Abstract
A bacteriocin produced by a vaginal isolate of Enterococcus faecium strain 62-6, designated enterocin 62-6, was characterized following purification and DNA sequence analysis and compared to previously described bacteriocins. Enterocin 62-6 was isolated from brain heart infusion (BHI) culture supernatants using ammonium sulfate precipitation followed by elution from a Sepharose cation exchange column using a continuous salt gradient (0.1–0.7 M NaCl). SDS-PAGE of an active column fraction resulted in an electrophoretically pure protein, which corresponded to the growth inhibition of the sensitive Lactobacillus indicator strain in the gel overlay assay. Purified enterocin 62-6 was shown to be heat- and pH-stable, and sensitive to the proteolytic enzymes α-chymotrypsin and pepsin. Results from mass spectrometry suggested that it comprised two peptides of 5206 and 5219±1 Da, which was confirmed by DNA sequence analysis. The characteristics of enterocin 62-6 as a small, heat- and pH-stable, cationic, hydrophobic, two-peptide, plasmid-borne bacteriocin, with an inhibitory spectrum against a broad range of Gram-positive but not Gram-negative bacteria, were consistent with its classification as a class IIc bacteriocin. Furthermore, its wide spectrum of growth inhibitory activity against Gram-positive bacteria of vaginal origin including lactobacilli, and stability under the acidic conditions of the vagina, are consistent with our hypothesis that it could have potential significance in disrupting the ecology of the vaginal tract and pave the way for the establishment of the abnormal microbiota associated with the vaginal syndrome bacterial vaginosis. This is the first class IIc bacteriocin produced by a strain of E. faecium of vaginal origin to be characterized.
Keywords: Bacterial vaginosis, bacteriocin, Enterococcus faecium, vaginal lactobacilli, microbial interactions
Introduction
There is currently a resurgence of interest in the small, ribosomally synthesized antimicrobial peptide bacteriocins produced by lactic acid bacteria (1,2). Many of these bacteriocins have an inhibitory spectrum against a broad range of Gram-positive bacteria. Given that these bacteria commonly reside in environments with other Gram-positive bacteria, the production of bacteriocins is considered to give producer strains a competitive advantage (2). Bacteriocins also have potential applications in the food industry as natural alternatives to the use of chemical preservatives in the control of food spoilage and food-borne pathogenic organisms (2–4). By contrast, our interest focuses on the possible significance of bacteriocins in the ecology of the human vaginal tract. Specifically, we are investigating the hypothesis that the introduction of Gram-positive bacteriocin-producing organisms into the vaginal tract may inhibit the growth of bacteria associated with the healthy vagina, particularly the lactobacilli, and thus be one mechanism that could potentially pave the way for the establishment of the abnormal micro-biota associated with the vaginal tract syndrome, bacterial vaginosis (BV) (5).
BV is a polymicrobial syndrome characterized by a shift in the ecology of the vaginal tract as reflected in the altered composition of the microbiota. Studies employing cultivation methods have shown that lactobacilli are dominant in many healthy women, but during BV they are replaced by the massive overgrowth of a mixture of organisms including Gardnerella vaginalis, Gram-positive and Gram-negative anaerobes, genital mycoplasmas, and Mobiluncus spp. (6). Concentrations of aerobes and anaerobes reach levels 100- and 1000-fold greater, respectively, than those seen in healthy subjects (7). Not only is BV the most common vaginal tract infection seen in primary health care in the United States (8), but the presence of BV is associated with preterm delivery and chorioamnionitis in pregnant women, pelvic inflammatory disease, and serious infections following obstetric or gynecologic surgery (8,9). Furthermore, individuals with BV are at increased risk for acquisition of HIV following heterosexual intercourse (10–12). Despite intense research efforts, the pathogenesis of BV - including factors that mediate the shift in composition of the vaginal microbiota - remains poorly understood (8,13,14) and the possibility exists that it may be polyetiologic.
Results from a clinical study (15) suggested that lactobacilli are the first population to decline in prevalence in a sequence of population changes culminating in BV. Since sexual activity has been a well-documented risk factor for BV acquisition (8,16) and sexual contact provides the opportunity for introduction of microorganisms into the vagina, we are investigating whether the introduction of bacteriocin-producing bacteria into the healthy vaginal tract could affect the ecology by causing the decline in concentration of lactobacilli. We have been evaluating bacteriocin production against vaginal strains of lactobacilli by genera of bacteria associated with the healthy vaginal tract, in particular streptococci and enterococci, since they would have the potential to establish a new host in the vagina (5).
We previously reported production of a bacteriocin-like inhibitor by E. faecium strain 62-6, which was antagonistic to the growth of 16 of 32 lactobacilli tested (including known hydrogen peroxide producers) as well as other Gram-positive bacteria of vaginal origin (5). The growth inhibitory effects of E. faecium 62-6 against the lactobacilli were shown to be independent of conditions of low pH alone and hydrogen peroxide production (5). Physicochemical characterization of the inhibitor using MRS broth culture supernatants from strain 62-6 indicated that the inhibitor was heat- (100°C, 30 min), and pH-(range 4–7) stable, and that it contained an essential proteinaceous component; properties which suggested that it was bacteriocin-like (5).
The aim of the current study was to further characterize the E. faecium strain 62-6 inhibitor following purification and DNA sequence analysis, to determine whether it was indeed a bacteriocin and to compare it to previously described bacteriocins. Consistent with the previous naming of bacteriocins produced by enterococci as enterocins, we propose the designation of this inhibitor as enterocin 62-6. While enterocins have been reported in the literature, this finding is, to the best of our knowledge, novel in that it is the first characterization of a class IIc bacteriocin produced by an isolate of Enterococcus of vaginal origin.
Materials and methods
Bacterial strains and culture conditions
Unless stated otherwise, all bacteria used in this study were of vaginal origin and had been identified as described previously (5). Cultivation was in a humid atmosphere at 35°C in the presence of 5% (v/v) CO2. The bacteriocin-producing strain E. faecium 62-6 was cultivated in BHI (Difco, Detroit, MI, USA). To scale up to 1 liter of culture, a 5 ml volume of an overnight (18–20 h) culture of strain 62-6 was inoculated into 100 ml BHI. Following incubation for 24 h, a 25 ml volume was inoculated into each of two 500 ml volumes of BHI, incubated for 24 h, and then the cultures were pooled. Throughout, the indicator bacteria routinely used for detection of bacteriocin activity by E. faecium 62-6 were the sensitive organism Lactobacillus acid-ophilus 4-1 and the resistant isolate L. rhamnosis 62-5 (5). Cultivation of all indicator bacteria was over-night (18–20 h) in 7 ml Lactobacilli MRS broth (Difco). MRS agar was made from MRS broth with the addition of 1.5% (w/v) agar (Difco). Stock cultures of each strain were stored at −80°C following the addition of glycerol (final concentration 10% (v/v) to overnight cultures) (5).
Detection of antibacterial activity
The well diffusion technique (5,17) was used to detect the growth inhibitory activity of liquid preparations against indicator bacteria. Briefly, 100 µl of each preparation was added to a well of 5 mm diameter cut into MRS agar, which had had its base sealed using a drop of molten agar. Following diffusion of the liquid into the agar medium, plates were surface-sterilized by exposure to chloroform vapors for 20 min, then air-dried for at least 30 min. Indicator bacteria were applied as lawn cultures then incubated for up to 44 h before being examined for the presence of zones of inhibited growth in the vicinity of each well. The diameter of any zone of inhibition, inclusive of the diameter of the well, was measured in millimeters.
Isolation and purification of enterocin 62-6
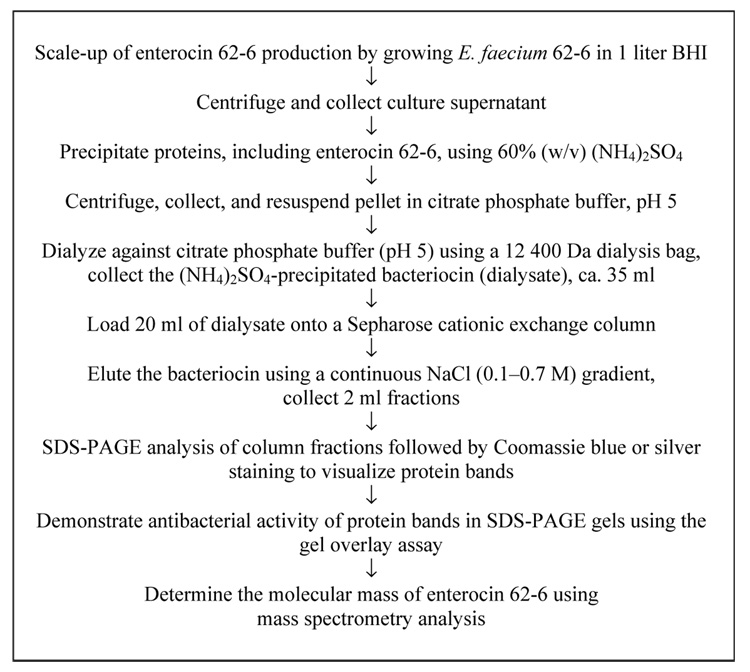
Methods for the isolation and purification of enterocin 62-6 are summarized in Figure 1. Enterocin 62-6 was isolated following the scale-up of growth of E. faecium 62-6 to 1 liter in BHI, and centrifuged using a Beckman biofuge (Palo Alto, CA, USA; 15 300 g, 4°C, 15 min) to remove bacterial cells. The supernatant was collected and the remaining proteins were precipitated at 4°C using 60% (w/v) ammonium sulfate and harvested by centrifugation (31 000 g, 4°C, 15 min). The pellet was resuspended in approximately 25 ml citrate-phosphate buffer (pH 5), placed in seamless cellulose dialysis tubing (12 400 Da cut-off; Sigma-Aldridge, St Louis, MO, USA) then dialyzed against 1 liter volumes of the same buffer at 4°C, with four buffer changes over 3 consecutive days. Each step of the isolation procedure was tested for the presence of antibacterial activity using the well diffusion technique.
Figure 1.
Flow diagram showing methods for the purification of enterocin 62-6.
Following dialysis, cation exchange chromatography was used to isolate enterocin 62-6 by loading a 20 ml volume of the dialysate onto 10 ml of a CM Sepharose® Fast Flow (Sigma-Aldridge) chromatography column (110 mm × 20 mm) that had previously been equilibrated with citrate-phosphate buffer (pH 5), at room temperature. Proteins were eluted from the column by employing a linear salt gradient of 0.1–0.7 M NaCl, similar to that used by Farías et al. (18) in citrate-phosphate buffer pH 5 (total volume 40 ml) with a flow rate of 8 ml/h. Fractions (2 ml) were collected and assayed for antibacterial activity by well diffusion. As a negative control, the antibacterial activity of the medium alone was tested in parallel. Column fractions showing antibacterial activity against the growth of L. acidophilus 4-1, but not L. rhamnosis 62-5, were fractionated using SDS-PAGE containing 15% (w/v) acrylamide (19) and stained either with Coomassie Blue R-250 (Bio-Rad Laboratories, Hercules, CA, USA) or by silver staining. The BenchMark™ prestained protein ladder (Invitrogen, Carlsbad, CA, USA) served as the molecular mass standards.
Gel overlay assay for detection of antibacterial activity in SDS-PAGE gels
SDS-PAGE gels were run in duplicate. The first gel was stained using either Coomassie blue or silver staining to visualize protein bands. The second gel was subject to the gel overlay technique (20) to correlate any zone of inhibition of L. acidophilus 4-1 to its corresponding protein seen on the stained gel, as follows. Gels were placed in 30 ml detoxifying solution of 20% (v/v) isopropyl alcohol, 10% (v/v) acetic acid in deionized water for 30 min, then washed with four changes of deionized water for 30 min each. Each gel was transferred to a sterile petri dish and overlaid with 10 ml soft 1% (w/v) MRS agar. Once solidified, 2 ml of an overnight culture of L. acidophilus 4-1 was flooded on top of the agar and the excess liquid was removed. The plates were incubated for up to 44 h before being examined for zones of inhibition. This technique was also carried out using strain 62-5 as a negative control.
Molecular mass determination
Mass spectrometry (MS) analysis was performed to determine the molecular mass of enterocin 62-6 in positive ion electrospray mode on a Q-Tof Micro (Waters Corporation, Milford, MA, USA) at the M.W. Keck Foundation Biotechnology Resource Laboratory (Yale University, New Haven, CT, USA). The spectrum containing multiply charged ions was processed using the Maxent1 transformation algorithm of the Masslynx software.
LC MS/MS analysis
LC MS/MS analysis of enterocin 62-6 was carried out at the M.W. Keck Foundation Biotechnology Resource Laboratory using a Waters (Milford, MA, USA) CapLC and a Waters Q-Tof mass spectrometer. An in-gel sample of the protein band corresponding to enterocin 62-6 was digested with trypsin. The tryptic peptides were extracted and analyzed by LC-MS/MS. The MS/MS spectra were analyzed using automated MASCOT searches or were manually interpreted.
PCR amplification
E. faecium strain 62-6 was grown overnight on blood agar (bioMérieux, Lombard, IL, USA) and a 1 µl suspension in water served as template for PCR. Primers used were designed based on the published sequence of enterocins L50A and L50B from E. faecium L50 (Genbank accession no. AJ223633) (21). The forward primer was Ent62-6ABF (5′-GT-GGAAAGCTAGTATTTGCAAC-3′) and Ent62-6ABR (5′-AGCGTTAAGCCGAATGTTTACAC-3′) was the reverse primer, they were homologous to the 1239–1260 and 1728–1750 regions, respectively, of AJ223633. PCR amplifications were performed in 50 µl reaction mixtures containing 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 0.01% gelatin, 0.2 mM deoxyribonucleotides, 100 pmol of each primer, and 1 U of Red-Taq DNA polymerase (Sigma-Aldrich). Samples were initially denatured (94°C for 10 min), followed by 30 cycles of denaturation (94°C for 1 min), annealing (49°C for 1 min), and elongation (72°C for 1 min), ending with a final elongation (72°C for 5 min), in an Eppendorf Mastercycler Gradient thermocycler (Eppendorf North America, New York, NY, USA). PCR products were separated by electrophoresis in 1.2% (w/v) agarose and visualized by ethidium bromide staining under UV light.
DNA sequence determination
The PCR product was gel-purified using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) and the DNA sequence was determined by the Biotechnology Resource Center at Cornell University (Ithaca, NY, USA) using Ent62-6ABF and Ent62-6ABR as sequencing primers.
Isolation of plasmid DNA and Southern blot analysis
The method of Woodford et al. (22) was used to isolate plasmid DNA from E. faecium 62-6 and a negative control strain, a strain of Enterococcus shown not to produce the bacteriocin, following overnight growth in 7 ml volumes of MRS broth. Digestion with restriction endonucleases, agarose gel electrophoresis, and Southern blotting were carried out according to standard procedures (23). The PCR product containing structural genes for enterocin 62-6 was used as a probe and labeled with the DIG high prime DNA labeling and detection starter kit (Roche Applied Science, Indianapolis, IN, USA).
Physicochemical characterization
Purified enterocin 62-6 from Sepharose column fractions was tested for its stability following exposure to heat and the proteolytic enzymes trypsin, pepsin, α-chymotrypsin, proteinase K, lysozyme, and lipase, using the well diffusion assay, as described previously (5). The pH stability of enterocin 62-6 was tested following a 1:1 dilution of the bacteriocin-containing column fractions in citrate (pH 4.0) or phosphate (pH 6.0 or 8.0) buffers.
Spectrum of inhibitory activity
The growth inhibitory activity of purified enterocin 62-6-containing column fractions was tested against Gram-positive bacteria isolated from the vaginal tract and Gram-negative bacteria from either the American Type Culture Collection (ATCC; Rockville, MD, USA) or Presque Isle Cultures (PI; Presque Isle, PA, USA), by well diffusion.
Nucleotide sequence accession no
The nucleotide sequence reported in this publication containing enterocins 62-6A and 62-6B has the GenBank accession no. EF112398.
Results
Purification of enterocin 62-6
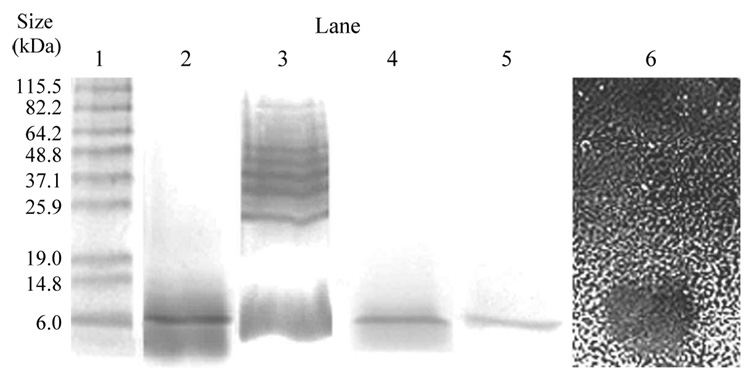
The inhibitor was isolated from 1 liter volumes of BHI culture supernatant using ammonium sulfate to precipitate proteins, then dialysed extensively (Figure 1). In initial experiments to isolate the bacteriocin, the dialysate was loaded onto a Sepharose cation exchange column and isolated using step-wise sodium chloride elution (0.05, 0.1, 0.2, 0.5, and 1.0 M). Samples from column fractions with inhibitory activity (detected by well diffusion) against the sensitive Lactobacillus indicator strain, L. acidophilus 4-1, but not the resistant indicator strain, were fractionated using SDS-PAGE gels and stained with Coomassie blue to confirm purity, a single distinct band was observed (Figure 2, lane 2) with an estimated molecular mass of around 6 kDa. However, when replicates of these gels were silver-stained a band of approximately 6 kDa was still visible, but five additional bands were observed with molecular masses in the approximate size range 25.9–64.2 kDa (Figure 2, lane 3), indicating that the fraction was impure.
Figure 2.
SDS-PAGE analysis of Sepharose column fractions with inhibitory activity against L. acidophilus 4-1 (as assayed by well diffusion) following step-wise NaCl elution (lanes 2 and 3) and continuous gradient NaCl elution (lanes 4 and 5) as shown for fraction 16 of Figure 3. Lanes 2 and 4 were stained using Coomassie blue and lanes 3 and 5 were silver stained. Using the gel overlay assay, the growth inhibitory activity of the ca. 6 kDa protein is shown against L. acidophilus 4-1 (lane 6). The black oval zone corresponds to growth inhibition of strain 4-1. Standard protein markers are shown in lane 1 with their molecular masses indicated to the left of the gel.
To purify enterocin 62-6 from the active column fractions above, we used HPLC with a reverse phase C18 column, since this method had frequently been used to obtain purified enterocins (18,24,25). However, the bacteriocin was never recovered from the column. A possible explanation was that due to its high hydrophobicity it remained bound, as has been reported previously for other hydrophobic bacteriocins (26).
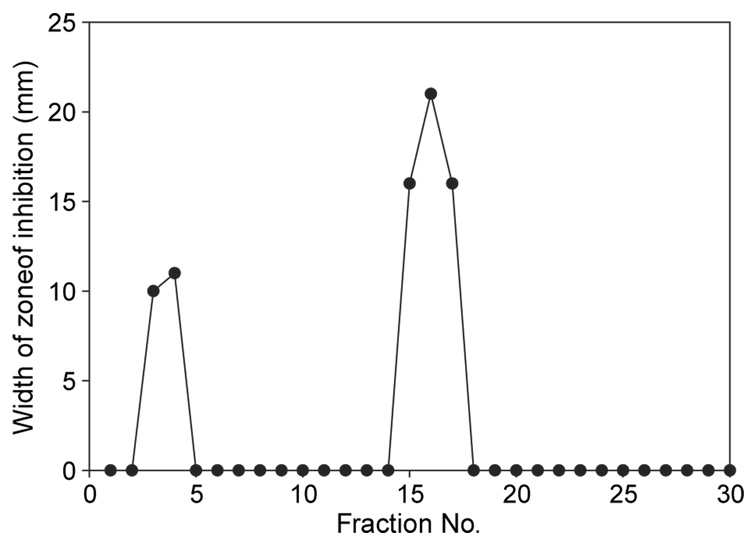
Since the active column fraction from the step-wise procedure (Figure 2, lanes 2 and 3) had been eluted from the Sepharose column when the concentration of sodium chloride was in the 0.2–0.5 M range, we then employed a continuous sodium chloride gradient (0.1–0.7 M; Figure 1) in an attempt to produce a column fraction containing only purified enterocin 62-6. The inhibitory activity of the column fractions collected during a typical continuous gradient elution is shown in Figure 3. The relatively small (10–11 mm diameter) zones of inhibition of column fractions 3 and 4 represent the bacteriocin that did not bind to the column and had eluted in the void volume. As fraction 16 from this column (Figure 3) contained the greatest inhibitory activity against the sensitive Lactobacillus indicator it was further checked for purity. As seen in Figure 2 (lanes 4 and 5), 15% SDS-PAGE fractionation followed by both Coomassie blue and silver staining suggested that this fraction contained only one protein band and estimated its molecular mass to be around 6 kDa. Using the gel overlay assay of an SDS-PAGE gel this approximately 6 kDa protein was shown to correspond to the growth inhibition of L. acidophilus 4-1 (Figure 4, lane 6) but not L. rhamnosis (results not shown), suggesting it was enterocin 62-6.
Figure 3.
Antibacterial activity of column fractions following continuous gradient sodium chloride elution from a Sepharose column against the sensitive indicator strain L. acidophilus 4-1, as detected using the well diffusion assay. Growth inhibitory activity against the negative control strain L. rhamnosis 62-5 was not detected.
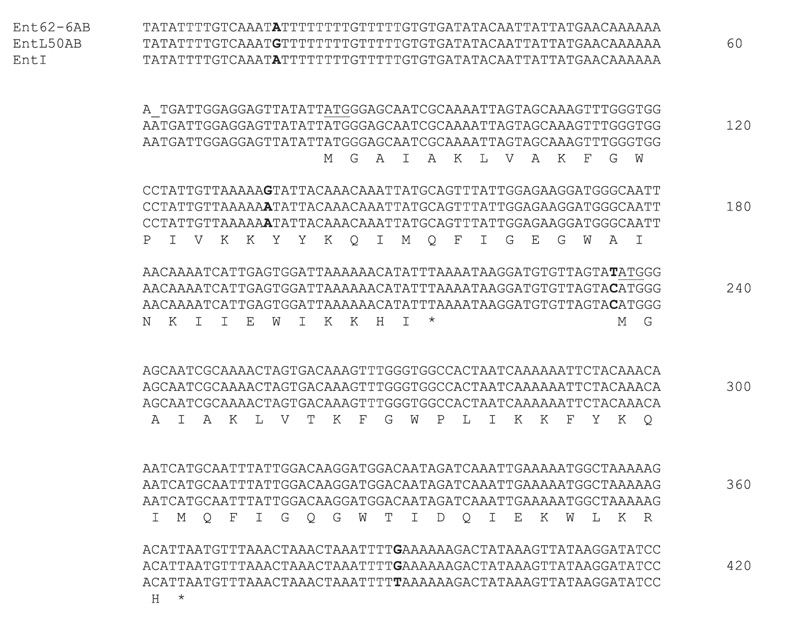
Figure 4.
Nucleotide sequence comparison between the genetic regions ent62-6AB, entL50AB, and entI corresponding to GenBank accession nos EF112398, AJ223633, and Y16413, respectively. The deduced amino acid sequence for each peptide is shown below the open reading frames. Start codons are underlined and stop codons are indicated with an asterisk. Nucleotide differences between the three sequences are indicated in bold.
Molecular mass determination
When electrospray MS was used to determine the molecular mass of the inhibitor present in the active column fraction (above) three peptides were observed corresponding to 5206, 5219, and 5235 Da (data not shown), with the experimental error in the range 0.01–0.02% (plus or minus 1 Da).
Amino acid sequence tag of enterocin 62-6
The column fraction containing purified enterocin 62-6 was initially subjected to automated Edman degradation to obtain amino acid sequence data, but the N terminus was shown to be blocked so data were not obtained. However, the following sequence tag, Ile-Gly-Gln-Gly-Trp-Thr-Ile-Asp, was generated by manual interpretation of an MS/MS spectrum, then searched using MS-pattern. The automated MASCOT search of this sequence tag indicated that it was a direct match to an 8 amino acid portion of the 43 amino acid bacteriocin, enterocin L50B, 1 of 2 peptides (enterocins L50A and L50B) produced by E. faecium L50 isolated from Spanish dry fermented sausage (21,24). The predicted sequence ions for this peptide were in agreement with the observed MS/MS fragment ions.
Genetic characterization of the enterocin 62-6 structural genes
Given that our eight amino acid sequence tag had directly matched enterocin L50B, to elucidate the structural gene sequence corresponding to enterocin 62-6, PCR amplification was carried out using primers based on the published sequence for enterocin L50 (GenBank accession no. AJ223633). The PCR product was purified and a 419 nucleotide stretch was sequenced and compared to enterocins L50A and L50B. It was also compared to another two-peptide bacteriocin, enterocin I, produced by E. faecium strain 6T1a isolated from a Spanish-style olive fermentation and known to have identical structural gene sequences to enterocins L50A and L50B (27). Figure 4 shows the comparative DNA sequence alignment between enterocins 62-6, L50, and I and the corresponding peptide sequences. While the DNA sequences corresponding to each of the two peptides were almost identical, one silent mutation was found in 62-6A and three further point mutations were shown to be present in the intergenic regions for all three enterococcal bacteriocins. Plasmid DNA was isolated from strain 62-6, digested with restriction enzymes, separated by agarose gel electrophoresis, and transferred to a nylon membrane by Southern blotting. The PCR product containing enterocin 62-6 was used as a probe. This probe was shown to hybridize to undigested plasmid DNA larger than 10 kb, and specifically to a 1.7 kb EcoRV fragment. Thus, it seems that the enterocin 62-6 structural genes are plasmid-borne.
Physicochemical characterization of enterocin 62-6
The antibacterial activity of column fractions containing purified enterocin 62-6 was tested following exposure to various physicochemical conditions (Table I). Enterocin 62-6 was stable following boiling for 30 min, and over the pH range tested (pH 4–8). Its demonstrated sensitivity to certain proteolytic enzymes (pepsin and α-chymotrypsin) suggested that it contained an essential proteinaceous component, while stability in the presence of both lysozyme and lipase indicated that it lacked carbohydrate and lipid moieties.
Table I.
Physicochemical characterization of purified enterocin 62-6 against the sensitive Lactobacillus indicator strain 4-1 and the resistant isolate 62-5.
| Inhibitory activity of enterocin 62-6 following exposure to | Growth inhibition of Lactobacillus indicator strain* | |
|---|---|---|
| 4-1 | 62-5 | |
| No treatment, positive control | + | − |
| Heat, 100°C for 30 min | + | − |
| Trypsin (pH 7) | + | − |
| Pepsin (pH 3 and 7) | − | − |
| α-Chymotrypsin (pH 8) | − | − |
| Proteinase K (pH 7) | + | − |
| Lysozyme | + | − |
| Lipase | + | − |
| pH 4 | + | − |
| pH 6 | + | − |
| pH 8 | + | − |
Assays for inhibitory activity were carried out on MRS agar using the well diffusion technique.
As assessed by the measurement of the diameter (mm) of the zone of inhibited growth of the indicator strain, inclusive of the 5 mm diameter of the well, where ‘+’ indicates a diameter of the zone of growth inhibition of ≥7 mm and ‘−’ indicates a diameter of inhibited growth of <7 mm.
Inhibitory spectrum of purified enterocin 62-6
The spectrum of inhibitory activity of enterocin 62-6 against a range of bacterial strains was assayed by well diffusion (Table II). Enterocin 62-6 was shown to inhibit the growth of a range of Gram-positive genera of bacteria from the vaginal tract, including lactobacilli, corynebacteria, streptococci, and enterococci, but not staphylococci. It also inhibited the growth of two of three Lactobacillus strains isolated from beer spoilage. It did not inhibit the growth of any of the Gram-negative bacteria tested.
Table II.
Spectrum of antibacterial activity of purified enterocin 62-6 as assayed by well diffusion on MRS agar.
| Bacterial strains | No. strains inhibited*/no. tested |
|---|---|
| Gram-positive vaginal isolates | |
| lactobacilli | 6/10 |
| corynebacteria | 2/3 |
| streptococci | 4/5 |
| enterococci | 3/3 |
| staphylococci | 0/5 |
| Gram-negative bacteria | |
| Escherichia coli ATCC 4157 | 0/1 |
| Citrobacter freundii PI 239 | 0/1 |
| Proteus mirabilis ATCC 7002 | 0/1 |
| Pseudomonas aeruginosa ATCC 10145 | 0/1 |
| Lactobacilli isolated from beer spoilage | |
| L. paracollinoides ATCC 8291 | 1/1 |
| L. buchneri ATCC 11307 | 1/1 |
| L. paraplantarum ATCC 700211 | 0/1 |
As assessed by the measurement of the diameter (mm) of the zone of inhibited growth of the indicator strain, inclusive of the 5 mm diameter of the well, where a positive result was indicated by a diameter of the zone of growth inhibition of ≥7 mm and a negative result was indicated by a diameter of inhibited growth of <7 mm.
Discussion
The current study describes the purification and characterization of the first class IIc enterococcal bacteriocin from an isolate of E. faecium of vaginal origin, enterocin 62-6. The inhibitory activity of Sepharose column fractions containing purified enterocin 62-6 was shown to be heat-stable, and stable over the range pH 4–8, sensitive to the proteolytic agents pepsin and α-chymotrypsin, but resistant to proteinase K and trypsin (Table I). These physico-chemical properties were consistent with those reported for inhibitor-containing culture supernatants from the growth of E. faecium 62-6-in MRS broth (5). Furthermore, the combined characteristics of enterocin 62-6, found in this study, as small (<10kDa), heat- and pH-stable, cationic, hydrophobic, two-peptide bacteriocin, with an inhibitory spectrum against a broad spectrum of Gram-positive but not Gram-negative bacteria (Table II), were consistent with its classification as a class IIc bacteriocin (21,28).
We had previously reported that MRS culture supernatants lost inhibitory activity following exposure to lipase (5), which could have indicated a lipid moiety of enterocin 62-6 and its classification as a class IV bacteriocin (1). Follow-up experiments using supernatants from the growth of strain 62-6 in BHI (data not presented) and, in the present study, with column fractions containing purified enterocin 62-6, did not demonstrate lipase sensitivity of the inhibitor (Table I). These findings appear to rule out the classification of enterocin 62-6 as a possible class IV bacteriocin. This was further supported by the identical peptide sequence derived from the enterocin 62-6 structural genes (as described below) to enterocins L50A and L50B (Figure 4), which are well-characterized, class IIc bacteriocins (21,24,28). The lipase sensitivity of the inhibitor demonstrated using the MRS culture supernatants was probably due to the high hydrophobicity of class II bacteriocins, which causes them to bind to medium components such as the Tween 80 present in MRS broth, as has been reported previously (1,26,29).
When subject to Edman degradation to determine the amino acid sequence, purified enterocin 62-6 was shown to be N-terminally blocked, which has been found for certain enterocins, including enterocins L50A and L50B and 4 (21,30). Following the direct match of the amino acid sequence tag Ile-Gly-Gln-Gly-Trp-Thr-Ile-Asp (generated by the in-gel cleavage of enterocin 62-6 with trypsin) to an eight amino acid sequence within enterocin L50B, a 43 amino acid peptide produced by E. faecium L50 (21,24) PCR was employed to amplify and sequence the putative structural genes for enterocin 62-6. Not only did this provide evidence that enterocin 62-6, like enterocin L50, comprised two peptides corresponding to two open reading frames, but also that their peptide sequences were identical and their DNA sequences were almost identical to enterocins L50A and L50B and the two enterocin I peptides (Figure 4) (27). Accordingly, they were named enterocins 62-6A and 62-6B. Similar to enterocins L50 and I, we also showed that the structural genes for enterocin 62-6 were plasmid-borne determinants (21,27).
To date, several enterocins from various sources and countries have been reported that are either identical or very closely related to enterocins L50A and L50B, as determined by comparative DNA or amino acid sequence analysis or by the binding of primers specific to the L50 structural genes to DNA preparations. This includes enterococcal strains isolated from Spanish dry fermented sausages (31), enterocin I from E. faecium 6T1a (Figure 4) isolated from a Spanish-style green olive fermentation (27), E. faecium F58 isolated from a Moroccan soft goat’s cheese, E. faecium strains B1 and B2 isolated from Malaysian tempeh (32), and more recently a strain of E. faecalis, MRR 10-3, isolated from the uropygial gland of the Hoopoe (Upupa epops) in Spain (33). While many of these isolates are from food sources some, including the avian isolate (E. faecalis MRR 10-3) and our vaginal strain, are clearly not. The apparent presence of these L50-related bacteriocins across the four continents reported here is noteworthy and raises the question of the ubiquity of this bacteriocin.
Electrospray MS of purified column fractions of enterocin 62-6 yielded molecular masses of 5206, 5219, and 5235 Da. Since the difference in molecular mass between the 5219 Da protein and the 5235 Da protein of 16 Da most likely corresponded to an oxidized form of the 5219 Da protein (W.M. Keck Foundation, personal communication) the data suggested that two distinct peptides had co-eluted in this column fraction. The molecular masses of enterocins 62-6A and 62-6B of 5219 and 5206 Da, respectively, differ from the masses calculated from their deduced amino acid sequences of 5190 and 5178 Da, corresponding to the 44 and 43 amino acid residue peptides (21). This discrepancy between calculated molecular masses and those assigned through MS has been routinely found for all of the L50A- and L50B-related enterocins (33). Various chemical modifications have been put forward to account for these observations, including alterations during the chemical purification process (33). But the most likely explanation is the retention of formylmethionine (29 Da), which is known to block Edman degradation (21) and which we experienced during attempts to sequence enterocin 62-6A and 62-6B. Taking this into account, when the molecular masses of the 5219 and 5206 Da purified peptides are reduced to 5190 and 5177 Da, respectively, these data are in agreement with their calculated masses of 5190 and 5178 Da when the experimental error inherent in MS of ±1 Da is factored in.
Given that the molecular mass of enterocins 62-6A and 62-6B were determined to be less than 5300 Da, it was interesting to note that as part of the purification process, they were retained inside a dialysis bag of 12 400 Da molecular mass cut-off (Figure 1). Similarly, we previously reported that the inhibitory activity of E. faecium strain 62-6 culture supernatants was retained inside the same size dialysis tubing (5). A possible explanation for this is the tendency for some of these bacteriocins to form aggregates, which has been previously reported for enterocins as well other bacteriocins produced by lactic acid bacteria (34).
Understanding the interactions, including both positive and negative symbioses, among bacteria inhabiting the lower genital tract has been proposed to answer some of the complexities surrounding the pathogenesis of BV (13,35–38). To date, while the production of antibacterial substances by lactobacilli that could contribute to their dominance in the healthy vaginal tract has been well researched (8,38,39), only a few studies have examined the converse (40,41), namely the production of antagonistic substances that could lead to the decline in concentration of vaginal lactobacilli. Such antagonistic interactions may be one mechanism leading to an alteration in the ecology of the vaginal tract by initiating a sequence of events culminating in the massive change in the vaginal microbiota characteristic of BV. Since the context of the current study was the production of substances by vaginal tract bacteria that could potentially disrupt the ecology of the healthy vagina, it was notable that enterocin 62-6 was stable over the pH values associated with both the healthy vaginal environment (ca. pH 4) and BV, greater than pH 4.5 (Table I) (42). In addition, its inhibitory activity against Lactobacillus strains associated with beer spoilage suggests a possible application in the food industry as a natural alternative for the control of contaminating bacteria.
Several enterocins have been characterized to date, many of which are produced by enterococci isolated from the food industry which are antagonistic to the growth of common food spoilage pathogens such as Listeria monocytogenes (21,31). While there has been a paucity of reports of other bacteriocin or bacteriocin-like inhibitors produced by enterococci from the vaginal environment (43–45), this is the first characterization of a class IIc bacteriocin produced by a vaginal strain of E. faecium. It is one of few microbial interactions reported that may be of significance to the pathogenesis of BV, specifically by disrupting the vaginal tract ecology by antagonizing the growth of vaginal lactobacilli, corynebacteria, streptococci, and enterococci (Table II). Since enterocins related to L50, including enterocin 62-6, appear to be present in representative enterococcal isolates worldwide from a variety of sources, clinical studies assessing the prevalence of this bacteriocin in vaginal isolates and its possible role in vivo in the pathogenesis of BV are warranted.
Acknowledgements
This research was supported by a research grant from the NIH/National Institute of Allergy and Infectious Disease, AI054402, and a McArthur grant from Kalamazoo College awarded to VP. M.J.M. and D.C.D. gratefully acknowledge the receipt of summer research fellowships from the Howard Hughes Medical Institute and the Heyl Fund, respectively. We are grateful to Bob Heinrickson, Proteos Laboratory, Kalamazoo, MI, for his guidance on protein purification and to Jackson School, Pfizer, Kalamazoo, MI for his assistance with reverse phase HPLC analysis.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 2.Eijsink VGH, Axelsson L, Diep DB, Håvarstein LS, Holo H, Nes IF. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie van Leeuwenhoek. 2002;81:639–654. doi: 10.1023/a:1020582211262. [DOI] [PubMed] [Google Scholar]
- 3.Jack RW, Tagg JR, Ray B. Bacteriocins of Gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casaus P, Nilsen T, Cintas LM, Nes IF, Hernández PE, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Micro-biology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MC, Mequio MJ, Pybus V. Inhibition of vaginal lactobacilli by a bacteriocin-like inhibitor produced by Enterococcus faecium 62-6: potential significance for bacterial vaginosis. Infect Dis Obstet Gynecol. 2003;11:147–156. doi: 10.1080/10647440300025513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koumans EH, Kendrick JS. Preventing adverse sequelae of bacterial vaginosis: a public health program and research agenda. Sex Transm Dis. 2001;28:292–297. doi: 10.1097/00007435-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980;303:601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- 8.Hillier S, Holmes KK. Bacterial vaginosis. In: Holmes KK, Sparling PF, Mårdh PA, editors. Sexually transmitted diseases. New York: McGraw-Hill; 1999. pp. 563–586. [Google Scholar]
- 9.French JI, McGregor JA. Bacterial vaginosis. In: Faro S, Soper DE, editors. Infectious diseases in women. Philadelphia: WB Saunders; 2001. pp. 221–239. [Google Scholar]
- 10.Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, Garcia P, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chaing Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 12.Warren D, Klein S, Sobel J, Kieke B, Brown W, Schuman P, et al. A multicenter study of bacterial vaginosis in women with or at risk for human immunodeficiency virus infection. Infect Dis Obstet Gynecol. 2001;9:133–141. doi: 10.1155/S1064744901000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faro S. Bacterial vaginosis: the quest continues. Infect Dis Obstet Gynecol. 2000;8:75. doi: 10.1002/(SICI)1098-0997(2000)8:2<75::AID-IDOG1>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobel J. Bacterial vaginosis. Annu Rev Med. 2000;51:349–356. doi: 10.1146/annurev.med.51.1.349. [DOI] [PubMed] [Google Scholar]
- 15.Rosenstein IJ, Morgan DJ, Sheehan M, Lamont RF, Taylor-Robinson D. Bacterial vaginosis in pregnancy: distribution of bacterial species in different gram-stain categories of the vaginal flora. J Med Microbiol. 1996;45:120–126. doi: 10.1099/00222615-45-2-120. [DOI] [PubMed] [Google Scholar]
- 16.Schwebke J, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis. 2005;32:654–658. doi: 10.1097/01.olq.0000175396.10304.62. [DOI] [PubMed] [Google Scholar]
- 17.Hynes WL, Tagg JR. Production of broad-spectrum bacteriocin-like activity by group A streptococci of particular M-types. Zentralbl Bakteriol Mikrobiol Hyg A. 1985;259:155–164. doi: 10.1016/s0176-6724(85)80046-8. [DOI] [PubMed] [Google Scholar]
- 18.Farías ME, Farías RN, de Ruiz Holgado AP, Sesma F. Purification and N-terminal amino acid sequence of enterocin CRL 35, a pediocin-like bacteriocin produced by Enterococcus faecium CRL 35. Lett Appl Microbiol. 1996;22:417–419. doi: 10.1111/j.1472-765x.1996.tb01193.x. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Du Toit M, Franz CMAP, Dicks LMT, Holzapfel WH. Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J Appl Microbiol. 2000;88:482–494. doi: 10.1046/j.1365-2672.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 21.Cintas LM, Casaus P, Holo H, Hernández PE, Nes IF, Håvarstein LS. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J Bacteriol. 1998;180:1988–1994. doi: 10.1128/jb.180.8.1988-1994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodford N, Morrison D, Cookson B, George RC. Comparison of high-level gentamicin-resistant Enterococcus faecium isolates from different continents. Antimicrob Agents Chemother. 1993;37:681–684. doi: 10.1128/aac.37.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. Cold Spring Harbor. 2nd edn. New York: Cold Spring Harbor Laboratory; 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- 24.Cintas LM, Rodreguez JM, MFernandez MF, Sletten K, Nes IF, Hernandez PE, et al. Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus adidilactici with a broad inhibitory spectrum. Appl Environ Microbiol. 1995;61:2643–2648. doi: 10.1128/aem.61.7.2643-2648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aymerich T, Holo H, Håverstein LS, Hugas M, Garriga M, Nes IF. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contreras BGL, de Vuyst L, Devreese B, Busanyova K, Raymaeckers J, Bosman F, et al. Isolation, purification, and amino acid sequence of lactocin A, one of two bacteriocins produced by Lactobacillus amylovorus LMG P-13139. Appl Environ Microbiol. 1997;63:13–20. doi: 10.1128/aem.63.1.13-20.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Floriano B, Ruiz-Barba JL, Jiménez-Díaz R. Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl Environ Microbiol. 1998;64:4883–4890. doi: 10.1128/aem.64.12.4883-4890.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nes IF, Diep DB, Holo H. Bacteriocin diversity in Streptococcus and Enterococcus. J Bacteriol. 2007;189:1189–1198. doi: 10.1128/JB.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aroutcheva AA, Simoes JA, Faro S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect Dis Obstet Gynecol. 2001;9:33–39. doi: 10.1155/S1064744901000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joostein HM, Nuñez M, Devresse B, Beeumen JV, Marugg JD. Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalis INIA 4. Appl Environ Microbiol. 1996;62:4220–4223. doi: 10.1128/aem.62.11.4220-4223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cintas LM, Casaus P, Herranz C, Hâvarstein LS, Holo H, Hernández PE, et al. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J Bacteriol. 2000;182:6806–6814. doi: 10.1128/jb.182.23.6806-6814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno MR, Leisner JJ, Tee LK, Ley C, Radu S, Rusul G, et al. Microbial analysis of Malaysian tempeh, and characterization of two bacteriocins produced by isolates of Enterococcus faecium. J Appl Microbiol. 2002;92:147–157. doi: 10.1046/j.1365-2672.2002.01509.x. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Platero AM, Valdivia E, Ruiz-Rodriguez M, Soler JJ, Martin-Vivaldi M, Maqueda M, et al. Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10-3, isolated from the uropygial gland of the hoopoe (Upupa epops) Appl Environ Microbiol. 2006;72:4245–4249. doi: 10.1128/AEM.02940-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Togawa Y, Shimosaka M, Okazaki M. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Appl Environ Microbiol. 2003;69:5746–5753. doi: 10.1128/AEM.69.10.5746-5753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobel JD. Bacterial vaginosis – an ecologic mystery. Ann Intern Med. 1989;111:551–553. doi: 10.7326/0003-4819-111-7-551. [DOI] [PubMed] [Google Scholar]
- 36.Pybus V, Onderdonk AB. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis. 1997;175:406–413. doi: 10.1093/infdis/175.2.406. [DOI] [PubMed] [Google Scholar]
- 37.Pybus V, Onderdonk AB. A commensal symbiosis between Prevotella bivia and Peptostreptococcus anaerobius involves amino acids: potential significance to the pathogenesis of bacterial vaginosis. FEMS Immunol Med Microbiol. 1998;22:317–327. doi: 10.1111/j.1574-695X.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 38.Pybus V, Onderdonk AB. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1999;1:285–292. doi: 10.1016/s1286-4579(99)80024-0. [DOI] [PubMed] [Google Scholar]
- 39.Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 40.Pavlova SI, Kiliç AO, Mou SM, Tao L. Phage infection in vaginal lactobacilli: an in vitro study. Infect Dis Obstet Gynecol. 1997;5:36–44. doi: 10.1155/S1064744997000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao L, Pavlova SI, Mou SM, Ma W, Kiliç AO. Analysis of Lactobacillus products for phages and bacteriocins that inhibit vaginal lactobacilli. Infect Dis Obstet Gynecol 1997. 1997;5:244–251. doi: 10.1155/S1064744997000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amsel R, Totten PA, Spiegel CA, Chen KCS, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 43.Pohunek M. Streptococci antagonizing the vaginal lactobacillus. J Hyg Epidemiol Microbiol Immunol. 1961;5:267–270. [PubMed] [Google Scholar]
- 44.James SM, Tagg JR. Streptococcal and enterococcal inhibition of endocervical lactobacilli. FEMS Microbiol Lett. 1987;41:321–326. [Google Scholar]
- 45.De Kwaadsteniet M, Fraser T, Van Reenen CA, Dicks LMT. Bacteriocin T8, a novel class IIa sec-dependent bacteriocin produced by Enterococcus faecium T8 isolated from vaginal secretions of children infected with human immunodeficiency virus. Appl Environ Microbiol. 2006;72:4761–4766. doi: 10.1128/AEM.00436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]