Abstract
Dopamine (DA) acts through five receptor subtypes (D1–D5). We compared expression levels and distribution patterns of all DA mRNA receptors in the spinal cord of wild-type (WT) and loss of function D3 receptor knockout (D3KO) animals. D3 mRNA expression was increased in D3KO, but no D3 receptor protein was associated with cell membranes, supporting the previously reported lack of function. In contrast, mRNA expression levels and distribution patterns of D1, D2, D4, and D5 receptors were similar between WT and D3KO animals. We conclude that D3KO spinal neurons do not compensate for the loss of function of the D3 receptor with changes in the other DA receptor subtypes. This supports use of D3KO animals as a model to provide insight into D3 receptor dysfunction in the spinal cord.
Keywords: Monoamines, Real-time PCR, In situ hybridization, Restless Legs Syndrome, Dopamine
Introduction
In genetically engineered animals in which the activity of a single gene has been removed (“knocked-out”), adaptive compensatory mechanisms may occur that can lead to surprising behavioral changes (Pich and Epping-Jordan 1998; Drago et al. 2003). The unpredictability of such complex changes resulting from the deletion of a single gene function underlines the growing need for understanding physiological functions in knockout animals (Branchi and Ricceri 2002). While a large body of literature has examined the physiological consequences in knockouts (Drago et al. 1998; Hollon et al. 2002; Karper et al. 2002; McDougall et al. 2005; Leggio et al. 2008), few studies assess the potential compensatory consequences of gene deletions in related genes or gene pathways at their expression level (Goody et al. 2002; Hannon et al. 2002), or that map the consequences of such gene deletions at the tissue level (Tien et al. 2003; Gan et al. 2004). There is evidence that in D2 receptor knockout animals D3 receptors compensate for some of the lacking D2 function (Jung et al. 1999). Using real-time polymerase chain reaction (PCR) and in situ hybridization (ISH) techniques we have recently demonstrated the expression levels and distribution patterns of all five DA receptors in the lumbar spinal cord of the 14-day-old mouse (Zhu et al. 2007). Here, we determine whether a disruption of the D3 receptor system in the loss of D3 receptor function knockout (D3KO) mouse (Accili et al. 1996) leads to similar compensatory changes gene expression or distribution patterns of the other four dopamine (DA) receptor subtypes.
The catecholaminergic neurotransmitter DA acts through five molecularly distinct receptors generally classified as D1-like (D1 and D5) and D2-like (D2, D3, and D4). A large body of literature has detailed the diverse functions of these receptor subtypes (Joyce 1983; Jackson and Westlind-Danielsson 1994; Jaber et al. 1996; Missale et al. 1998), often using knockouts for each specific receptor subtype (Karasinska et al. 2000; Park et al. 2001; Hollon et al. 2002; Centonze et al. 2003; Kobayashi et al. 2004; Tran et al. 2005). In the D3KO mouse, spinal cord function is modulated differently by DA, when compared to its JAX-recommended wild-type (WT) control, C57BL/6J (http://jaxmice.jax.org/strain/002958.html), suggesting a role for the D3 receptor in limiting spinal cord excitability (Clemens and Hochman 2004). Behaviorally, the D3KO mouse displays hyperactivity, increased locomotor activity, and hypertension (Accili et al. 1996; Asico et al. 1998). There is evidence that the D3 receptor system may also be involved in the regulation of anxiety (Steiner et al. 1998), mood disorders (Leggio et al. 2008) and the sleep disorder Restless Legs Syndrome (RLS) (Clemens et al. 2006; Zhao et al. 2007). While the disruption of the single D3 gene can lead to complex and wide-ranging changes in behavior, there is still a lack of understanding if these behavioral changes are accompanied by corresponding changes in expression levels and the distribution patterns of the other functionally intact DA receptors in the CNS (Jung et al. 1999). Here we explore this issue in the spinal cord of D3KO animals.
Our data in spinal cord show that the mRNA expression levels and distribution patterns of the other four DA receptors (D1, D2, D4, and D5) were unaltered in D3KO from WT control. Thus, at least in the spinal cord, the expression of other DA receptors does not appear to be altered following the loss of D3 receptor function.
Materials and methods
Animals and slide preparations
A total of 20 D3KO mice (B6.129S4-Drd3/J, Jackson Laboratory, Bar Harbor, ME, USA), postnatal day 14 and approximate WT controls (C57BL/6J) (n = 17) were used in this study. For the quantification of the D3 receptor subtype levels in the spinal cord, three WT and three D3KO animals were anesthetized with i.p. injection of urethane (2 mg/kg body mass) and decapitated. The complete spinal cords were quickly dissected out and stored in RNA later (Qiagen, Valencia, CA, USA) until use, when they were extracted to process for real-time PCR (Zhu et al. 2007). For the ISH experiments, animals (WT: n = 5; D3KO: n = 4) were anesthetized and perfused with 4% paraformaldehyde, 0.1 M PO3, pH 7.4, and cryoprotected in 10% sucrose, 0.1 M PO3 (RNAse free) for 1 h. Whole cords were dissected out and stored at −80°C in OCT compound (Sakura Finetek, Torrance, CA, USA) for later sectioning. For immunohistochemistry, animals (WT: n = 4; D3KO: n = 3) were anesthetized, perfused with 1:3 volume/body weight ice-cold 0.9% NaCl, 0.1% NaNO2, 1 unit/ml heparin, followed by equal volume/body weight of modified Lana’s fixative (4% paraformaldehyde, 0.2% picric acid, 0.16 M PO3, pH 6.9). Spinal cords were removed and cryoprotected in 10% sucrose containing 0.1 M PO3 until sectioning.
Quantitative real-time RT-PCR
All protocols have been described in detail previously (Zhu et al. 2007). In short, total RNA was extracted from these cords by using Qiagen RNeasy Mini kits (Qiagen, Valencia, CA, USA) and cDNA was synthesized by using the High Capacity cDNA Archive Kit (Applied Biosystems, Bedford, MA, USA), allowing us to compare the levels of gene expression relative to the housekeeping gene glyceraldehyde 3-phosphated dehydrogenase (GAPDH) (Law et al. 1999), using the same primers as previously (Zhu et al. 2007). To normalize the amount of total mRNA present in each reaction, levels of the GAPDH in WT and D3KO animals were monitored in parallel samples. Results are expressed as relative levels of mRNA, normalized to GAPDH expression (Livak and Schmittgen 2001).
Non-radiographic in situ hybridization
Total RNA was extracted from the mouse spinal cord by using Qiagen RNeasy Mini kits (Qiagen, Valencia, CA, USA). Five microgram of total RNA was subjected to cDNA synthesis with oligo-dT15 primer and Superscript II Reverse transcriptase (Invitrogen, Carlsbad, CA, USA) for 1 h at 42°C. The reverse transcriptase was inactivated, and RNA was degraded by heating at 95°C for 5 min. Of the 20 µl of cDNA obtained from the synthesis reaction 5 µl were directly added to the PCR reaction using a PCR Mastermix kit (Eppendorf, Hamburg, Germany) containing 1 µM gene-specific primers. The primers used in this study were designed by the Invitrogen-OligoPerfect™ Designer program (Invitrogen, Carlsbad, CA, USA). We then determined the five DA receptor probe sequences and performed the PCR reactions and ISH protocol as described previously (Zhu et al. 2007). In most cases, as in our previous study, we stopped the color development reaction for each receptor subtype when a just visible reaction product was observed in the sense probe of the sense/antisense probe pairs. However in two animals for each strain we also time-matched the color development reaction of the D3 receptor between WT and D3KO animals (set at 8 h), to allow for a comparison of the signal intensities.
Immunohistochemistry
Spinal cords were isolated and post-fixed 1 h in modified Lana’s fixative, cryoprotected in 10% sucrose, 0.1 M PO3, pH 7.4 until sectioning in 10-µm thick slices on a cryostat (Leitz 1720). All incubations were performed in 0.1 M PO3 buffered saline containing 0.3% Triton X-100 (PBS-T) with 1% donkey serum and washes were performed without serum. Tissue was washed overnight in PBS-T at 4°C followed by incubation in rabbit anti-D3 receptor (Chemicon Inc.; diluted 1/250) for 48–72 h. Tissue was washed at room temperature 3× 30 min, followed by incubation in biotinylated donkey anti-rabbit (Jackson Immunoresearch; diluted 1/250) for 1.5 h. Slides were washed 3× 30 min and incubated in Cy3 conjugated extravidin (Sigma, diluted 1:1,000) for 1.5 h. Subsequently, slides were washed 20 min in PBS-T followed by 2× 20 min in 50 mM Tris–HCl, pH 7.4 and coverslipped with vectashield.
Data and image analysis
For the real-time PCR analysis, all values are given as mean ± SE. We used SigmaPlot and SigmaStat (Systat Software Inc., San Jose, CA, USA) to analyze the data and test for significant differences between WT and D3KO receptor subtypes, ANOVA and paired t-test procedures were applied as appropriate. Differences were considered significant if P < 0.05.
For ISH, images were digitized with Nikon ACT-1 software, using identical settings for both sense and antisense ISH probes and for the immunohistochemical study respectively, before being converted into gray scale images. Image editing and montages were performed subsequently using CorelDraw software (Corel, Ottawa, ON, Canada).
For immunohistochemistry, slides were visualized on a confocal microscope (Zeiss LSM 510) under identical settings and images were transformed using Zeiss LSM (Carl Zeiss Inc., Thornwood, NY, USA) and CorelDraw software.
Results
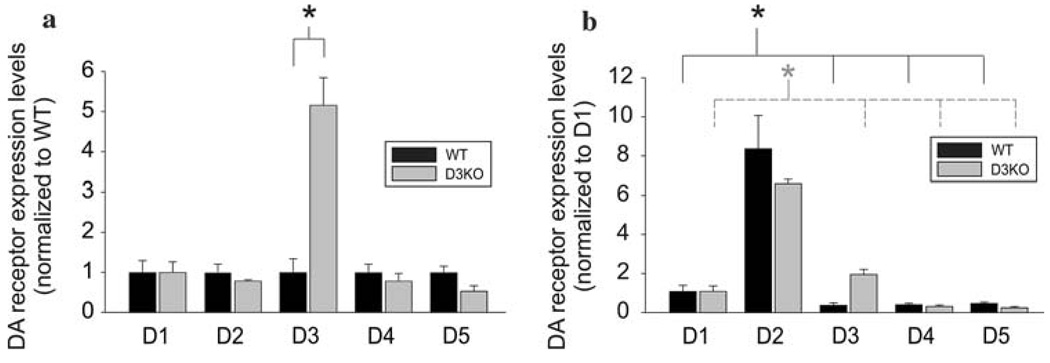
We first performed real-time PCR to assess the expression levels of DA receptor mRNAs in the cord and to compare these levels between WT and D3KO animals (n = 3 for each). Figure 1 shows the expression values of all five DA receptor mRNAs in the whole spinal cord normalized to WT (Fig. 1a) and D1 expression (Fig. 1b). In both WT and D3KO animals all five receptor mRNAs are expressed, and we observed a strong increase in D3 receptor mRNA in the D3KO animals (P = 0.005, Fig. 1a). Importantly however, we did not find any significant differences in receptor mRNA expression between WT and D3KO for D1, D2, D4, and D5 receptor subtypes (Fig. 1a). This suggests that the system-wide dysfunction of the D3 receptor in the D3KO animal does not lead to compensation in mRNA expression levels of the other four DA receptor subtypes in the spinal cord. We further found that, as in WT animals (Zhu et al. 2007), D2 mRNAs in the D3KOs were expressed at significantly higher values than the other DA receptors (P < 0.002, Fig. 1b). Thus the D2 receptor appears to be the dominant receptor subtype expressed in spinal cord neurons of both WT and D3KO animals.
Fig. 1.
Quantitative real-time PCR of dopamine (DA) receptor mRNA expression in wild-type (WT) (black bars) and D3 receptor knockout animals (D3KO, n = 3 each) (gray bars). a Comparison of expression levels between WT and D3KO normalized to WT expression. There was no significant difference between WT and D3KO for D1, D2, D4, and D5 receptor subtypes (D1: P = 0.99; D2: P = 0.37; D4: P = 0.48; D5: P = 0.09). In contrast, D3 receptor mRNA expression was significantly enhanced in D3KO (P = 0.005). Values are expressed as mean ± SE. b Expression levels are normalized to D1 expression levels of WT and D3KO, respectively. In both WT and D3KO, D2 receptor mRNA expression was significantly greater than all other DA receptor expression values respectively (P < 0.005 for both WT and D3KO comparisons)
To assess the distribution of the five DA receptors in the lumbar spinal cord between WT and D3KO, we next initiated a comparative ISH study of D3 receptor expression in the spinal cord. Antisense and sense receptor probes were generated as described earlier (Zhu et al. 2007), but only antisense data are illustrated here. To compare labeling intensities of the D3 receptor mRNA between WT and D3KO animals, we time-matched the color reactions in this study (cf. Sect. ”Materials and methods,” n = 2 for WT and D3KO each).
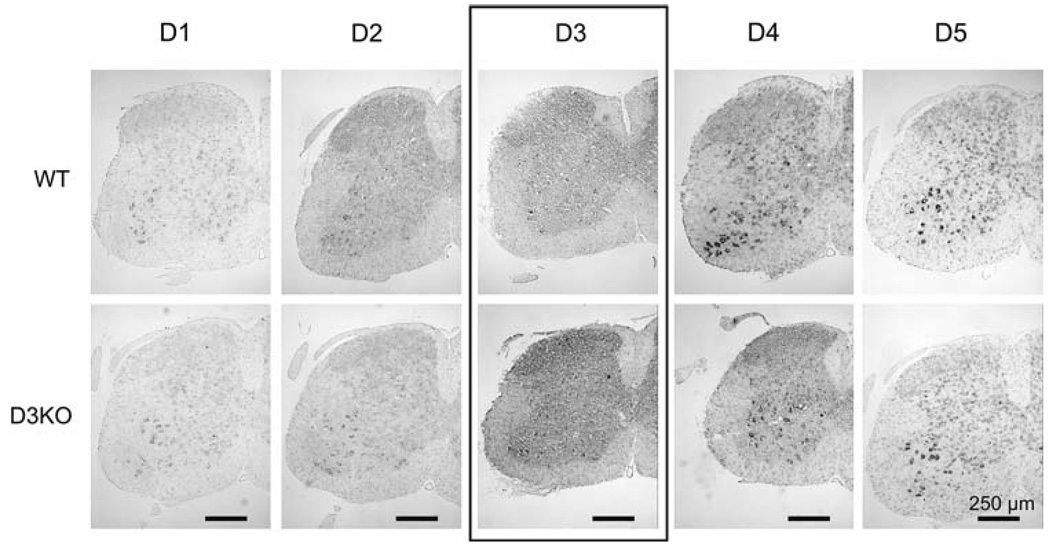
Figure 2 shows low-power views of lumbar spinal cords of a representative WT and D3KO animal at lumbar level L2 for all DA receptor antisense probes. Using identical exposure and time-matched settings, staining intensities and distribution patterns of D1, D2, D4, and D5 receptor subtypes are similar between WT and D3KO, whereas the staining intensities of the D3 receptor subtype appear to be stronger in the D3KO animal than in the WT. This staining pattern was similar throughout the lumbar region examined (L2–L5). The apparent stronger staining in D3KO mice is consistent with the real-time PCR data showing increased D3 receptor mRNA expression in the D3KO animals.
Fig. 2.
Anti-sense DA receptor mRNA labeling in the lumbar spinal cord of WT and D3KO animals (shown here only: lumbar segment L2). Low power magnification reveals that all DA receptors are present in both WT and D3KO animals, and that overall distribution and staining intensities are similar for the D1, D2, D4, and D5 receptor subtypes. In contrast, D3 receptor mRNA expression appears to be more prominent in the D3KO animals
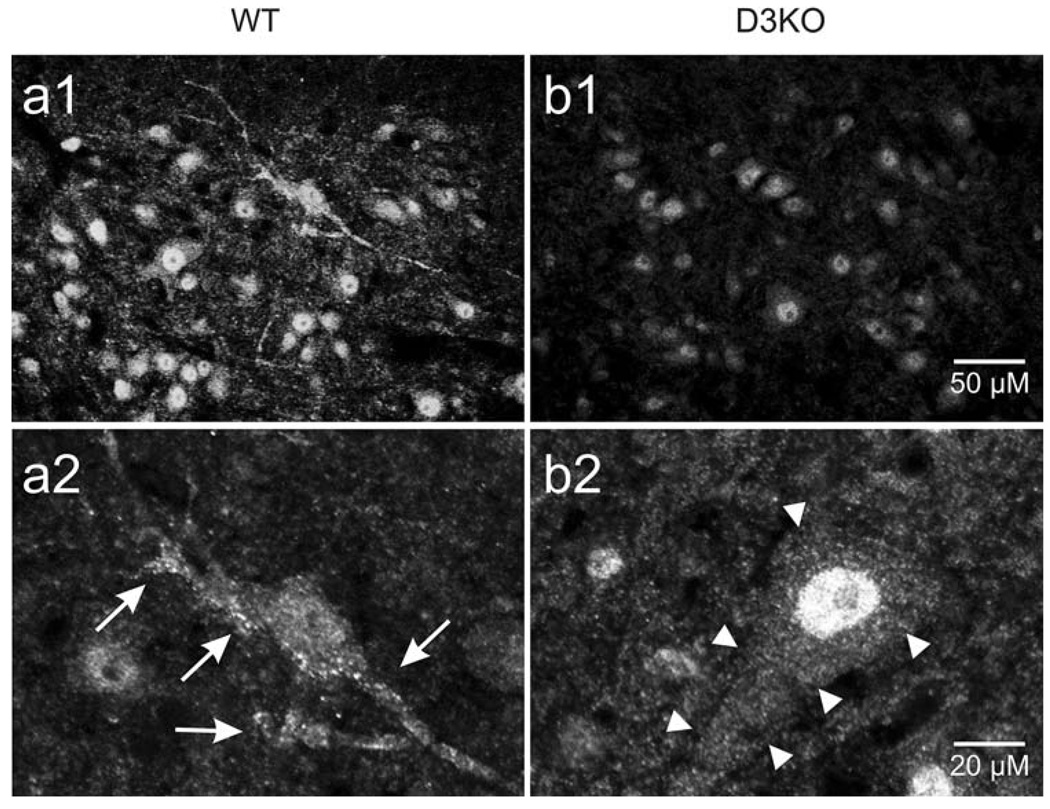
To generate the D3KO animals, a premature chain-termination mutation was introduced in the second predicted intracellular loop of the D3 receptor gene of mouse embryonic stem cells (Accili et al. 1996). This intervention does not prevent mRNA transcription of the D3 receptor, but it obstructs translation of the gene into a functional protein. We therefore used immunolabeling to assess the D3 receptor labeling patterns of the D3 receptor protein in WT and D3KO animals (Fig. 3). In all WT animals (n = 3), we regularly found evidence of membrane-delineated D3 receptor distribution in both unidentified neurons (Fig. 3a 1,2 arrows) and motoneurons (not shown). In comparison, we never observed such a punctate staining in D3KO animals (Fig. 3b 1,2 arrowheads). These findings support previous binding studies reporting that in D3KO the D3 mRNA is not translated into functional proteins (Accili et al. 1996).
Fig. 3.
Representative anti-D3 receptor immunolabeling in the ventral horn of the lumbar spinal cord of WT and D3KO animals. a 1,2 In WT animals, we regularly observed punctated D3 receptor labeling aligned along the membrane of unidentified neurons (s. arrows). b 1,2 In contrast, in D3KO animals, overall labeling was weaker and we did never observed a membrane-associated punctated labeling as in WT (s. arrowheads). Scale bar in B1 applies to top row and in B2 for bottom row
Taken together our data show that a system-wide loss of function of the DA D3 receptor does not affect the presence and expression levels of the other 4 DA receptor subtypes in spinal cord neurons.
Discussion
This study used real-time PCR, ISH and immunohistochemistry to compare the expression levels and distribution patterns of all known DA receptors in the lumbar spinal cord between WT and D3KO mice. The principal findings are that (1) all five receptor mRNAs are expressed in the lumbar spinal cord in both WT and D3KO, (2) D1, D2, D4, and D5 receptors are expressed and distributed similarly, with D2 receptor expression being dominant in both WT and D3KO, and (3) while D3 mRNA expression levels are increased in D3KO mice, membrane bound D3 receptor protein appears to be absent in these animals. The increase of D3 mRNA expression might be due to an interruption of a feedback signal associated with membrane insertion. Overall, these data suggest that, in contrast to the D2 receptor knockout (Jung et al. 1999), the D3KO animal does not compensate for the loss of D3 receptor function with corresponding changes in the functional DA receptor population.
There is evidence for both excitatory and inhibitory actions of DA in the spinal cord (Carp and Anderson 1982; Gajendiran et al. 1996; Barriere et al. 2004; Clemens and Hochman 2004; Han et al. 2007), and these actions are thought to be mediated by excitatory D1-like or inhibitory D2-like pathways, respectively, possibly as a function of the different receptor affinities for DA (Clemens and Hochman 2004; Clemens et al. 2006). The D3 receptor has very high affinity for DA (http://pdsp.med.unc.edu/pdsp.php), and we recently reported in the D3KO mouse a DA-mediated unmasking of excitatory actions in the spinal cord (Clemens and Hochman 2004). The finding that expression levels and distribution patterns of the functional DA receptors present in the spinal cord are unchanged in D3KO animals (Fig. 1, Fig. 2) suggests that the alterations in spinal DA actions on reflexes observed in D3KO mice (Clemens and Hochman 2004) are not due to a compensatory effect of any of the other four DA receptor subtypes.
A loss of D3 receptor function is not only involved in hyperactivity, increased locomotor activity, and hypertension (Accili et al. 1996; Asico et al. 1998), there is also evidence that reduced D3 receptor function may be involved in the pathophysiology of RLS (Montplaisir et al. 1999; Barriere et al. 2005; Ondo et al. 2007). RLS is a sleep disorder of CNS origin that involves abnormal limb sensations with a strong circadian pattern and increased spinal cord excitability, and which is relieved by D3 receptor-preferring agonists (Bara-Jimenez et al. 2000; Montplaisir et al. 2000; Odin et al. 2002; Zucconi and Ferini-Strambi 2004). The lack of compensatory responses in expression and distribution of D1, D2, D4, and D5 receptors in the D3KO animals supports further use of these mice to study the consequence of D3 receptor loss on dopaminergic modulatory actions in the spinal cord (Clemens et al. 2005; Clemens et al. 2006).
Acknowledgments
Funding for this study was awarded from the National Institutes of Health, NINDS, Grant NS045248. All experimental procedures complied with the “Principles of animal care,” publication No. 86-23, revised 1985 by the NIH, and the Emory Institutional Animal Care and Use Committee. We thank Dr Dapeng Cui for assistance with real-time PCR.
Abbreviations
- D3KO
D3 receptor knockout
- DA
Dopamine
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- ISH
In situ hybridization
- PBST
PBS containing 0.3% Triton X-100
- PCR
Polymerase chain reaction
- WT
Wild-type
Contributor Information
Stefan Clemens, Email: scleme2@emory.edu.
Shawn Hochman, Email: shochm2@emory.edu.
References
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–1616. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- Barriere G, Cazalets JR, Bioulac B, Tison F, Ghorayeb I. The restless legs syndrome. Prog Neurobiol. 2005;77:139–165. doi: 10.1016/j.pneurobio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Barriere G, Mellen N, Cazalets JR. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J Neurosci. 2004;19:1325–1335. doi: 10.1111/j.1460-9568.2004.03210.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, Ricceri L. Transgenic and knock-out mouse pups: the growing need for behavioral analysis. Genes, brain, and behavior. 2002;1:135–141. doi: 10.1034/j.1601-183x.2002.10301.x. [DOI] [PubMed] [Google Scholar]
- Carp JS, Anderson RJ. Dopamine receptor-mediated depression of spinal monosynaptic transmission. Brain Res. 1982;242:247–254. doi: 10.1016/0006-8993(82)90307-9. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci. 2004;24:11337–11345. doi: 10.1523/JNEUROSCI.3698-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Sawchuk MA, Hochman S. Reversal of the circadian expression of tyrosine-hydroxylase but not nitric oxide synthase levels in the spinal cord of D3 receptor knockout mice. Neuroscience. 2005;133:353–357. doi: 10.1016/j.neuroscience.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- Drago J, Padungchaichot P, Accili D, Fuchs S. Dopamine receptors and dopamine transporter in brain function and addictive behaviors: insights from targeted mouse mutants. Dev Neurosci. 1998;20:188–203. doi: 10.1159/000017313. [DOI] [PubMed] [Google Scholar]
- Drago J, McColl CD, Horne MK, Finkelstein DI, Ross SA. Neuronal nicotinic receptors: insights gained from gene knockout and knockin mutant mice. Cell Mol Life Sci. 2003;60:1267–1280. doi: 10.1007/s00018-003-2259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajendiran M, Seth P, Ganguly DK. Involvement of the presynaptic dopamine D2 receptor in the depression of spinal reflex by apomorphine. Neuroreport. 1996;7:513–516. doi: 10.1097/00001756-199601310-00033. [DOI] [PubMed] [Google Scholar]
- Gan L, Falzone TL, Zhang K, Rubinstein M, Baldessarini RJ, Tarazi FI. Enhanced expression of dopamine D(1) and glutamate NMDA receptors in dopamine D(4) receptor knockout mice. J Mol Neurosci. 2004;22:167–178. doi: 10.1385/JMN:22:3:167. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci. 2007;27:13192–13204. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon JP, Petrucci C, Fehlmann D, Viollet C, Epelbaum J, Hoyer D. Somatostatin sst2 receptor knock-out mice: localisation of sst1–5 receptor mRNA and binding in mouse brain by semi-quantitative RT-PCR, in situ hybridisation histochemistry and receptor autoradiography. Neuropharmacology. 2002;42:396–413. doi: 10.1016/s0028-3908(01)00186-1. [DOI] [PubMed] [Google Scholar]
- Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, III, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther. 1994;64:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Joyce JN. Multiple dopamine receptors and behavior. Neurosci Biobehav Rev. 1983;7:227–259. doi: 10.1016/0149-7634(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, El-Ghundi M, Fletcher PJ, O’Dowd BF. Modification of dopamine D(1) receptor knockout phenotype in mice lacking both dopamine D(1) and D(3) receptors. Eur J Pharmacol. 2000;399:171–181. doi: 10.1016/s0014-2999(00)00347-2. [DOI] [PubMed] [Google Scholar]
- Karper PE, De la Rosa H, Newman ER, Krall CM, Nazarian A, McDougall SA, Crawford CA. Role of D1-like receptors in amphetamine-induced behavioral sensitization: a study using D1A receptor knockout mice. Psychopharmacology (Berl) 2002;159:407–414. doi: 10.1007/s00213-001-0936-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, Vitale C, Westphal H, Drago J, Borrelli E. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci USA. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AK, Pencea V, Buck CR, Luskin MB. Neurogenesis and neuronal migration in the neonatal rat forebrain anterior subventricular zone do not require GFAP-positive astrocytes. Dev Biol. 1999;216:622–634. doi: 10.1006/dbio.1999.9498. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Micale V, Drago F. Increased sensitivity to antidepressants of D3 dopamine receptor-deficient mice in the forced swim test (FST) Eur Neuropsychopharmacol. 2008;18:271–277. doi: 10.1016/j.euroneuro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Reichel CM, Cyr MC, Karper PE, Nazarian A, Crawford CA. Importance of D(1) receptors for associative components of amphetamine-induced behavioral sensitization and conditioned activity: a study using D(1) receptor knockout mice. Psychopharmacology (Berl) 2005;183:20–30. doi: 10.1007/s00213-005-0146-9. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52:938–943. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Denesle R, Petit D. Pramipexole in the treatment of restless legs syndrome: a follow-up study. Eur J Neurol. 2000;7 Suppl 1:27–31. doi: 10.1046/j.1468-1331.2000.0070s1027.x. [DOI] [PubMed] [Google Scholar]
- Odin P, Mrowka M, Shing M. Restless legs syndrome. Eur J Neurol. 2002;9 Suppl 3:59–67. doi: 10.1046/j.1468-1331.9.s3.8.x. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Zhao HR, Le WD. Animal models of restless legs syndrome. Sleep Med. 2007;8:344–348. doi: 10.1016/j.sleep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Park Y, Ho IK, Fan LW, Loh HH, Ko KH. Region specific increase of dopamine receptor D1/D2 mRNA expression in the brain of mu-opioid receptor knockout mice. Brain Res. 2001;894:311–315. doi: 10.1016/s0006-8993(01)02001-7. [DOI] [PubMed] [Google Scholar]
- Pich EM, Epping-Jordan MP. Transgenic mice in drug dependence research. Annals of medicine. 1998;30:390–396. doi: 10.3109/07853899809029939. [DOI] [PubMed] [Google Scholar]
- Steiner H, Fuchs S, Accili D. D3 Dopamine receptor-deficient mouse: evidence for reduced anxiety. Physiol Behav. 1998;63:137–141. doi: 10.1016/s0031-9384(97)00430-7. [DOI] [PubMed] [Google Scholar]
- Tien LT, Park Y, Fan LW, Ma T, Loh HH, Ho IK. Increased dopamine D2 receptor binding and enhanced apomorphine-induced locomotor activity in mu-opioid receptor knockout mice. Brain Res Bull. 2003;61:109–115. doi: 10.1016/s0361-9230(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci USA. 2005;102:2117–2122. doi: 10.1073/pnas.0409726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhu W, Pan T, Xie W, Zhang A, Ondo WG, Le W. Spinal cord dopamine receptor expression and function in mice with 6-OHDA lesion of the A11 nucleus and dietary iron deprivation. J Neurosci Res. 2007;85:1065–1076. doi: 10.1002/jnr.21207. [DOI] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk M, Hochman S. Expression and distribution of all dopamine receptor subtypes (D1–D5) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885–897. doi: 10.1016/j.neuroscience.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5:293–299. doi: 10.1016/j.sleep.2004.01.004. [DOI] [PubMed] [Google Scholar]