Abstract
The challenge of designing a construct for the repair of focal cartilage defects such that it mimics the mechanical properties of and can integrate with native cartilage has not been met by existing technologies. Herein we describe a novel construct consisting of a non-degradable poly-vinyl alcohol scaffold to provide long-term mechanical stability, interconnected pores to allow for the infiltration of chondrocytes and poly-lactic glycolic acid microspheres for the incorporation of growth factors to enhance cellular migration. The objective of this study was to characterize the morphological features and mechanical properties of our porous PVA-PLGA construct as a function of PLGA content. Varying the PLGA content was found to have a significant effect on the morphological features of the construct. As PLGA content increased from 10 – 75%, samples exhibited a six-fold increase in average percent porosity, an increase in average microsphere diameter from 8 – 34 µm, and an increase in average pore diameter from 29 – 111 µm. The effect of PLGA content on Aggregate Modulus and Permeability was less profound. Our findings suggest that that morphology of the construct can be tailored to optimize cellular infiltration and the dynamic mechanical response.
Introduction
Over 800,000 patients undergo various non-arthroplasty treatments for articular cartilage damage in the United States annually, at a cost to the economy of approximately $400 million (Jackson, et al., 2001). However, patients diagnosed with articular cartilage defects are at increased risk for the early development of osteoarthritis (Gillogly et. al., 1998). Successfully treating the defect has the potential to halt this progression. The clinical standard-of-care involves the transplantation of autogenous osteochondral plugs from non-weight bearing areas. This approach has had reasonable short-term success (Sanders et al., 2001; Fitzpatrick and Morgan, 1998; Horas et al., 2000) but also faces many unresolved technical challenges including defect size limitations, donor site morbidity, the challenge of matching donor site and host site curvature (Bartz et al., 2001) and regional differences in cartilage properties (Lane et al., 2001, Jurvelin et al., 2000). Microfracture of the subchondral plate is another clinically performed procedure that attempts to elicit a fracture healing response (Gillogly et al., 1998), but results have been variable (Mithoefer et al., 2005) with complications such as dislocation of the tissue that fills the defect (Gudas et. al., 2005). Expanded autogenous chondrocytes have been implanted into defects in an attempt to grow cartilage in situ, but problems of uncontrolled tissue overgrowth have been reported (Wood et. al. 2006) and results appear to be heavily dependent on surgical technique (Gillogly and Myers, 2005).
The concept of growing articular cartilage ex vivo and implanting the tissue into cartilaginous defects has become a major area of research. Typically, cells are seeded into degradable hydrogels, such as chitosan (Griffon et al., 2006; Li et. al., 2004; Sechriest, et al., 2000) or alginate (Elisseeff et al., 2002). The constructs are cultured in the presence of chondroinductive growth factors (Yasuda et al., 2006; DeFail et al., 2005) or under deformational loading conditions (Hung et al., 2004) in an attempt to optimize matrix generation within the scaffolds. In order for the newly formed cartilage to function as a load supporting structure when implanted, its mechanical properties should ideally approach that of native tissue. Furthermore, integration with surrounding tissues is vital for long-term fixation. To date however, despite the wide range of conditions to which cell-seeded constructs have been exposed, their mechanical properties have been far inferior to that of articular cartilage (Hung et al., 2004) and integration between the constructs and surrounding native tissue has been problematic (Ramaswamy, et al 2006; Giurea, et al 2002; Obradovic, et al 2001).
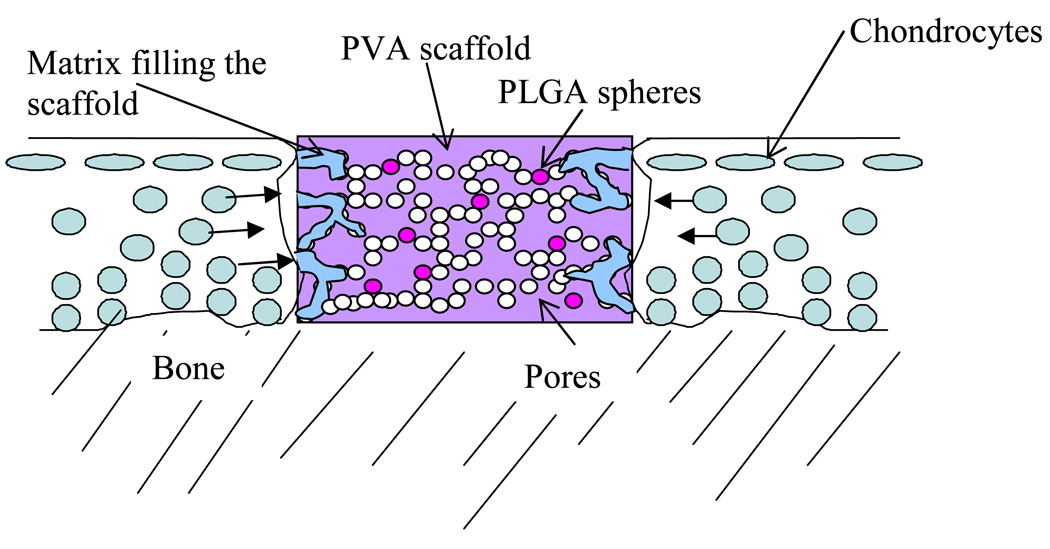
The use of non-degradable synthetic materials to repair cartilage defects has been suggested and explored in animal models (Noguchi et al., 1991, Oka et al., 1997). Non-degradable synthetics are potentially attractive because of an ability to define mechanical characteristics by controlling manufacturing processes. However, integration with surrounding tissue remains an unsolved problem (Ushio, et al 2004). In this study we describe a partly-degradable porous hydrogel construct to replace damaged cartilage. The construct is made of non-degradable poly-vinyl alcohol (PVA) to provide long-term mechanical stability and the interconnected pores will allow for the infiltration of chondrocytes and thus construct/tissue integration. In addition, degradable poly-lactic glycolic acid (PLGA) microspheres are seeded within the construct for incorporation of growth factors to enhance cellular migration and matrix generation (Figure 1). Manufacture of the PVA-PLGA constructs herein proposed involves producing an emulsion of PLGA dissolved in dichloromethane, and PVA dissolved in de-ionized water. We hypothesized that the post-solidification morphology of the constructs would be affected by the relative amounts of PLGA and PVA solutions. The objective of this study was to characterize the morphology and mechanical behavior of porous PVA-PLGA constructs as a function of PLGA content.
Figure 1.
Schematic of porous PVA hydrogel with PLGA microspheres in a cartilage defect. Chondrocytes from surrounding tissue will ideally migrate into the PVA scaffold.
Materials & Methods
Manufacture
The PVA-PLGA constructs were manufactured by mixing 10 g of PVA powder (Elvanol© 71-30, Mw ~ 96,000; DuPont, Wilmington, DE) with 90 g of double distilled de-ionized water. The mixture was autoclaved for one hour at 120 °C to produce 10% weight PVA solution. The solution was allowed to cool to room temperature. PLGA powder (Medisorb® 5050DL 3.5A, Alkermes Inc., Cambridge, MA) was dissolved in dichloromethane, DCM (Sigma Aldrich, St. Louis, MO) to create a solution of 10% weight PLGA. The PLGA solution was sonicated in an ultrasonic cleaner (Branson 1510, Branson, Danbury, CT) for 10 minutes, then added drop-wise to the PVA solution. The amount of PLGA solution added was varied as a weight percent of PVA to produce the following groups: 10%, 20%, 50%, and 75% PLGA (n=10 per group). The combined mixture was magnetically stirred at 300 rpm for 10 minutes. The emulsion was poured into wells of a 24-well polystyrene plate, sealed with parafilm and subjected to five cycles of 23 hours of freezing (−20°C) followed by 1 hour of thawing at room temperature (25°C) in a MicroClimate™ chamber (Cincinnati Sub-Zero, Cincinnati, OH). Samples were kept in a −20°C freezer until testing.
Morphology
A disc of material approximately 5 mm thick was sliced from the middle of each construct for morphological analysis (n=5 per group). The discs were placed in an environmental chamber of a scanning electron microscope (FEI Philips, Hillsboro, OR). Two images, one at 250× and one at 500× magnification were analyzed using J-image (National Institutes of Health, Bethesda, MD). Using the 500× images, pores in the PVA were identified and the diameters recorded. Similarly, using the 250× images, microspheres were identified and diameters measured. The percent porosity of the PVA was calculated from the 250× images, as the ratio of the total area of pores to area of the field of view.
Mechanical Testing
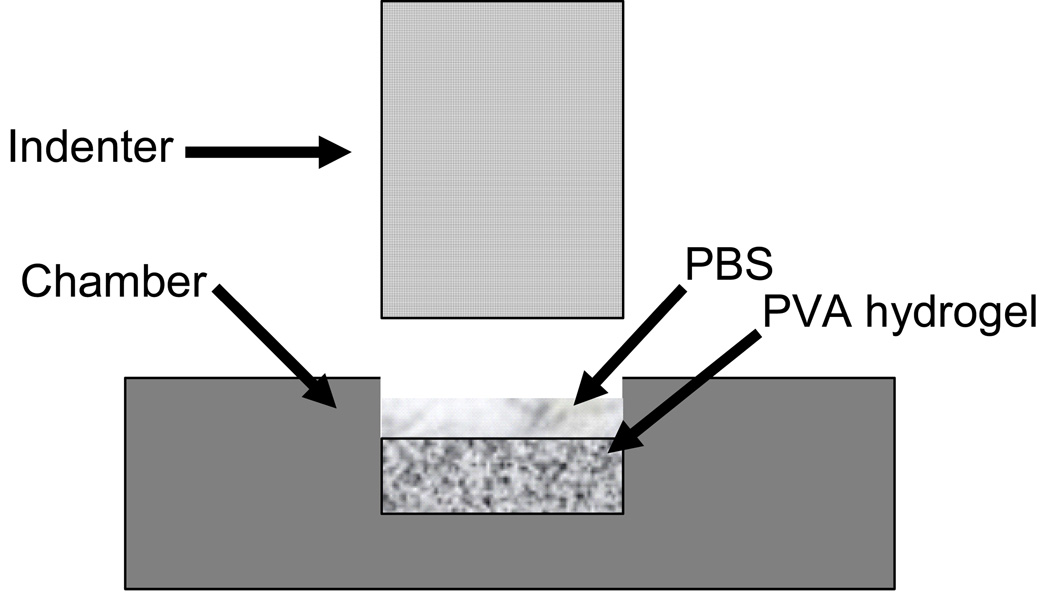
Constructs (n=5 per group) were cored using a 5 mm diameter biopsy punch and sliced to a thickness of 2–3 mm on a freezing stage microtome to ensure flat parallel surfaces. A creep test (figure 2) was performed using a custom-made test apparatus, the Compression Computer Automated Soft Tissue Test System (CCASTTS), (Torzilli, 1990). Samples were confined in a 5 mm diameter stainless-steel well and compressed by a porous 5 mm diameter brass filter. The confined samples were rapidly compressed at a rate of 150 µm/s to a load of 50 g. Thereafter the load was held constant and samples were allowed to creep for one hour. Throughout testing the creep displacement was recorded. The data was analyzed using the biphasic theory (Mow et al.) to calculate the Aggregate Modulus, Ha, and Permeability, κ. The stress (σ) vs. strain (ε) relationship for the initial phase of rapid loading was linear up to 8 % strain for all samples, thus the slope was defined as the Dynamic Modulus.
Figure 2.
Schematic of the experimental test setup for confined compressive testing. Samples were confined in a 5 mm diameter stainless-steel well and compressed by a porous 5 mm diameter brass filter. A pre-defined load was rapidly applied, and thereafter the load was held constant for one hour to allow the sample to creep.
Full thickness discs of articular cartilage (n=5, 5 mm diameter × 1.5 mm height) were harvested from the trochlea of mature bovine knees. A biopsy punch was used to core the tissue, after which a scalpel was used to remove the cartilage disk from a close as possible to the underlying bone. A freezing stage sledge microtome was used to remove a small amount of tissue from the base of the plug to ensure that the top and bottom surfaces were parallel.
Methods of Statistical Analysis
The relationships between PLGA content and morphological features (porosity, pore diameter, microsphere diameter) and mechanical properties (Aggregate Modulus, Dynamic Modulus and Permeability) were analyzed as follows. Aggregate Modulus, Dynamic Modulus and Permeability and porosity were analyzed using a one way ANOVA with %PLGA as the independent variable and Bonferroni corrected post-hoc comparisons were run on the data. Pore diameter and microsphere diameter data were logarithmically transformed to produce normally distributed data. A one factor ANOVA with samples nested within the factor, PLGA content, was run. The results for the multiple comparisons were corrected with a Bonferroni adjustment.
Results
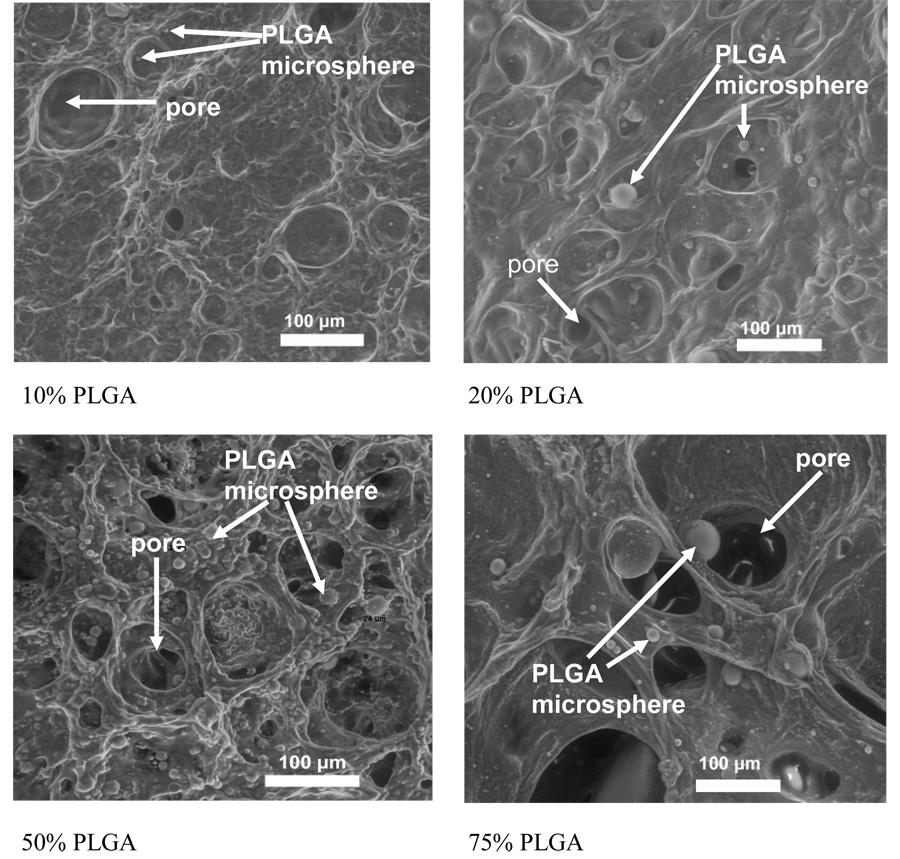
We successfully manufactured porous, microsphere seeded PVA-PLGA constructs using the methods herein described and measured the morphological changes in the constructs as a function of PLGA content (Figure 3). Porosity increased from 8 ± 3 % to 54 ± 19 %, pore diameter from 29± 21 µm to 111 ± 85 µm and microsphere diameter from 9 ± 2 µm to 33 ± 35 µm as PLGA content increased from 10 % to 75 % (Table 1).
Figure 3.
SEM images of 10%, 20%, 50% & 75% PLGA hydrogel surfaces. In these representative pictures it can be seen that as PLGA content increases, the porosity, pore diameter, and microsphere diameter also increase.
Table 1.
Porosity, Micro-Sphere Diameter and Pore Diameter (average ± standard deviation) as a function of %PLGA content
| % PLGA | Porosity | Micro-Sphere Diameter (µm) | Pore Diameter (µm) |
|---|---|---|---|
| 10 | 8±3 | 8.5±2.1 | 29 ± 21 |
| 20 | 17±5 | 10.8±3.9 | 35 ± 22 |
| 50 | 49±20 | 25.4±25.5 | 95 ± 82 |
| 75 | 54±19 | 33.7±34.7 | 111 ± 85 |
Porosity, pore diameter, and microsphere diameter were not significantly different between the 50 % and 75 % PLGA samples, nor between the 10 % and 20 % PLGA samples (see Table 2). The 50 % and 75% PLGA constructs had a higher porosity, larger pores and larger microspheres than the 10% and 20% PLGA constructs.
Table 2.
Summary of statistical analysis of inter-group differences of porosity, microsphere diameter and pore diameter
| % PLGA | Porosity | Micro-Sphere Diameter | Pore Diameter |
|---|---|---|---|
| 10 vs. 20 | 50 =75 (p=1.0) | 50 =75 (p=0.2) | 20 > 10 (p=0.01) |
| 10 vs. 50 | 50 > 10 (p=0.002) | 50 > 10 (p<0.001) | 50 > 10 (p<0.001) |
| 10 vs. 75 | 75 >10 (p<0.001) | 75 >10 (p<0.001) | 75 >10 (p<0.001) |
| 20 vs. 50 | 50 > 20 (p=0.02) | 50 > 20 (p<0.001) | 50 > 20 (p<0.001) |
| 20 vs. 75 | 75 >20 (p=0.003) | 75 >20 (p<0.001) | 75 >20 (p<0.001) |
| 50 vs. 75 | 50 = 75 (p=1.0) | 50 = 75 (p=0.4) | 50 = 75 (p=0.2) |
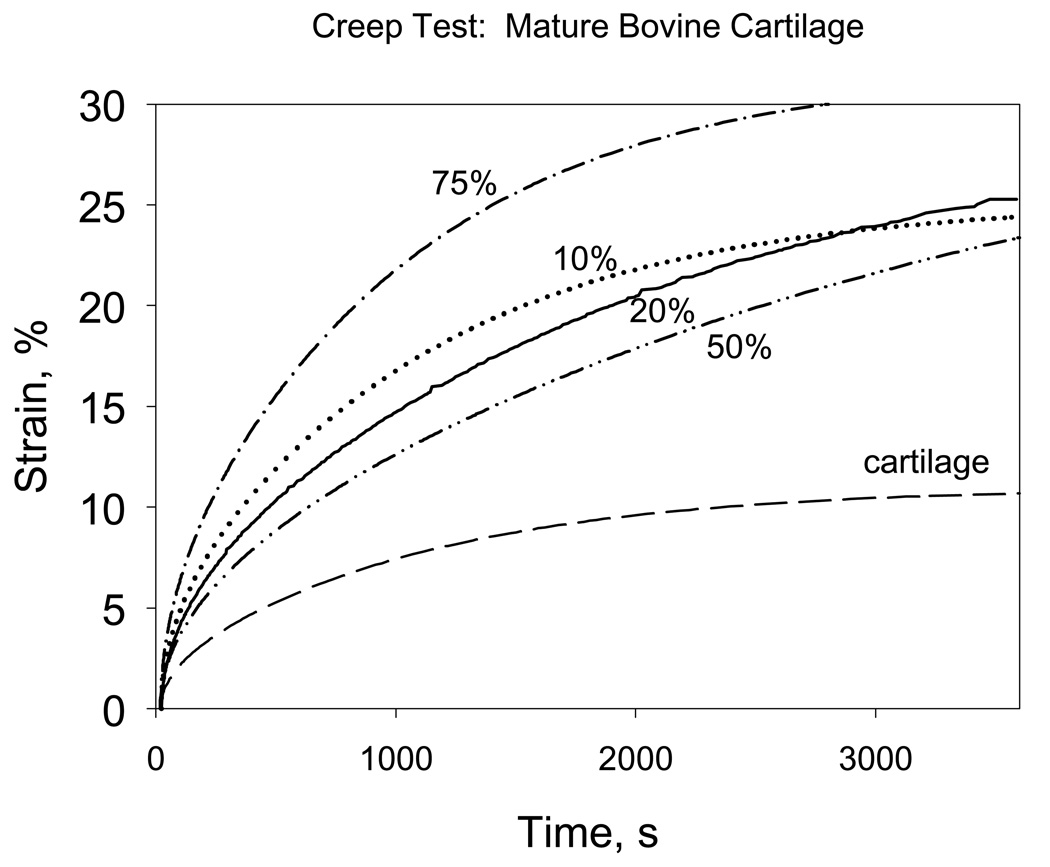
The biphasic-theory fitted creep response of the PLGA/PVA constructs and mature articular cartilages are illustrated in Figure 4. The Aggregate Modulus ranged from 0.086 ± 0.0075 to 0.102 ± 0.013 MPa and was independent of PLGA content, p=0.15 (Table 3). Articular cartilage had a significantly higher Aggregate Modulus (0.311 ± 0.062 MPa) compared to all PLGA-PVA constructs, regardless of PLGA content.
Figure 4.
Strain versus time plot for a 10, 20, 50, and 75% PLGA 5mm diameter hydrogel core using a 5mm diameter porous brass indenter in a confined creep test.
Table 3.
Aggregate Modulus and Permeability (average ± standard deviation) as a function of %PLGA content
| PLGA % | Aggregate Modulus, Ha | Ave. Permeability, k (× 10−14 m4/Ns) |
|---|---|---|
| 10 | 0.102 ± 0.013 | 2.74± 0.56 |
| 20 | 0.087 ± 0.0075 | 1.53 ± 0.68 |
| 50 | 0.101 ± 0.015 | 1.39 ± 0.39 |
| 75 | 0.097 ± 0.0087 | 4.32 ± 1.05 |
| cartilage | 0.311 ± 0.062 | 0.42 ± 2.51 |
The Permeability ranged from 1.39 ± 039 × 10−14 to 4.32 ± 1.05 × 10−14 m4/Ns (Table 3). The 75% PLGA construct had a higher permeability when compared to the other constructs (10, 20 and 50% PLGA). However, there were no other inter-group differences. The articular cartilage was an order of magnitude less permeable when compared to all constructs.
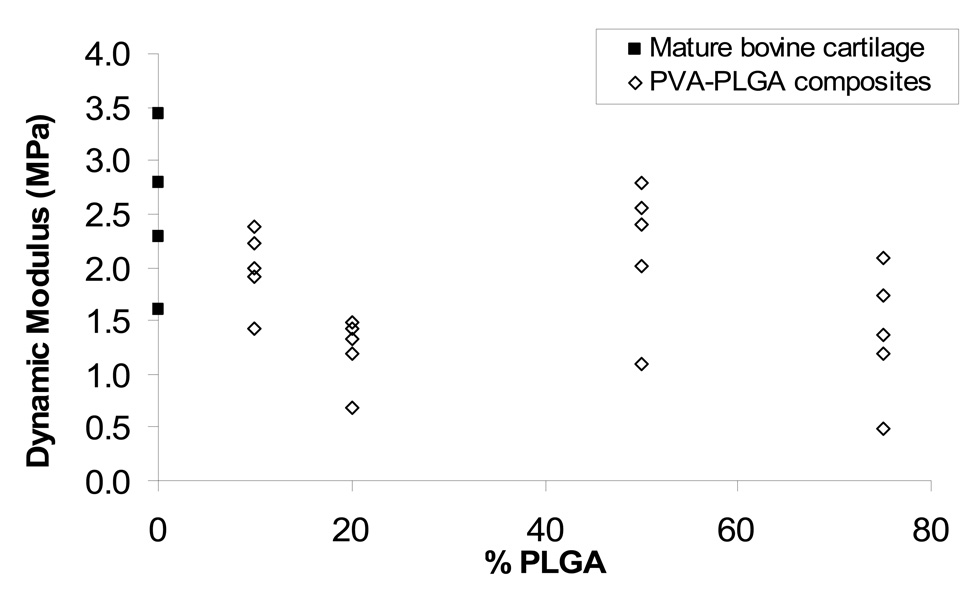
The Dynamic Modulus for 20% PLGA constructs was significantly lower than that or articular cartilage (p=0.03), but all other constructs had Dynamic Modulus values similar to that of articular cartilage (p>0.06). Dynamic Modulus values are presented in Figure 5.
Figure 5.
Dynamic Modulus at 5% strain of mature bovine articular cartilage and PVA-PLGA composites as a function of %PLGA.
Discussion
The challenge of designing a construct for the repair of focal cartilage defects such that it mimics the mechanical properties of and can integrate with native cartilage has not been met by existing technologies. Our concept is to utilize a porous non-degradable construct capable of providing immediate and enduring load carrying ability to the defect site, while at the same time encouraging cellular infiltration from adjacent tissue. The ability of the construct to meet these criteria will depend on its morphology and mechanical properties. Our objective was to characterize the morphological features and mechanical properties of a new class of porous PVA-PLGA constructs as a function of PLGA content.
In choosing materials for the manufacture of a non-degradable porous construct, long-term biocompatibility is a requisite. PLGA is a biodegradable and biocompatible polymer commonly used to encapsulate growth factors for drug delivery (Mercier et al., 2005; Kang et al., 2005; Elisseeff et al., 2001). PVA is a non-degradable biocompatible hydrogel that has been used in several biomedical applications including contact lenses, ophthalmic materials and tendon repair (Corkhill et al., 1990; Noguchi et al., 1991; Chang et al., 1994; Oka et al., 2000; Oka et al., 1997). Non-degradable poly(vinyl alcohol) has long been suggested for the repair of articular cartilage defects (Peppas and Merrill, 1977). Non-porous PVA hydrogel plugs have also been used for the repair of focal cartilage defects in animal models (Noguchi et al., 1991, Oka et al., 1997), but lack of integration with surrounding tissue has been problematic. The concept of using a porous PVA scaffold to encourage integration with articular cartilage is new.
The manipulation of the mechanical properties of non-porous PVA by altering its composition, structure and method of manufacture has been well characterized (Moroni et al, 2006; Woodfield et al. 2004). For example, dehydration has been shown to increase compressive stiffness (Thomas et. al., 2004). Decreasing the water content (Stammen, et al, 2001) and gamma-irradiating the material have also been shown to increase Compressive Modulus (Peppas & Merrill, 1977). Methods to control the mechanical properties of porous PVA have been less characterized. The mode of manufacture of the PVA/PLGA construct herein described is such that PLGA is first dissolved in an organic solvent (DCM) and then added to a PVA solution. As the solutions are emulsified, the DCM evaporates leaving pores. By varying the PLGA content, a corresponding change in DCM content is required and results in changes in the morphology and mechanical properties of the composite.
The percent of PVA in the final construct was less than 10% weight and the remainder was water. As a porous, fluid-filled construct, load is carried via the pressurization of the entrapped fluid within the construct and the solid construct itself. The Aggregate Modulus (Modulus of the solid construct at equilibrium), Dynamic Modulus (Modulus of the construct during a phase of rapid loading) and Permeability of the construct, which are well characterized for articular cartilage, were measured in this study. The strain vs. time data generated from each creep test was fit with the biphasic theory of Mow et al., 1980. A key assumption in this theory is that the solid matrix does not exhibit an intrinsic viscoelastic response. Due to the small percent of solid PVA included in the construct utilization of the biphasic theory to compute the Aggregate Modulus and Permeability of the construct is considered appropriate.
The wide range of articular cartilage mechanical properties reported in literature has been attributed to variability in the species and location from which articular cartilage was harvested (Armstrong and Mow, 1982; Jurvelin et al., 2000), variation in collagen, water and proteoglycan content of the tissue (Appleyard et al., 2003), and to variation in the type of test, rate of loading and % strain to which the sample was loaded (Li et al., 2002; Korhonen et al., 2002). Given the strong dependency of mechanical properties on test conditions and sample type, to enable a comparative assessment of the mechanical properties of the hydrogel scaffolds to that of articular cartilage, all samples were tested under the same loading conditions. Of note, due to the difficulty of obtaining undamaged human articular cartilage samples, bovine articular cartilage was tested in this study. This limits our ability to equate the construct mechanical properties to that of human articular cartilage.
The Dynamic Modulus of the hydrogel constructs measured in this study was within the range of that measured for the mature bovine articular cartilage (2.5 ± 0.8 MPa) and within the larger range of a previously reported compressive modulus of human articular cartilage 1.9 – 14.4 MPa (Stammen et al., 2001). Constructs with PLPGA contents of 10%, 50% and 75% had Dynamic Modulus values similar to that of articular cartilage, suggesting that PLGA content can be chosen to maximize the release of growth factors without significantly affecting the time-zero mechanical properties of the scaffold. It is unclear why 20% PLGA constructs had Dynamic Moduli that were significantly lower than that of articular cartilage; larger sample sizes may clarify this effect.
The Aggregate Modulus was independent of PLGA content, ranging from 0.087 ± 0.008 to 0.102 ± 0.013 MPa. These values are lower than the Ha measured for mature bovine articular cartilage in this study (0.311 ± 0.062 MPa), but within the wider range of 0.01 to 0.8 MPa reported in the literature (Armstrong et al., 1982; Athanasiou et al., 1991; Chen et al., 2001; Mow et al., 1980; Torzilli, 1976; Wang et al., 2001). Variations in PLGA content of less than 75% had little effect on construct permeability; however at 75% PLGA the construct had a significantly higher permeability compared to all other groups. Construct permeability ranged from 1.39 ± 0.39 to 4.32 ± 1.05 × 10−14m4/Ns which is one order of magnitude higher than that measured for mature bovine articular cartilage 0.42 ± 2.51 × 10−14 m4/Ns, but within the range of values reported in the literature 0.01 – 0.5 × 10−14 m4/Ns (Armstrong et al., 1982; Athanasiou et al., 1991; Mow et al., 1980).
The ability of chondrocytes to migrate into, remain viable and synthesize extracellular matrix within a scaffold is influenced by its pore diameter and porosity (Chang et al., 2003; Oh et al., 2003). The pore diameter of the constructs was significantly influenced by PLGA content, where higher PLGA content led to larger pores. Pore diameters in the 50% PLGA (95 ± 82 µm) and 75% PLGA (111 ± 85 µm) groups are within the range of pore diameters used by others in cell seeded constructs. For example, Lee et al. (2006) used scaffolds with pore diameters that ranged from 50 – 250 µm, Oh et al. (2003) used scaffolds with pore sizes in the 200 – 300 µm range, and Chang et al. (2003) used scaffolds with average pore diameters of 180 µm. It has recently been suggested that although large pore diameters may be needed to encourage chondrocyte migration into a scaffold, smaller pores may be better to optimize cell-cell interactions and chondrogenesis (Vikers et al., 2006).
The porosity of our constructs also increased with increasing PLGA content, ranging from 8 ± 3% at 10% PLGA to 54 ± 19% at 75% PLGA. Scaffolds ranging from 75% (Chang et al., 2003) to 90% porosity (Oh et al., 2003) have been successfully seeded with chondrocytes, suggesting that further studies are warranted to determine cell seeding ability in our PVA-PLGA composite.
To encourage construct integration with the surrounding cartilage tissue it is envisaged that the PLGA microspheres will be filled with growth factors that encourage cellular migration into the construct. As PLGA content increased from 10–75%, we demonstrated a four-fold increase in microsphere diameter. Change in sphere diameter will likely influence the kinetics of drug release (DeFail et al., 2006), the control of which should lead to optimized cellular infiltration. Furthermore, it is envisaged that by functionalizing the PVA scaffold walls with adhesion peptides or fibronectin, chondrocytes will be able to adhere to the matrix to further facilitate scaffold-matrix integration (Oh, et al, 2003; Zajaczkowski, et al, 2003; Nuttelmann, et al, 2001).
In conclusion, we developed a porous, semi-degradable biocompatible PVA-PLGA construct with variable porosity and pore diameter and mechanical properties similar to that of articular cartilage. Varying the PLGA content was found to have a profound effect on the morphological features of the constructs. As PLGA content increased from 10 to 75%, constructs exhibited a six-fold increase in average percent porosity, a four-fold increase in microsphere diameter and a four-fold increase in pore diameter. The effect of the PLGA content on the Aggregate Modulus, Permeability was and Dynamic Modulus was less profound. This suggests that that the morphology of the construct can be tailored to optimize cellular infiltration and the release of growth factors, without detrimentally affecting scaffold mechanical properties.
Acknowledgments
This project was funded by the MacArthur, Kirby, and Beinecke Foundations and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, NIH. The authors would like to acknowledge support from NIH AR046121. We would also like to thank Kathya Carrillo for her assistance with mechanical testing and Dr. Steven Doty and Tony Labissiere for assistance with SEM.
Footnotes
The experiments herein presented were conducted at Hospital for Special Surgery.
References
- 1.Appleyard RC, Burkhardt D, Ghosh P, Read R, Cake M, Swain MV, Murrell GA. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthritis Cartilage. 2003 Jan;11(1):65–77. doi: 10.1053/joca.2002.0867. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong C, Mow V. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration and water content. Journal of Bone and Joint Surgery. 1982;64A:88–94. [PubMed] [Google Scholar]
- 3.Athanasiou K, Rosenwasser M, Buckwalter J, Malinin T, Mow V. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. Journal of Orthopaedic Research. 1991;9:330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 4.Bartz RL, Kamaric E, Noble PC, Lintner D, Bocell J. Topographic matching of selected donor and recipient sites for osteochondral autografting of the articular surface of the femoral condyles. Am. J. Sports Med. 2001;29(2):207–212. doi: 10.1177/03635465010290021501. [DOI] [PubMed] [Google Scholar]
- 5.Chang CH, Liu HC, Lin CC, Chou CH, Lin FH. Gelatin-chondroitin-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials. 2003;24(26):4853–4858. doi: 10.1016/s0142-9612(03)00383-1. [DOI] [PubMed] [Google Scholar]
- 6.Chang YS, Oka M, Kobayashi M, Gu HO, Li ZL, Kitsugi T, Nakamura T. Bone formation and remodeling around implanted materials under load-bearing conditions. Clin Mater. 1994;17(4):181–187. doi: 10.1016/0267-6605(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. Journal of Biomechanics. 2001;34(1):1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 8.Corkhill PH, Trevett AS, Tighe BJ. The Potential of Hydrogels as Synthetic Articular Cartilage. J. Eng. Medicine: Proc. Inst. Mech. Eng. 1990;204(3):147–155. doi: 10.1243/PIME_PROC_1990_204_249_02. [DOI] [PubMed] [Google Scholar]
- 9.DeFail AJ, Chu CR, Izzo N, Marra KG. Controlled release of bioactive TGF-beta 1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27(8):1579–1585. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19(6):1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 11.Elisseeff JH, Lee A, Kleinman HK, Yamada Y. Biological response of chondrocytes to hydrogels. Ann New York Academy of Science. 2002;961:118–122. doi: 10.1111/j.1749-6632.2002.tb03062.x. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick PL, Morgan DA. Fresh osteochondral allografts: a 6–10-year review. Australian and New Zealand Journal of Surgery. 1998;68(8):573–579. doi: 10.1111/j.1445-2197.1998.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 13.Gillogly SD, Myers TH. Treatment of full-thickness chondral defects with autologous chondrocyte implantation. Ortho. Clin. North Amer. 2005;36(4):433–446. doi: 10.1016/j.ocl.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Gillogly SD, Voight M, Blackburn T. Treatment of Articular Cartilage Defects of the Knee with Autologous Chondrocyte Implantation. J. Orthop. Sports Phys. Therapy. 1998;28(4):241–251. doi: 10.2519/jospt.1998.28.4.241. [DOI] [PubMed] [Google Scholar]
- 15.Giurea A, DiMicco MA, Akeson WH, Sah RL. Development-associated differences in integrative cartilage repair: roles of biosynthesis and matrix. J Orthop Res. 2002;20(6):1274–1281. doi: 10.1016/S0736-0266(02)00084-0. [DOI] [PubMed] [Google Scholar]
- 16.Griffon DJ, Seddighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomaterials. 2006;2(3):313–320. doi: 10.1016/j.actbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surgery Sports Traumatol Arthrosc. 2006 September;14(9):834–842. doi: 10.1007/s00167-006-0067-0. [DOI] [PubMed] [Google Scholar]
- 18.Horas U, Schnettler R, Pelinkovic D, Herr G, Aigner T. Osteochondral transplantation versus autogenous chondrocyte transplantation. A prospective comparative clinical study. Chirurg. 2000;71(9):1090–1097. doi: 10.1007/s001040051184. [DOI] [PubMed] [Google Scholar]
- 19.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004 January;32(1):35–49. doi: 10.1023/b:abme.0000007789.99565.42. Review. Erratum in: Ann Biomed Eng. 32 (3), 510, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage regeneration: The impact in the new Millennium. Clinical Orthopaedics. 2001;391 Supplement:S14–S25. [PubMed] [Google Scholar]
- 21.Jurvelin JS, Arokoski JP, Hunziker EB, Helminen HJ. Topographical variation of the elastic properties of articular cartilage in the canine knee. Journal of Biomechanics. 2000;33(6):669–675. doi: 10.1016/s0021-9290(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 22.Kang SW, Jeon O, Kim BS. Poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for cartilage tissue engineering. Tissue Eng. 2005 March–April;11(3–4):438–447. doi: 10.1089/ten.2005.11.438. [DOI] [PubMed] [Google Scholar]
- 23.Korhonen RK, Laasanen MS, Toyras J, Rieppo J, Hirvonen J, Helminen HJ, Jurvelin JS. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002 Jul;35(7):903–909. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 24.Lane JG, Tontz WL, Jr, Ball ST, Massie JB, Chen AC, Bae WC, Amiel ME, Sah RL, Amiel D. A morphologic, biochemical, and biomechanical assessment of short-term effects of osteochondral autograft plug transfer in an animal model. Arthroscopy. 2001;17(8):856–863. doi: 10.1016/s0749-8063(01)90010-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee CT, Huang CP, Lee YD. Biomimetic porous scaffolds made from Poly(L-lactide)-g-chondroitin sulfate blend with Poly(L-lactide) for cartilage tissue engineering. Biomacromolecules. 2006;7(7):2200–2209. doi: 10.1021/bm060451x. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Buschmann MD, Shirazi-Adl A. The role of fibril reinforcement in the mechanical behavior of cartilage. Biorheology. 2002;39(1–2):89–96. [PubMed] [Google Scholar]
- 27.Li Q, Williams CG, Sun DD, Wang J, Leong K, Elisseeff JH. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68(1):28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 28.Mercier NR, Costantino HR, Tracy MA, Bonassar LJ. Poly(lactide-co-glycolide) microspheres as a moldable scaffold for cartilage tissue engineering. Biomaterials. 2005;26(14):1945–1952. doi: 10.1016/j.biomaterials.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 30.Moroni L, de Wijn JR, van Blitterswijk CA. 3D Fiber-deposited Scaffolds for Tissue Engineering: Influence of Pores Geometry on Dynamic Mechanical Properties. Biomaterials. 2006;27(7):974–985. doi: 10.1016/j.biomaterials.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 31.Mow V, Kuei S, Lai W, Armstrong C. “Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments.”. Journal of Biomechanical Engineering. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi T, Yamamuro T, Oka M, Kumar P, Kotoura Y, Hyon S-H, Ikada Y. Poly(vinyl Alcohol) hydorgel as an artificial cartilage: evaluation of biocompatibility. Journal of Applied Biomaterials. 1991;2:101–107. doi: 10.1002/jab.770020205. [DOI] [PubMed] [Google Scholar]
- 33.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J. Orthoep. Res. 2001;19(6):1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 34.Oh SH, Kang SG, Kim ES, Cho SH, Lee JH. Fabrication and Characterization of Hydrophilic Poly(lactic-co-glycolic acid) / Poly(vinyl alcohol) Blend Cell Scaffolds by Melt-Molding Particulate-Leaching Method. Biomaterials. 2003;24:4011–4021. doi: 10.1016/s0142-9612(03)00284-9. [DOI] [PubMed] [Google Scholar]
- 35.Oka M, Chang Y-S, Nakamura T, Ushio K, Toguchi J, Gu H-O. Synthetic Osteochondral Replacement of the Femoral Articular Cartilage. Journal of Bone and Joint Surgery. 1997;79-B(6):1003–1007. doi: 10.1302/0301-620x.79b6.7606. [DOI] [PubMed] [Google Scholar]
- 36.Oka M, Ushio K, Kumar P, Ikeuchi K, Hyon SH, Nakamura T, Fujita H. Development of artificial articular cartilage. Proceedings of the Institute of Mechanical Engineers. 2000;214(H):59–68. doi: 10.1243/0954411001535246. [DOI] [PubMed] [Google Scholar]
- 37.Peppas NA, Merrill EW. Development of Semicrystalline Poly(vinyl alcohol) Hydrogels for Biomedical Applications. J. Biomed. Mater. Res. 1977;11:423–434. doi: 10.1002/jbm.820110309. [DOI] [PubMed] [Google Scholar]
- 38.Peretti GM, Xu JW, Bonassar LJ, Kirchhoff CH, Yaremchuk MJ, Randolph MA. Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Eng. 2006;12(5):1151–1168. doi: 10.1089/ten.2006.12.1151. [DOI] [PubMed] [Google Scholar]
- 39.Ramaswamy S, Wang DA, Fishbein KW, Elisseeff JH, Spencer RG. An analysis of the integration between articular cartilage and nondegradable hydrogel using magnetic resonance imaging. J. Biomed Mater Res B Appl Biomater. 2006;77(1):144–148. doi: 10.1002/jbm.b.30404. [DOI] [PubMed] [Google Scholar]
- 40.Sanders TG, Mentzer KD, Miller MD, Morrison WB, Campbell SE, Penrod BJ. Autogenous osteochondral "plug" transfer for the treatment of focal chondral defects: postoperative MR appearance with clinical correlation. Skeletal Radiology. 2001;30(10):570–578. doi: 10.1007/s002560100371. [DOI] [PubMed] [Google Scholar]
- 41.Sechriest VF, Miao YJ, Niyibizi C, Westerhausen-Larson A, Matthew HW, Evans CH, Fu FH, Suh JK. GAG-Augmented Polysaccharide Hydrogel: A Novel Biocompatible and Biodegradable Material to Support Chondrogenesis. J. Biomed. Mater. Res. 2000;49(4):534–541. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Stammen JA, Williams S, Ku DN, Guldberg RE. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials. 2001;22(8):799–806. doi: 10.1016/s0142-9612(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 43.Thomas J, Gomes K, Lowman A, Marcolongo M. The Effect of Dehydration History on PVA/PVP Hydrogels for Nucleus Pulposus Replacement. J Biomed Mater Res B Appl Biomater. 2004;69(2):135–140. doi: 10.1002/jbm.b.20023. [DOI] [PubMed] [Google Scholar]
- 44.Torzilli P. “Measurement of the compressive properties of thin cartilage slices: evaluating tissue inhomogeneity.”. In: Maroudas AK, K, editors. Cartilage Methods. New York: Academic Press; 1990. pp. 304–308. [Google Scholar]
- 45.Torzilli P. “The lubrication of human joints: a review.”. In: Fleming DF, BN, editors. Handbook of Engineering in Medicine and Biology. Ohio: CRC Press; 1976. pp. 225–251. [Google Scholar]
- 46.Ushio K, Oka M, Hyon SH, Hayami T, Yura S, Matsumura K, Toguchida J, Nakamura T. Attachment of Artificial Cartilage to Underlying Bone. Biomed. Mater. Res. Part B: Appl. Biomaterials. 2004;68B:59–68. doi: 10.1002/jbm.b.10076. [DOI] [PubMed] [Google Scholar]
- 47.Wang CC, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. Journal of Biomechanics. 2001;34(1):75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 48.Wood JJ, Malek MA, Frassica FJ, Polder JA, Mohan AK, Bloom ET, Braun MM, Cote TR. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Association. J Bone Joint Surg Am. 2006;88(3):503–507. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]
- 49.Woodfield TBF, Malda J, de Wijn J, Peters F, Resle J, van Blitterswijk CA. Design of Porous Scaffolds for Cartilage Tissue Engineering using a Three-Dimensional Fiber-Deposition Technique. Biomaterials. 2004;25:4149–4161. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 50.Yasuda A, Kojima K, Tinsley KW, Yoshioka H, Mori Y, Vacanti CA. In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors. Tissue Eng. 2006 May;12(5):1237–1245. doi: 10.1089/ten.2006.12.1237. [DOI] [PubMed] [Google Scholar]