Abstract
Metachromatic leukodystrophy is a lysosomal storage disorder with an estimated incidence of 1:40,000. Magnetic resonance imaging at time of diagnosis often shows symmetric white matter involvement sparing the arcuate fibers. A 25 month old female presented with a cranial neuropathy, a spastic gait, decreased leukocyte arylsulfatase-A activity and elevated urinary sulfatides. Magnetic resonance imaging revealed multiple cranial nerve enhancement, without intraparenchymal white matter involvement.
INTRODUCTION
Metachromatic leukodystrophy (MLD) is an autosomal recessive neurodegenerative disorder characterized by deficient activity of the enzyme arylsulfatase-A. The responsible gene is ARSA located on chromosome 22q13.31. Arylsulfatase-A catalyzes the hydrolysis of sulfatide, the sulfate ester of cerebroside. Sulfatide is primarily present in the membranes of myelin-producing cells and in myelin. Decreased activity of arylsulfatase-A results in the inability to breakdown and utilize myelin in the central and peripheral nervous systems. The accumulation of the metachromatic lipid material, galactosylceramide sulfatide, impairs the function of macrophages and Schwann cells and is thought to result in myelin destruction [1].
The disease encompasses three clinical subtypes: late infantile (40% of the patients with MLD), juvenile (40%), and adult (20%). In the infantile (onset 18–24 months) and juvenile (onset 4–10 years) variants, patients develop a progressive spastic ataxia, cranial nerve abnormalities and bulbar dysfunction. Peripheral neuropathy may be present even in the early stages of the disease and the conduction velocity of peripheral nerves is markedly reduced on electrophysiologic testing [1,2,3]. The typical radiographic appearance of MLD is that of radial “stripes” and round foci in the white matter, reminiscent of the skin pattern of tigers and leopards. The pattern is best seen as hypointense on T2-weighted images on the background of abnormally T2 bright white matter. The subcortical u-shaped white matter fibers are typically preserved (dark on T2). Neuropathologic correlation has shown that the stripes represent myelin near venules that is relatively spared by the disease process, as well as lipid-containing cells. Although this is a very sensitive imaging finding, this pattern can also be found in other lysosomal storage disorders, such as globoid cell dystrophy and infantile GM1 gangliosidosis (GM1), and is thus not specific for MLD [4].
CASE REPORT
The patient is a 25 month old Caucasian female who presented with a 2 month history of a progressively unsteady gait and 8 month history of right esotropia. She had been diagnosed and followed by ophthalmology for a right esotropia and right amblyopia, and came to the attention of a neurologist when gait abnormalities were noted. She was born to a G3P3 mother via spontaneous vaginal delivery with jaundice in the perinatal period. Of note were some delays in gross motor skills: she sat up at 9 months and walked at 15 months. Social and language development were within normal limits. At age two she was able to put two words together with a total vocabulary of 50 words. Her family history was positive for a maternal step-aunt (who shared the same father as the patient’s mother) who died at age 5 from metachromatic leukodystrophy. There was no family history of consanguinity.
On physical examination, her head circumference was 47.75 cm (50th percentile). Cranial nerve exam revealed a right lateral rectus palsy with limited abduction past the midline, and fine nystagmoid movements on visual pursuit bilaterally. No optic atrophy was visualized. Facial weakness was noted bilaterally with effacement of the nasolabial folds and decreased strength of eye closure on the right. Deep tendon reflexes were trace in the upper extremities, present in the patellar and absent at the Achilles bilaterally. There was dysmetria in reaching for objects bilaterally. Tone of the lower extremities was increased with no clonus. Gait was wide based and spastic-ataxic with toe walking.
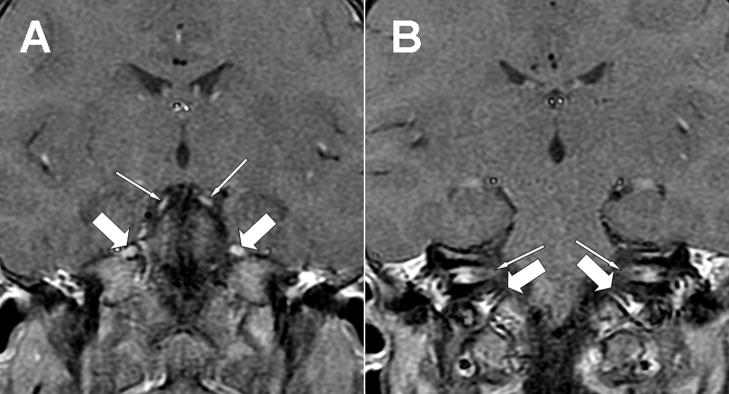
A diagnostic evaluation was performed. An extensive infectious and inflammatory workup of the blood and CSF was negative, except for an elevated CSF protein of 80 mg/dL (reference range 15–45 mg/dL). Nerve conduction studies revealed a prolongation of the left median and tibial distal motor latencies, along with minimally prolonged left medial plantar and left median sensory latencies, suggesting a demyelinating sensorimotor polyneuropathy. Magnetic resonance imaging revealed enhancement bilaterally of cranial nerves III, V, VII, VIII and IX (Fig. 1). Cortical white matter was considered normal (Fig. 2). Leukocyte testing for lysosomal enzyme activities revealed decreased arylsulfatase- A levels, with a specific activity of 8.9 nmol/mg protein per hour (normal values ≥ 70). Confirmatory testing revealed elevated urine sulfatides, present on TLC (thin layer chromatography) plates.
Figure 1.
Two consecutive postcontrast coronal T1 magnetic resonance images (GE, 1.5 T, TR 516, TE 10) of the brain, A and B. Note the bright signal representing contrast enhancement of oculomotor (A, thin arrows) and trigeminal (A, thick arrows) nerves, as well as bilateral facial and vestibulocochlear nerve enhancement in the internal auditory canals (B, thin arrows) and cranial nerve enhancement in the jugular foramen (B, thick arrows).
Figure 2.
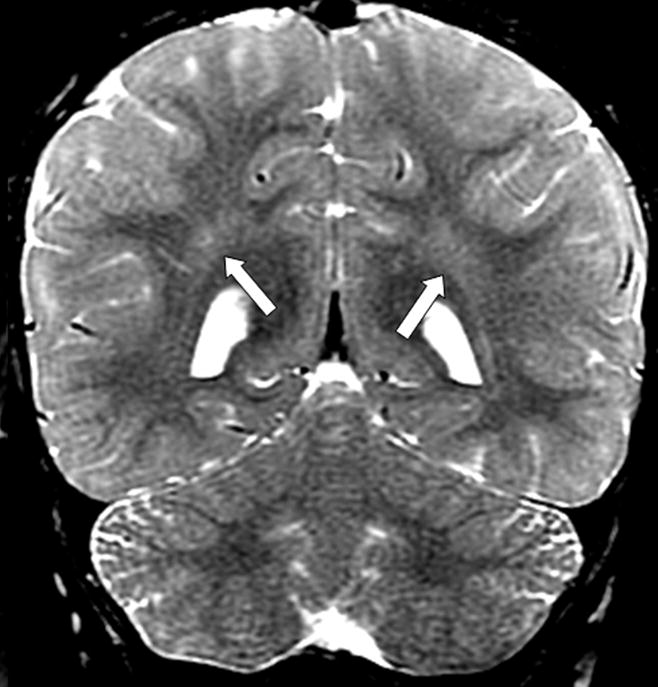
Coronal T2 weighted images (GE, 1.5T, TR 3800, TE 105) demonstrating normal dark signal of myelinated white matter, shown in the parietal lobes. Note symmetrical stripes of bright signal in the deep parietal white matter; these are frequently seen in normal patients, most of the time in conjunction with prominent perivascular (Virchow-Robin) spaces.
Since diagnosis, the patient’s clinical course has continued to deteriorate. Her lower extremity spasticity has progressed with the development of tight heel cord contractures, requiring botulinum toxin injections, along with swallowing and choking difficulties requiring the placement of a gastrostomy tube for enteral feeding.
DISCUSSION
This report describes a 2 year old female who presented with cranial neuropathy and a progressively spastic-ataxic gait. Lower extremity nerve conduction velocities were decreased, characteristic of a peripheral neuropathy. A diagnosis of MLD was suggested by the family history and clinical presentation. Neuroimaging abnormalities in MLD are well characterized [5,6], and consist of confluent periventricular white matter abnormalities sparing the arcuate fibers. Abnormal white matter can have a pattern of radiating stripes. In later stages, pyramidal tract involvement may be seen in the brainstem. Cerebellar involvement is variable. A posterior predominance has been described in early-onset MLD cases [4]. However, typically white matter changes of MLD are first seen in the corpus callosum before affecting the periventricular/deep white matter [1,4]. In this patient the corpus callosum was normal and it was thought that the white matter changes were unlikely related to MLD. Thus, MR imaging did not reveal any intraparenchymal white matter abnormalities, but instead symmetrical enhancement of multiple cranial nerves.
Cranial nerve enhancement may be present in a variety of demyelinating, neoplastic, or autoimmune diseases [7]. Cranial nerve enhancement has recently been described in metachromatic leukodystrophy, but in conjunction with cortical white matter involvement [8]. It has also been demonstrated, with cortical white matter abnormalities, in Krabbe’s disease [9]. It has been proposed that cranial nerve enhancement may be due to disruption of the myelin sheath and accumulation of the metachromatic lipid material, affecting the cranial nerves in a manner similar to the peripheral nerves [7,8]. Isolated peripheral nerve enhancement on MRI early in the course of the disease has been seen in Krabbe’s [10] and striking peripheral nerve enhancement is also known to occur in MLD [11,12]. In this patient, the eye findings were due to cranial nerve involvement, and the upper motor signs of the leg suggested subcortical involvement, although not evident on imaging. This is the first case to our knowledge describing cranial nerve enhancement without any intraparenchymal involvement, at the time of diagnosis of metachromatic leukodystrophy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Knaap MS. Metachromatic Leukodystrophy. In: van der Knaap MS, Valk J, editors. Magnetic Resonance of Myelination and Myelin Disorders. 3. Berlin: Springer; 2005. pp. 74–81. [Google Scholar]
- 2.Martinez AC, Ferrer MT, Fueyo E, Galdos L. Peripheral neuropathy detected on electrophysiological study as first manifestation of metachromatic leucodystrophy in infancy. J Neurol Neurosurg Psychiatry. 1975;38:169–74. doi: 10.1136/jnnp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fullerton PM. Peripheral Nerve Conduction in Metachromatic Leukodystrophy (Sulphatide Lipidosis) J Neurol Neurosurg Psychiatry. 1964;27:100–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Voorn JP, Pouwels PJ, Kamphorst W, Powers JM, Lammens M, Barkhof F, van der Knaap MS. Histopathologic correlates of radial stripes on MR images in lysosomal storage disorders. Am J Neuroradiol. 2005;26:442–46. [PMC free article] [PubMed] [Google Scholar]
- 5.Cheon JE, Kim IO, Hwang YS, Kim KJ, Wang KC, Cho BK, Chi JG, Kim CJ, Kim WS, Yeon KM. Leukodystrophy in children: a pictorial review of MR imaging features. Radiographics. 2002;22:461–76. doi: 10.1148/radiographics.22.3.g02ma01461. [DOI] [PubMed] [Google Scholar]
- 6.Faerber EN, Melvin J, Smergel EM. MRI appearances of metachromatic leukodystrophy. Pediatr Radiol. 1999;29:669–72. doi: 10.1007/s002470050672. [DOI] [PubMed] [Google Scholar]
- 7.Saremi F, Helmy M, Farzin S, Zee CS, Go JL. MRI of cranial nerve enhancement. Am J Roentgenol. 2005;185:1487–97. doi: 10.2214/AJR.04.1518. [DOI] [PubMed] [Google Scholar]
- 8.Maia AC, Jr, da Rocha AJ, da Silva CJ, Rosemberg S. Multiple cranial nerve enhancement: a new MR imaging finding in metachromatic leukodystrophy. Am J Neuroradiol. 2007;28:999. doi: 10.3174/ajnr.A0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal OG, Lenn N. Multiple cranial nerve enhancement in early infantile Krabbe’s disease. Neurology. 2000;54:2348–9. doi: 10.1212/wnl.54.12.2348. [DOI] [PubMed] [Google Scholar]
- 10.Vasconcellos E, Smith M. MRI nerve root enhancement in Krabbe disease. Pediatr Neurol. 1998;19:151–2. doi: 10.1016/s0887-8994(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 11.Toldo I, Carollo C, Batistella PA, Laverda AM. Spinal cord and cauda equina MRI findings in metachromatic leukodystrophy: case report. Neuroradiology. 2005;47:572–5. doi: 10.1007/s00234-005-1369-5. [DOI] [PubMed] [Google Scholar]
- 12.Coulter-Mackie MB, Applegarth DA, Toone JR, Gagnier L, Anzarut AR, Hendson G. Isolated peripheral neuropathy in atypical metachromatic leukodystrophy: a recurrent mutation. Can J Neurol Sci. 2002;29:159–63. [PubMed] [Google Scholar]