. In contrast, TH expression was inverted in D3KO mice, with TH levels consistently lower than in WT throughout the day, but strongly increased temporarily 1 h prior to daylight.
TH is the rate-limiting enzyme for the production of dopamine. Spinal dopamine dysfunction is implicated in a sleep disorder called restless legs syndrome (RLS). RLS follows a circadian rhythm and is relieved clinically by dopamine D3 receptor agonists. Our observations of an altered circadian pattern in spinal dopamine synthesis in D3KO animals may provide insight into putative dopaminergic mechanisms contributing to RLS.
Keywords: dopamine, circadian, restless legs syndrome, spinal cord, sleep disorder, animal model
Mammalian nervous system activity is under strong circadian control from the hypothalamic suprachiasmatic nuclei (SCN) (Moore, 1996; LeSauter and Silver, 1998). One SCN projection is to the dorsomedial hypothalamic (A11) dopaminergic nucleus (Reuss, 1996; Abrahamson and Moore, 2001). A11 neurons are the sole source of spinal dopamine, with strong projections to the sympathetic preganglionic neurons (SPNs) in the intermediolateral nucleus (IML) (Skagerberg and Lindvall, 1985; Holstege et al., 1996; Abrahamson and Moore, 2001). This bineuronal link between the SCN to SPNs via A11 neurons suggests that spinal dopaminergic actions are under circadian influence, presumably to modulate sympathetic autonomic function correspondingly.
Dopamine dose-dependently reduces spinal cord excitability in part via dopamine D3 receptors and, in the presence of low dopamine, dopaminergic depression is converted to excitation in dopamine D3 receptor knockout (D3KO) mice (Clemens and Hochman, 2004). Brain dopamine levels are lowest at night and early morning (Carlsson et al., 1980) and a similar cycling in spinal dopamine synthesis could modulate cord excitability in a corresponding manner. Hence, we sought to determine the local circadian expression of the dopamine-producing enzyme tyrosine-hydroxylase (TH) in the murine spinal cord and to compare these levels between wild type (WT) and D3KO mice.
The distribution of nitric oxide synthase (NOS) synthesizing neurons in the spinal cord suggests that nitric oxide can also modulate spinal pathways (Saito et al., 1994) including in the IML (Dun et al., 1992; Saito et al., 1994). Like dopamine, NOS follows a circadian pattern in the brain (Ayers et al., 1996; Tunctan et al., 2002). Therefore, for comparison, we also measured NOS levels in the IML of WT and D3KO mice. Some of these data have been published in abstract form (Sawchuk et al., 2004).
EXPERIMENTAL PROCEDURES
Dissection and electrophysiology of spinal cords
All procedures complied with the NIH guidelines for animal care and the Emory Institutional Animal Care and Use Committee, and care was taken to minimize the number of animals used and their suffering. Young (6–8 weeks, 20–24 g) male D3KO mice (TB6.129S4-Drd3J, Jackson Laboratory, Bar Harbor, ME, USA) (n=18) and their associated WT (C57BL/6 mice) (n=18) were held under a strict 12-h light/dark cycle (07:00–19:00 h) with free access to food and water. One week prior to the experiments, animals were isolated in individual cages, to minimize external disturbances. Two days before the experiments, animals received a single i.p. injection of 50 µl Fluorogold (FG) (1% in 0.9% NaCl), to retrogradely label preganglionic sympathetic neurons (Anderson and Edwards, 1994). On the day of the experiments, animals were collected in 4 h intervals, starting at 06:00 h in the morning (1 h before lights on) and lasting until the following day at 02:00 h. During light-off times, red light filters were used to avoid exposure to daylight. Animals were anesthetized with Isofluorane inhalant, and decapitated within 5 min of anesthesia. Spinal cords were removed, the thoracic segments T7–T10 blocked up and frozen on cryostat chucks. The tissue was cut in 10 µm-thick sections on a cryostat (OTF5000, Vibratome Company, St Louis, MO, USA) and thaw-mounted onto microscopy slides. Tissues from three animals were used for each time point in WT and D3KO animals, and at least 10 sections were analyzed from each animal for TH and NOS labeling. Representative sections were photographed for the presence of IML FG staining.
Immunolabeling
Slides were immersion-fixed in modified Lana’s fixative for 10 min (4% paraformaldehyde, 0.16 M phosphate buffer, 0.2% picric acid, pH 6.9), washed overnight in PBS-T (0.1 M phosphate buffer, 0.9% NaCl, 0.3% Triton X-100, pH 7.4) and incubated for 48–72 h. To distinguish dopaminergic from adrenergic input, slides were incubated in mouse anti-dopamine-beta-hydroxylase (DBH) (Chemicon, Temecula, CA, USA; 1:1000 dilution) and rabbit anti-TH (Chemicon; 1:1000 dilution). Slides were washed 3×30 min in PBS-T, and incubated with FITC-conjugated donkey anti-mouse (Jackson Immunoresearch, West Grove, PA, USA; 1:100 dilution) or Cy3-conjugated donkey anti-rabbit (Jackson Immunoresearch, 1:250 dilution). Slides were washed 20 min in PBS-T followed by 2×20 min in 50 mM Tris–HCl (pH 7.4), coverslipped with vectashield (Vector Laboratories, Burlingame, CA, USA) and photographed. For densitometric studies, slides were incubated in either rabbit anti-TH or rabbit anti-neuronal NOS (Chemicon, 1:250 dilution). Slides were washed 3×30 min in PBS-T followed by secondary incubation in goat anti-rabbit (Sternberger Monoclonals Inc., Lutherville, MD, USA; 1:100 dilution) for 1.5 h. Tissue was washed 3×20 min in PBS-T and incubated in rabbit PAP for 1.5 h. (Sternberger Monoclonals Inc., 1:500 dilution). Slides were washed 20 min in PBS-T and 2×20 min in 50 mM Tris–HCl. Peroxidase detection was performed in 50 mM Tris–HCl with 0.02% di-amino-benzidine tetrahydrochloride and 0.0045% hydrogen peroxide and stopped in 50 mM Tris–HCl. Slides were dehydrated, defatted and coverslipped with permount. Control slides were treated identically but received no primary antibody.
Image analysis
IML areas (minimum of 10 per animal) were digitized with neurolucida software (MicroBrightField Inc., Williston, VT, USA) and the IML region was examined for grayscale density (0–255, with 0=white and 255=black). Control areas the size of the IML region were also analyzed, to account for the differences in background labeling. Time sets of WT and D3KO animals were processed in parallel, and a correction factor for the gray scale density was calculated with the following formula:
with λ= average of gray scale values of the IML area,λmin=average of gray scale values of the background area, λmax=maximum gray value.
Data analysis
All values are given as mean±S.E. We used SigmaPlot and SigmaStat (both: SPSS Science, Chicago, IL, USA) to analyze the data, and we tested for significant differences in the course of an experiment using two-way ANOVA tests. Differences were considered significant if P<0.05.
RESULTS
We found that the overall expression of TH and NOS levels changed significantly between WT and D3KO animals. In a cumulative sum comparison, TH levels decreased in D3KO to 76% of the levels observed in WT, while cumulative NOS levels in D3KO were increased 20% over WT control.
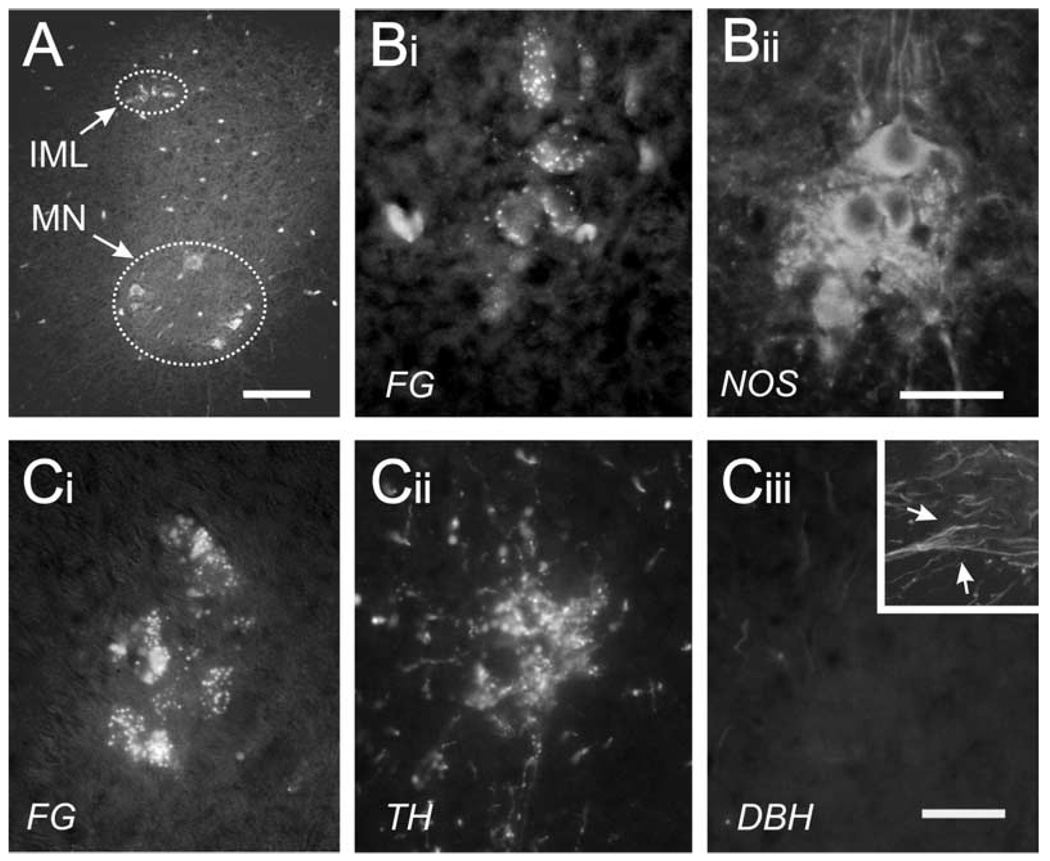
Intraperitoneal injection of FG labels all spinal motor efferents (Fig. 1A, arrows). SPNs originate in the IML and motoneurons (MNs) in the ventral horn. FG-positive IML neurons were positive for NOS (Fig. 1B). Processing for TH and DBH revealed that the FG-positive IML neurons were surrounded by TH but not DBH immunoreactive arborizations (Fig. 1C). Since DBH is required to convert dopamine to noradrenaline, these data demonstrate that the TH found in the IML region reflects descending dopaminergic fibers and not adrenergic projections.
Fig. 1.
Identification of IML region and circadian levels of TH and NOS. (A) I.p. injection with FG selectively labeled efferent neurons in the IML and MN in the ventral horn. (B) Double-staining for FG and NOS revealed that IML neurons were positive for FG (Bi) and NOS (Bii). (C) Triple-staining revealed TH but not DBH in the FG-positive IML region. Note that neuropil surrounding FG+ sympathetic neurons are TH+ but DBH− identifying these as descending dopaminergic axonal arborizations. As a positive control for DBH, the inset in Ciii shows an area in the ventral horn with several DBH immunoreactive fibers (arrows). Note that panels were obtained from D3KO mice perfused with Lana’s fix, to improve cellular resolution. Scale bars=100 µm A, B and C: 25 µm.
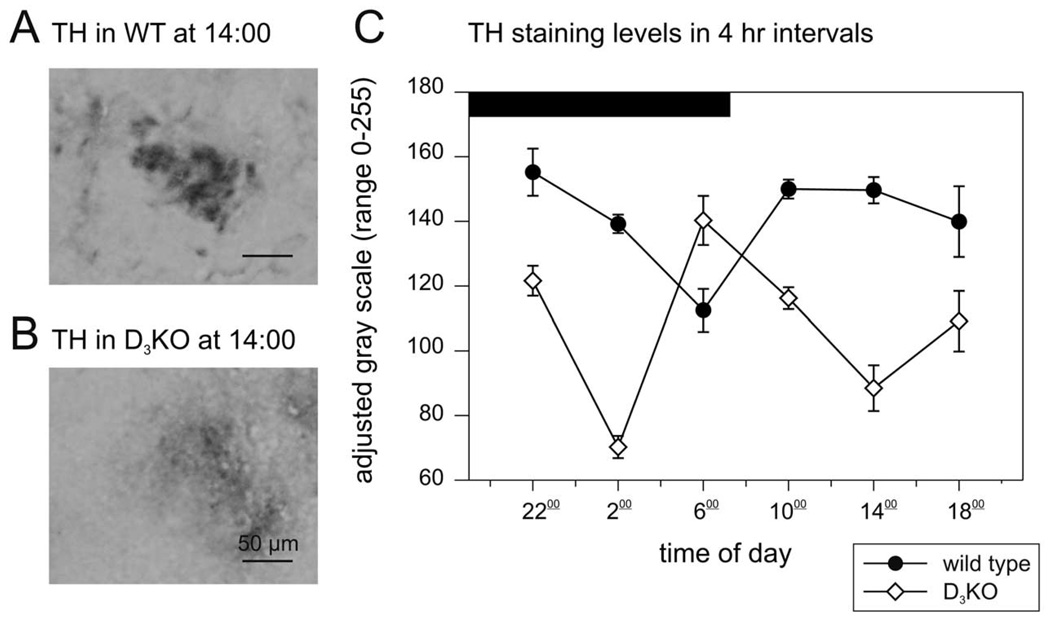
We digitized the TH labeling in the IML to gray scale values, to assess the respective intensities of the TH labeling (Fig. 2A, B). Representative sections of the IML region of WT and D3KO animals at the same time points reveal strong differences in TH intensities. In D3KO, TH labeling was significantly weaker than in WT, but expressed its highest value at the 06:00 time point when TH was at its lowest in WT mice (Fig. 2C). This suggests an inversion of circadian TH expression in D3KO animals.
Fig. 2.
Circadian levels of TH. (A) Representative gray-scale image of a TH-labeled IML region in a WT animal at 14:00 h. (B) Representative gray-scale image of a TH-labeled IML region in a D3KO animal at the same time point. The IML displays a much weaker TH labeling than in WT. (C) Circadian pattern of TH-labeling in WT and D3KO animals. In WT (black circles), TH levels were generally stable and decreased only temporarily 1 h before daylight. In contrast, TH levels were significantly different at all time points in the D3KO animals (open triangles, P<0.001, two-way ANOVA with Tukey post hoc comparison), and their pattern was inverted with regard to the WT animals, with lower levels of TH throughout the day and a temporary peak in TH expression 1 h before daylight.
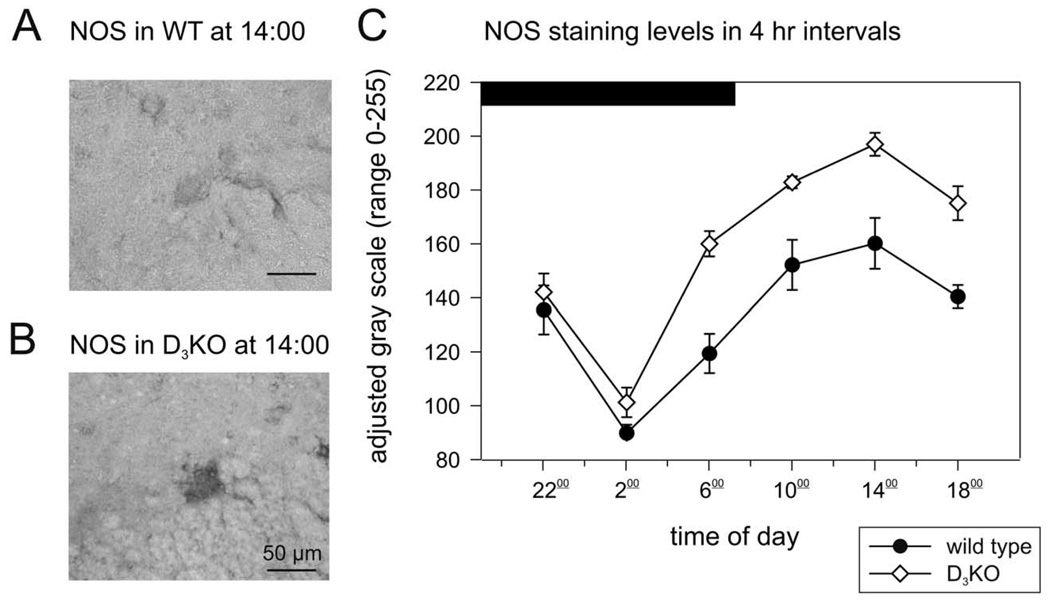
Analysis of the levels of NOS labeling in WT (Fig. 3A) and D3KO (Fig. 3B) animals also revealed a circadian pattern. However, unlike the reversal observed for TH in D3KO, the patterns were similar in both animal types, with a trough at 02:00 h and a peak at 14:00 h (Fig. 3C). Additionally, although labeling intensities were significantly higher in D3KO during daytime, they were similar between WT and D3KO at night.
Fig. 3.
Circadian levels of NOS. (A) Representative gray-scale image of a NOS-labeled neuron in the IML region of a WT animal at 14:00 h. (B) Representative gray-scale image of a NOS-labeled neuron in the IML region of a D3KO animal at 14:00 h. IML neurons in D3KO animals display a much stronger labeling of NOS than observed in WT animals. (F) Circadian pattern of NOS-labeling in WT and D3KO animals. In WT (black circles), NOS levels display a circadian, with a trough at 06:00 h and a peak at 14:00 h. This circadian pattern was the same for the D3KO animals (open triangles), although the intensity levels of NOS labeling was significantly higher during daylight (P<0.001, two-way ANOVA with Tukey post hoc comparison).
DISCUSSION
The IML in the thoracic spinal cord houses the spinal sympathetic preganglionics and is the major sympathetic output of the CNS. Here we show that the expression of the transmitter synthesizing enzymes TH and NOS undergoes circadian variations in the IML and that these variations differ between WT and D3KO. In comparison to WT, overall TH levels were decreased in D3KO mice, while overall NOS expression was increased. Despite significant differences in NOS levels, the circadian pattern was similar in WT and D3KO, with a nadir at 02:00 h and a peak at 14:00 h. In contrast, TH levels were stable throughout the day in WT, but decreased at the 06:00 time point, the period of maximum activity in mice. This TH pattern was inverted in D3KO animals, with significantly lower levels in TH throughout the day, but a spike in TH expression at 06:00 h.
Our data on circadian TH expression are consistent with the observation that brain dopamine content in mouse cycles with peaks at midnight dropping significantly by 04:00 h (Huie et al., 1989). However our observations differ from a study on TH mRNA undertaken in the rat substantia nigra pars compacta (Weber et al., 2004) where TH levels gradually increased from 04:00–16:00 h and declined 1 h before lights off. Two possible explanations for observed differences are that (i) CNS regions serving different functions require a different circadian regulation of TH, and (ii) axonal transport of cytosolic protein complexes can be slow (2–8 mm/day, e.g. Brown, 2003) resulting in distance-dependent expression lag times in the circadian cycling of somatic TH synthesis.
Our data show that TH and thus presumably dopamine levels are lower in D3KO animals at most time points. Since dopamine largely decreases spinal cord excitability (Carp and Anderson, 1982; Gajendiran et al., 1996; Svensson et al., 2003; Clemens and Hochman, 2004) this reduction in descending inhibitory drive from the brain should coincide with an increase in spinal excitability. Indeed, D3KO mice are hyperactive and display increased locomotor activity (Accili et al., 1996; Asico et al., 1998).
The observations in D3KO mice of circadian dopaminergic dysfunction in concert with increased cord excitability is intriguing in relation to the restless legs syndrome (RLS) phenotype in humans. RLS is a sleep disorder of CNS origin that involves abnormal limb sensations with a marked circadian pattern (worst at night), with evidence of increase spinal cord excitability, and is relieved particularly by DB3B receptor-preferring agonists (Bara-Jimenez et al., 2000; Montplaisir et al., 2000; Odin et al., 2002; Zucconi and Ferini-Strambi, 2004). Since hypothalamic dopamine levels are at their lowest in the early morning hours (Carlsson et al., 1980) when RLS symptoms peak, it is tempting to suggest that a reduced dopaminergic activity in spinal cord is responsible for the increase in excitability observed in RLS patients and in D3KO mice.
We observed that NOS levels in the mouse IML underwent a strong circadian cycle with highest levels observed during the light phase (14:00 h). In contrast, NOS circadian rhythms in the rat and mouse brain show higher NOS levels during their dark phase (Ayers et al., 1996; Tunctan et al., 2002). Again, these timing differences could be due to different local functions of NOS in the spinal cord and/or cytosolic transport. NOS activity is involved in the regulation of body temperature and motor activities (Kamerman et al., 2002), and both body temperature and physical activity peak conjointly (Refinetti and Menaker, 1992). Thus the inversion of the TH circadian pattern and the increase in NOS levels in the IML of D3KO animals during subjective nighttime could indicate an upregulation of these functions consistent with the behavioral changes observed in D3KO mice.
In summary, our data demonstrate that transmitter synthesis in spinal sympathetic neurons is under circadian controls and that synthesis is altered in D3KO mice. Importantly an inverted circadian cycling in TH expression in D3KO mice could lead to changes in autonomic function and cord excitability, two observed features of the RLS phenotype in humans. These observations introduce alterations in circadian TH expression as a possible component of dopamine dysfunction in RLS patients and support a circadian component to the spinal dopamine dysfunction observed in D3KO mice (Clemens and Hochman, 2004), further validating D3KO mice as a potential animal model of RLS.
Acknowledgments
S.H. was supported by NINDS NS045248 and the Restless Legs Syndrome Foundation, and S.C. received support from the Christopher Reeve Paralysis Foundation (CB2-0205-1B). We thank David Rye, MD, PhD, Dept. Neurology, Emory Univ. Sch. Med. for providing D3KO mice for breeding, Barbara Breen, PhD, for help in the numerical analysis of the Neurolucida data, and Maggie Hatcher and Megan Daugherty for expert technical support.
Abbreviations
- DBH
dopamine-beta-hydroxylase
- D3KO
dopamine D3 receptor knockout
- FG
Fluorogold
- IML
intermediolateral nucleus
- MN
motoneurons
- NOS
nitric oxide synthase
- RLS
restless legs syndrome
- SCN
suprachiasmatic nucleus
- SPNs
sympathetic preganglionic neurons
- TH
tyrosine-hydroxylase
- WT
wild type
REFERENCES
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CR, Edwards SL. Intraperitoneal injections of Fluorogold reliably labels all sympathetic preganglionic neurons in the rat. J Neurosci Methods. 1994;53:137–141. doi: 10.1016/0165-0270(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hyper-tension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers NA, Kapas L, Krueger JM. Circadian variation of nitric oxide synthase activity and cytosolic protein levels in rat brain. Brain Res. 1996;707:127–130. doi: 10.1016/0006-8993(95)01362-8. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–1616. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Svennerholm L, Winblad B. Seasonal and circadian monoamine variations in human brains examined post mortem. Acta Psychiatr Scand Suppl. 1980;280:75–85. [PubMed] [Google Scholar]
- Carp JS, Anderson RJ. Dopamine receptor-mediated depression of spinal monosynaptic transmission. Brain Res. 1982;242:247–254. doi: 10.1016/0006-8993(82)90307-9. [DOI] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci. 2004;24:11337–11345. doi: 10.1523/JNEUROSCI.3698-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Forstermann U, Tseng LF. Nitric oxide synthase immunoreactivity in rat spinal cord. Neurosci Lett. 1992;147:217–220. doi: 10.1016/0304-3940(92)90599-3. [DOI] [PubMed] [Google Scholar]
- Gajendiran M, Seth P, Ganguly DK. Involvement of the presynaptic dopamine D2 receptor in the depression of spinal reflex by apomorphine. Neuroreport. 1996;7:513–516. doi: 10.1097/00001756-199601310-00033. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Van Dijken H, Buijs RM, Goedkengt H, Gosens T, Bongers CM. Distribution of dopamine immunoreactivity in the rat, cat, and monkey spinal cord. J Comp Neurol. 1996;376:631–652. doi: 10.1002/(SICI)1096-9861(19961223)376:4<631::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Huie JM, Sharma RP, Coulombe RA., Jr Diurnal alterations of catecholamines, indoleamines and their metabolites in specific brain regions of the mouse. Comp Biochem Physiol C. 1989;94:575–579. doi: 10.1016/0742-8413(89)90115-1. [DOI] [PubMed] [Google Scholar]
- Kamerman P, Mitchell D, Laburn H. Circadian variation in the effects of nitric oxide synthase inhibitors on body temperature, feeding and activity in rats. Pflugers Arch. 2002;443:609–616. doi: 10.1007/s00424-001-0738-0. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Output signals of the SCN. Chronobiol Int. 1998;15:535–550. doi: 10.3109/07420529808998706. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Denesle R, Petit D. Pramipexole in the treatment of restless legs syndrome: a follow-up study. Eur J Neurol. 2000;7 Suppl 1:27–31. doi: 10.1046/j.1468-1331.2000.0070s1027.x. [DOI] [PubMed] [Google Scholar]
- Moore RY. Entrainment pathways and the functional organization of the circadian system. Prog Brain Res. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- Odin P, Mrowka M, Shing M. Restless legs syndrome. Eur J Neurol. 2002;9 Suppl 3:59–67. doi: 10.1046/j.1468-1331.9.s3.8.x. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav. 1992;51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Reuss S. Components and connections of the circadian timing system in mammals. Cell Tissue Res. 1996;285:353–378. doi: 10.1007/s004410050652. [DOI] [PubMed] [Google Scholar]
- Saito S, Kidd GJ, Trapp BD, Dawson TM, Bredt DS, Wilson DA, Traystman RJ, Snyder SH, Hanley DF. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience. 1994;59:447–456. doi: 10.1016/0306-4522(94)90608-4. [DOI] [PubMed] [Google Scholar]
- Sawchuk MA, Clemens S, Hochman S. Tyrosine-hydroxylase levels in spinal sympathetic regions: Circadian variation and greatly reduced staining in D3 knockout mice. Proceedings of the San Diego Society for Neuroscience. 2004;546:15. [Google Scholar]
- Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985;342:340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- Svensson E, Wikstrom MA, Hill RH, Grillner S. Endogenous and exogenous dopamine presynaptically inhibits glutamatergic reticulospinal transmission via an action of D2-receptors on N-type Ca2+ channels. Eur J Neurosci. 2003;17:447–454. doi: 10.1046/j.1460-9568.2003.02466.x. [DOI] [PubMed] [Google Scholar]
- Tunctan B, Weigl Y, Dotan A, Peleg L, Zengil H, Ashkenazi I, Abacioglu N. Circadian variation of nitric oxide synthase activity in mouse tissue. Chronobiol Int. 2002;19:393–404. doi: 10.1081/cbi-120002915. [DOI] [PubMed] [Google Scholar]
- Weber M, Lauterburg T, Tobler I, Burgunder JM. Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neurosci Lett. 2004;358:17–20. doi: 10.1016/j.neulet.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5:293–299. doi: 10.1016/j.sleep.2004.01.004. [DOI] [PubMed] [Google Scholar]