Abstract
Canine leptospirosis has been described as having re-emerged in North America around the mid-1990s, with a change in the epidemiology of the infecting serovars responsible for the disease emergence. A retrospective case-control study was conducted to examine the re-emergence of seroprevalent cases of canine leptospirosis in Ontario using serology submission records from 1406 dogs from January 1, 1998 to December 31, 2006. The data collected [results of the microscopic agglutination test (MAT), veterinary clinic postal code, age, sex, neutering status, and breed] were analyzed by multivariable logistic regression, generalized linear mixed modeling, and Cochran-Armitage test for trends in proportions. Dogs in urban areas appeared to be at significantly higher risk than dogs in rural areas for the entire study period [odds ratio (OR) = 1.6, confidence interval (CI) = 1.2–2.3], though this was not as marked as in other studies. Results indicated that canine leptospirosis in Ontario is a disease of all breeds and ages, regardless of gender. No geographic clustering was noted, but clustering of cases by clinic within geographic areas suggested differences in awareness or in diagnosis by veterinarians. A distinctive seasonal pattern of leptospirosis, with more cases occurring during the summer and fall, as found in previous studies, was also observed in this study. The temporal trend analysis was consistent with an increasing proportion or re-emergence of seroprevalent cases of canine leptospirosis since 1998, suggesting that the putative increase in canine leptospirosis has been genuine.
Résumé
La leptospirose canine est décrite comme étant en réémergence en Amérique du Nord depuis le milieu des années 1990, avec un changement dans l’épidémiologie des sérovars infectant responsables de l’émergence de la maladie. Une étude rétrospective de cas-témoin a été menée pour examiner la réémergence des cas séro-prévalents de leptospirose canine en Ontario en utilisant les données des demandes de sérologie de 1406 chiens pour la période allant du 1er janvier 1998 au 31 décembre 2006. Les données amassées [résultats de l’épreuve d’agglutination microscopique (MAT), le code postal de la clinique vétérinaire, l’âge, le sexe, l’état de stérilisation et la race] ont été analysées par régression logistique multivariée, modélisation linéaire mixte généralisée et test de Cochran-Armitage pour les tendances dans les proportions. Les chiens en régions urbaines ont semblé être à risque d’une manière significativement plus élevée que les chiens en régions rurales pour toute la durée de l’étude [rapports de cotes (OR) = 1,6, intervalle de confiance (CI) = 1,2–2,3], bien que ceci ne soit pas aussi marqué que dans d’autres études. Les résultats indiquent que la leptospirose canine en Ontario est une maladie de toutes les races et tous les âges, indépendamment du genre. Aucune grappe géographique n’a été notée, mais un regroupement des cas par clinique à l’intérieur d’une région géographique suggérait une différence dans la sensibilisation ou le diagnostic par les vétérinaires. Un patron saisonnier distinct des cas de leptospirose, plus de cas étant rencontrés à l’été et à l’automne, tel que retrouvé dans des études précédentes, a également été noté dans la présente étude. L’analyse de la tendance temporelle était en accord avec une augmentation des proportions ou une réémergence des cas séroprévalents de leptospirose canine depuis 1998, ce qui suggère que l’augmentation présumée de leptospirose canine est réelle.
(Traduit par Docteur Serge Messier)
Introduction
Canine leptospirosis is a bacterial zoonosis with worldwide distribution (1–4), causing renal and hepatic disease, coagulopathies and other abnormalities (5–7), with a case fatality rate of 10–20% in dogs (8–10). Leptospirosis is caused by pathogenic spirochetes once classified as the single species Leptospira interrogans (11), now subdivided into at least 16 genomospecies (4). Within the leptospires, there are at least 23 different serogroups and 218 serovars, which do not reliably relate to the causative genomospecies (2,4).
In North America, leptospires are maintained by a wide range of reservoir hosts without clinical signs or symptoms, including many wildlife sources such as skunks, raccoons, and rats, and domestic animal sources such as cattle, pigs and, historically, dogs (3,10). Reservoir hosts typically carry and transmit specific host-adapted serovar(s) (3,12). Transmission generally occurs after a susceptible animal is directly exposed to leptospires in an infected host’s urine or contaminated water, mud, or moist soil (4,7,12). In their reservoir host, leptospires escape the immune system in the proximal convoluted renal tubules allowing the infected animal to become a persistent shedder (13). Following shedding in urine, these bacteria may survive for some months given appropriately temperate moist or wet environments; however, survival is very poor in dry or cold environments (3,7).
Shedding animals pose a public health risk. Leptospires can be transmitted to humans through contact with urine-contaminated environments, particularly water. Human leptospirosis is usually associated with direct contact with shedding companion animals, occupational exposure (sewage and agricultural workers, veterinarians, for example) or outdoor recreational activities such as swimming, boating, or endurance competitions (3,12,14).
Historically, the serovars canicola and icterohaemorrhagiae were of major concern for dogs in North America, but there was a sharp decline in dogs infected with these serovars from the 1970s to the mid-1990s (9). This drop has been attributed to the introduction of an effective canine vaccine in the early 1970s (14,15). Since the mid-1990s, however, there have been many reports of the apparent re-emergence of canine leptospirosis associated with a change in the infecting serovars (9). The serovars most commonly reported since the re-emergence of leptospirosis have been serovars common to wildlife hosts, principally grippotyphosa and pomona (1,11). This prompted an introduction in 2001 of a canine vaccine, which contains serovars grippotyphosa and pomona in addition to canicola and icterohaemorrhagiae. It has been postulated that the re-emergence of leptospirosis and change in epidemiology of infecting serovars may be due to urbanization of rural areas in the 1980s and 1990s, which provided greater opportunity for contact between animals and wildlife reservoirs (11). However, an increased awareness and testing of dogs by veterinarians, and increased infection in raccoons as well as climate change might also be involved (15).
Investigations of the resurgence of canine leptospirosis revealed conflicting findings for risk factors such as age, sex, and breed of dogs (1,11,16). Conversely, environmental risk factors such as increased precipitation, warmer temperatures, and seasonality of cases have been fairly consistently identified (5,6,15–17).
The objectives of this study were: 1) to describe the current epidemiology of the serovars and seasonal trends of canine leptospirosis in Ontario; 2) to examine host risk factors; 3) to assess whether there is clustering of infected dogs; 4) and to examine if there has genuinely been a re-emergence of seroprevalent cases of leptospirosis in Ontario over the last decade.
Materials and methods
Data source
Microscopic agglutination test (MAT) results were selected from the Animal Health Laboratory (AHL) database at the University of Guelph from tests performed between January 1, 1998 and December 31, 2006. In accordance with international standards, serological positivity for leptospira antibodies was based on MAT titers with a value of 100 or greater as a positive titer cutoff (18). Serum samples were analyzed for agglutinating antibodies against 7 serovars: autumnalis, bratislava, canicola, grippotyphosa, hardjo, icterohaemorrhagiae, and pomona. Because only 13 of the 1406 samples were tested for the hardjo, the serovar was excluded from the analysis. Further information on age, sex, neutering status, breed, date of test result, and veterinary clinic postal code information was extracted from the AHL database.
Demographics
All cases and non-cases were analyzed for any differences in sex, neutering status, breed category, age, rural or urban environment, as well as veterinary clinic postal code. Sex was categorized as male or female. Neutering status was answered as either yes or no. Breed was categorized into 7 classes as defined by the Canadian Kennel Club (19), including: herding, hound, sporting, non-sporting, terrier, toy, working, along with 2 additional categories for mixed and dogs of unknown or unspecified breeds. Age of dogs was categorized into 4 groups: < 1 y, 1 to 3 y, 4 to 7 y, and ≥ 8 y. The rural and urban environments were classified by using the 2nd character of the postal code of the veterinary clinic from which the serum originated. If the number was a zero, the environment was specified as rural. If the number was 1 to 9, the environment was specified as urban (20).
Selection and exclusion criteria
Dogs were excluded from the study if the veterinary clinic postal code was not in Ontario. The first entry was retained for dogs that occurred more than once in the database during the study period and subsequent entries were excluded.
Data analysis
Test for trends in proportions
The proportion of leptospirosis cases and non-cases from 1998–2006 were compared using the Cochran-Armitage test for trends in proportions (21) to assess whether leptospirosis cases were increasing over the 8-year period. The test is designed to detect a linear trend in a response proportion. The slope was estimated using a weighted least squares method. A level of significance of α= 0.05 was used.
Logistic regression
The presence or absence of antibodies against leptospiras (yes/no) based on the MAT results for all dogs were analyzed using logistic regression. The effect of age category (< 1 y as the reference category), breed category (unknown or unspecified breeds as the reference category), neutering status (not neutered as the reference category), sex (males as the reference category), and rural versus urban (rural as the reference category) were included in the model to evaluate their association with the disease outcome.
All covariates were evaluated for significance (α = 0.10) individually using a univariable logistic regression. The significant covariates were included in the model. The remaining covariates were added using stepwise forward selection and evaluated using a likelihood ratio test (α = 0.05). If significant, the variable was added to the model; otherwise the covariates were excluded from the final model. All covariates excluded from the model were evaluated for confounding using a 30% or more change in the individual coefficient values. All models were compared using the Akaike Information Criterion (AIC) value. The model with the lowest AIC value was deemed to fit best. Deviance chi-squared goodness-of-fit test was used to assess the fit of the model using a significant (P < 0.05) result to indicate a poor fit. The model was assessed for influential observations and outliers using residuals versus fitted, normal Q-Q, scale-location, Cook’s distance, and residuals versus leverage plots.
Models were created for the entire study period from 1998 to 2006 as well as samples submitted from 1998 to 2000 and from 2001 to 2006 to evaluate differences in risk factors before and after the introduction of the canine vaccine containing grippotyphosa and pomona serovars in 2001. Odds ratios from the final models were estimated together with the respective 95% confidence intervals.
Generalized linear mixed modeling
The presence/absence of antibodies against leptospiras for all dogs was assessed using a generalized linear mixed modeling with normal random effects via Penalized Quasi-Likelihood methods (22). The fixed effects modeled were the same as the logistic regression models described previously. The veterinary clinic postal code was modeled as a random effect using the first 3 characters [so-called Canada Post Forward Sortation Areas (FSA)] of the veterinary clinic postal code to account for any spatial clustering or effects due to the environment in that location. The results of the mixed modeling were compared with that of the multivariable logistic regression model to identify any differences in coefficients and P-values of the independent variables.
Geographic clustering and cluster analysis
All case and non-case dogs were aggregated by the forward sortation area. The centroid of each FSA was geo-located in Cartesian coordinates. The data were smoothed using empirical Bayesian estimation. Moment estimators were used within a binomial model (23).
Moran’s I statistic (23) was calculated for the regional count data to evaluate the extent of spatial autocorrelation. This method is a spatially weighted form of the Pearson Correlation Coefficient, which summarizes the extent to which similar observations occur near each other over the entire study area (23). The neighborhood structure was determined using the first 2 neighboring FSA regions as determinants of adjacency. Moran’s I was then estimated using Empirical Bayes Index Modification of Moran’s I using random permutations.
The spatial dependence structure was then modeled using a semivariogram (23). The semivariogram cloud and empirical semivariogram were estimated using the smoothed data for the maximum distance of 400 km. An exponential semivariogram model was fit to the robust empirical semivariogram using maximum likelihood. Since the data had already been smoothed, no measurement error variance was expected and therefore the nugget effect was set to zero, and the trend in the data was specified as constant. By removing any other influences in the data, the true effect of the spatial correlation could be isolated. The empirical semivariogram estimation resulted in parameter values for the sill, which measures the overall variance in the data, and the range, which indicates the distance up to which spatial correlation is occurring.
Location and size of spatial clusters were then examined using a spatial scan test. The scan test is a likelihood ratio test, which is based on scanning windows of various sizes and positions and highlights the most likely clusters in the data. The regional count data was exported for use in SaTScan (24). A binomial model was used to investigate purely spatial clusters in the data. The maximum spatial cluster size was estimated to be 50% of the population at risk in order to capture all possible clusters of cases. Based on the statistically significant results from the spatial scan statistic (α = 0.05).
Statistical software
All data analysis was performed using the R statistical package (25) and SaTScan (24).
Results
Descriptive results
A total of 1406 dogs were selected for the study, with 802 (57%) case dogs, and 604 (43%) non-case dogs. The “non-case” dogs were dogs from which serum was submitted for diagnosis of canine leptospirosis but which had MAT titers to all serovars of < 100. For the 75 “non-case” dogs in which a second (“convalescent”) serum was tested by MAT, only 6 became positive, justifying the choice of this group as “non-cases.” With respect to the MAT titers of the 802 case dogs, the most strongly reacting serovars are shown in Table I.
Table I.
Most highly reacting leptospiral serovars for 802 dogs in Ontario from 1998–2006
| Highly reacting serovara | Number of cases |
|---|---|
| autumnalis | 251 (31.3%) |
| bratislava | 127 (15.8%) |
| grippotyphosa | 98 (12.2%) |
| icterohaemorrhagiae | 63 (7.9%) |
| canicola | 48 (6.0%) |
| pomona | 6 (0.75%) |
| Most strongly co-reactingb serovar | Number of cases |
| L. Aut/Grip | 37 (4.6%) |
| L. Aut/Bra | 31 (3.8%) |
| L. Aut/Bra/Grip | 20 (2.5%) |
| L. Bra/Grip | 19 (2.3%) |
| L. Aut/Ict | 15 (1.9%) |
| L. Aut/Can | 12 (1.5%) |
| L. Bra/Ict | 9 (1.1%) |
| L. Aut/Can/Grip | 8 (1.0%) |
| L. Aut/Pom | 8 (1.0%) |
| L. Grip/Ict | 7 (0.9%) |
| L. Can/Ict | 7 (0.9%) |
| L. Can/Grip | 7 (0.9%) |
| L. Aut/Grip/Ict | 5 (0.6%) |
| L. Aut/Bra/Can | 4 (0.5%) |
| L. Aut/Bra/Ict | 4 (0.5%) |
| L. Bra/Can | 3 (0.4%) |
| L. Aut/Bra/Pom | 3 (0.4%) |
| L. Bra/Grip/Ict | 2 (0.2%) |
| L. Aut/Grip/Pom | 2 (0.2%) |
| L. Can/Grip/Ict | 1 (0.1%) |
| L. Can/Grip/Pom | 1 (0.1%) |
| L. Bra/Can/Grip | 1 (0.1%) |
| L. Aut/Can/Pom | 1 (0.1%) |
| L. Bra/Pom | 1 (0.1%) |
“highly reacting serovar” = serovar with the highest titer as reported by the MAT.
“co-reacting serovar” = 2 or more serovars with equally high titers as reported by the MAT.
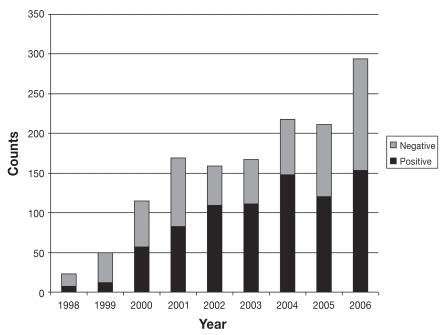
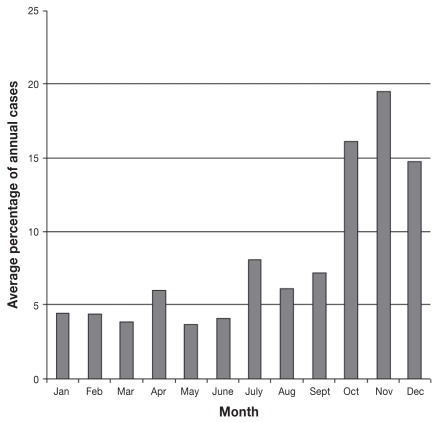
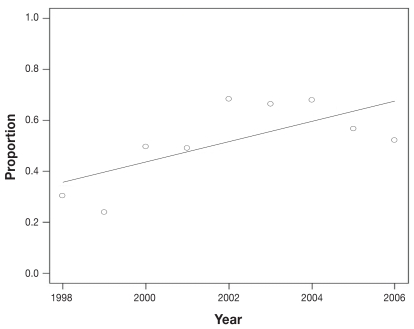
Temporal analysis from 1998 to 2006 showed an increasing trend in the number of submissions per year, from 23 submissions in 1998 to 294 submissions in 2006. The number of cases diagnosed each year also increased over the study period, from 7 cases diagnosed in 1998 to 153 cases diagnosed in 2006 (Figure 1). The monthly percentages of annual canine leptospirosis cases averaged over the study period (Figure 2) show a distinct seasonal pattern with an increased percentage of cases diagnosed from July to December. Results of the Cochran-Armitage test for trend in proportions indicate a significant increasing proportion of leptospirosis over the study period (P < 0.01) (Figure 3).
Figure 1.
Annual submission of samples for the microscopic agglutination test (MAT) and counts of positive and negative results for leptospirosis from January 1, 1998 to December 31, 2006.
Figure 2.
Monthly percentages of annual canine leptospirosis cases averaged over the years 1998–2006.
Figure 3.
Regression line showing the linear increase in the proportion of positive tested dogs as confirmed by the Cochran-Armitage trend test in Ontario 1998 to 2006.
Logistic regression
Results of the multivariable logistic regression (Table II) for samples submitted from 1998–2006 indicated that dogs had a statistically significant increased risk of leptospirosis if they were from an urban rather than a rural area (OR = 1.64, 95% CI = 1.17–2.29). No other variables were found to be significant or improve the fit when added to the model. The deviance goodness-of-fit test did not indicate serious model inadequacies (P = 0.48).
Table II.
Results of multivariable logistic regression for canine leptospirosis in Ontario in 1998–2006, 1998–2000, and 2001–2006
| Covariate | Coefficient | Odds ratio | P-value | 95% CI |
|---|---|---|---|---|
| Logistic regression model for 1998–2006 | ||||
| Urban | 0.4942 | 1.64 | 0.003 77 | 1.17–2.29 |
| Logistic regression model for 1998–2000 | ||||
| Herding breeds | −0.6624 | 0.52 | 0.225 950 | 0.17–1.49 |
| Hound breeds | −0.6624 | 0.52 | 0.440 294 | 0.08–2.78 |
| Mixed breeds | −0.7314 | 0.48 | 0.162 993 | 0.17–1.33 |
| Non-sporting breeds | 0.1643 | 1.18 | 0.789 719 | 0.35–4.06 |
| Sporting breeds | −2.1992 | 0.11 | 0.000 398 | 0.03–0.35 |
| Terrier breeds | −0.9343 | 0.39 | 0.206 187 | 0.08–1.62 |
| Toy breeds | −0.5978 | 0.55 | 0.441 525 | 0.11–2.53 |
| Working breeds | −0.9343 | 0.39 | 0.114 190 | 0.12–1.22 |
| Logistic regression model for 2001–2006 | ||||
| Urban | 0.6112 | 1.84 | 0.000 984 | 1.28–2.66 |
CI — confidence interval.
Results of the multivariable logistic regression (Table II) for MAT samples submitted from 1998–2000 found breed to be statistically significant. Specifically, dogs of the sporting breed classification (pointer, cocker spaniel, golden retriever, Irish setter, and vizsla) (19) were found to have a protective effect (OR = 0.11, 95% CI = 0.03–0.35) compared with unknown or unspecified breed classifications. No other variables were found to be significant or improve the fit when added to the model. The deviance goodness-of-fit test did not indicate serious model inadequacies (P = 0.31).
The multivariable logistic regression (Table II) for the time period from 2001–2006 again found that dogs from urban areas (OR = 1.84, 95% CI = 1.28–2.66) were, again, more likely to have leptospirosis than dogs from rural areas. No other variables were found to be significant or improve the fit when added to the model. The deviance goodness-of-fit test did not indicate serious model inadequacies (P = 0.48).
Generalized linear mixed modeling
The generalized linear mixed model analysis with veterinary clinic FSA as a random effect for samples submitted from 1998–2006, as well as before and after 2001, produced the same significant variables as the logistic regression models (Table III). For the study period 1998–2006, dogs were more likely to have leptospirosis if they were from urban areas (OR = 1.52, CI = 0.94–2.45) compared with dogs from rural areas, although this was of borderline statistical significance. Sporting breed classification was again protective (OR = 0.12, 95% CI = 0.04–0.42) compared with the unknown or unspecified breed class for the study period before 2001. Dogs with samples submitted from 2001 onwards were more likely to have leptospirosis if they were from urban areas (OR = 1.83, 95% CI = 1.17–2.86) compared with dogs from rural areas. When results of the generalized linear mixed model (Table III) were compared with the results of the logistic regression analyses (Table II), the individual coefficients changed only slightly. Overall, confidence intervals were mostly wider in the generalized linear mixed model, but most model variables remained statistically significant, except for the urban covariate in the mixed model from 1998–2006. The variance component of the random effect indicates evidence of clustering in the clinic FSA throughout the study period.
Table III.
Results of a generalized linear mixed modeling of canine leptospirosis in Ontario with veterinary clinic postal code modeled as the random effect in 1998–2006, 1998–2000, and 2001–2006
| Covariate | Coefficient | Odds ratio | P-value | 95% CI |
|---|---|---|---|---|
| Generalized linear mixed model for 1998–2006 | ||||
| Urban | 0.4177 | 1.52 | 0.0881 | 0.94–2.45 |
| Random effect variance = 0.272, residual = 0.988 | ||||
| Generalized linear mixed model for 1998–2000 | ||||
| Herding breeds | −0.5959 | 0.55 | 0.2922 | 0.19–1.64 |
| Hound breeds | −0.6299 | 0.53 | 0.4763 | 0.10–2.92 |
| Mixed breeds | −0.6694 | 0.51 | 0.2224 | 0.18–1.47 |
| Non-sporting breeds | 0.2429 | 1.27 | 0.7039 | 0.37–4.37 |
| Sporting breeds | −2.1028 | 0.12 | 0.0014 | 0.04–0.42 |
| Terrier breeds | −0.8194 | 0.44 | 0.2908 | 0.10–1.96 |
| Toy breeds | −0.4309 | 0.65 | 0.5973 | 0.13–3.13 |
| Working breeds | −0.8441 | 0.43 | 0.1703 | 0.13–1.40 |
| Random effect variance = 0.156, residual = 0.984 | ||||
| Generalized linear mixed model for 2001–2006 | ||||
| Urban | 0.6034 | 1.83 | 0.0084 | 1.17–2.86 |
| Random effect variance = 0.123, residual = 0.993 |
CI — confidence interval.
Geographic clustering and cluster analysis
The results of the Moran’s I statistic (not shown) give no evidence for spatial clustering. Results of the semivariogram modeling (not shown) indicate a range value of 0 that is an estimate as to maximum distance up to which 2 observations are spatially correlated. The results of the scan test do not identify any statistically significant disease clusters in Ontario.
Discussion
This study expands and extends the epidemiological aspects of an earlier description of the re-emergence of canine leptospirosis in Ontario (15). Importantly, the Cochran-Armitage trend test found an increasing trend of leptospirosis cases from 1998 to 2006 (Figure 3), supporting the many claims that leptospirosis is a re-emerging disease (15,16,26,27) rather than just being the result of increased awareness by veterinarians. Interestingly, also, the proportion of cases to non-cases peaked in 2002, but seems to have stabilized in the past 2 years at levels higher than that of the late 1990s (Figure 1). It is plausible that the stabilization might be the result of the use of a grippotyphosa and pomona vaccine that was licensed in Canada in 2001; however, vaccine status and usage data in the study population were not collected, therefore, no definitive conclusions can be made. There are no details available on the extent of immunization of dogs with the 4 serovar vaccines in Ontario. The introduction of canine vaccination with serovars canicola and icterohaemorrhagiae in the early 1970s was followed by a dramatic reduction in the disease (16). The apparent stabilization might also result from reduced use of laboratory confirmation of infection as more veterinarians become familiar with the disease and are more confident in their ability to make a diagnosis clinically rather than using expensive laboratory confirmation.
The Cochran-Armitage trend test is based on the relative changes in proportions of cases and non-cases over time, and detects a trend in the data. This assumes that the underlying reason for submission by the veterinary clinics throughout the study period was that the veterinarian suspected the dog had been exposed to leptospiras and was showing common signs and symptoms of acute leptospirosis. If there were changes in the reasons for submissions of samples during the study period (for example, veterinarians were screening all dogs in their clinics) the results of the trend test would be biased against a re-emergence. There were no data available to assess this possible bias in sample submissions for the study period.
Leptospirosis is a disease that is difficult to study using typical epidemiological approaches because of uncertainties surrounding this disease, and particularly because of problems associated with sample collection and testing. Many research studies, including this study, use serological test results to categorize dogs as either clinical cases or controls. This approach assumes that positive titers indicate acute infection, which is not always correct. Although high titers usually indicate recent exposure, titers of > 100 only indicate that dogs have been exposed, but do not indicate when this exposure occurred, and therefore may not represent acute disease. However, in this study we assume that veterinarians submitting serological samples for testing by the MAT are doing so because the dog is showing acute clinical signs of leptospirosis.
The gold standard for the MAT is based on paired serum collection. If a single titer is used for diagnosis, the interpretation can become complicated. Although the MAT is the most widely accepted standard test for canine leptospirosis, improper usage of the test may result in false-negative results and cross-reaction. False-negative results early in the course of the disease are common because it usually takes 5 to 7 d after onset of clinical symptoms for antibodies to develop (4). The sensitivity and specificity of the MAT also varies depending on the stage of the disease. In a recent study, McBride et al (28) found that if the MAT sample was taken during the first week of the illness, the sensitivity of the test was only 45.8–68.8%, although the specificity was 100%. However, when convalescent samples were taken, the sensitivity increased to 100% (95% CI = 91.1–100) and specificity was 100% (95% CI = 94.3–100). This may lead to misclassification, especially of “non-case” dogs, and demonstrates the importance of convalescent samples when diagnosing leptospirosis. In the AHL database only 75 dogs had 2 or more MAT tests completed. Furthermore, using a cutoff titer of 100 to distinguish cases from “non-cases” among those 75 dogs with multiple samples, only 6 (8%) of the dogs that were negative on their first test sero-converted to positive on their subsequent tests. This suggests that a titer of 100 is an appropriate cutoff value, and that only a small proportion of the “non-case” dogs were misclassified. A significant proportion of human patients with severe leptospirosis have been found to be sero-positive for several years after infection (29). That study found that more than 20% of cases infected with the serogroup autumnalis retained titers of > 800, 4 y after the acute illness. If this applies to dogs, this complicates the distinction between acute and sub-clinical cases.
The most consistent risk factor for leptospirosis in the study population was sociographical, with urban dogs being at higher risk for leptospirosis than rural dogs (Tables II, III). This may be due to the higher population density of infected raccoons in urban areas. Broadfoot et al (30) found that the raccoon and skunk populations are more concentrated in urban rather than rural areas. This suggests a greater risk of contact with such leptospiral carriers and, therefore, risk of leptospirosis for dogs as well as people living in urban areas. There is, however, a need to better understand the epidemiology of leptospirosis in urban wildlife in Ontario before one can confirm the suspected central role of raccoons rather than that of mice, skunks, squirrels, and other urban wildlife. The greater exposure in urban environments may reflect the higher density of people, dogs, raccoons, and veterinary clinics in urban settings. Although canine leptospirosis was found to be of higher risk in urban areas in this study, the disease is not solely an urban disease. It can be found throughout Ontario affecting all breeds, ages, and gender of dogs.
This study also examined spatial clustering of leptospirosis in Ontario. When the logistic regression analysis was compared with the generalized linear mixed model analysis, the variance component of the random effect was not equal to zero, indicating a clustering effect at the level of the veterinary clinic FSA. A spatial cluster analysis using Moran’s I statistic and the semivariogram to check for spatial clustering and the spatial scan statistic to detect disease clusters (23) revealed no spatial clustering of leptospirosis in Ontario during 1998–2006. Ward (6) found spatial clustering at a higher spatial aggregation level, with clusters identified in the mid-west of the United States as well as in Canada. The clustering found in the mixed model analysis (Table III) may be due to certain veterinary clinics with greater awareness of or more inclination to test for leptospirosis. This could cause a clustering effect of the disease at the clinic level. The present study suggests that canine leptospirosis is evenly distributed throughout Ontario; there is no indication for any disease clusters nor spatial trend. However there may be differences in awareness or diagnosis of the disease among veterinary practices.
The most strongly reacting serovars, those that showed the highest titers by the MAT, were autumnalis (31.3%), bratislava (15.8%), and grippotyphosa (12.2%). The results for this study are in agreement with previous investigations (1,11,31,32), except for the ordering of the most strongly reacting serovars. It has been suggested that the serovar bratislava is a host-adapted serovar to dogs (32). Prescott et al (33) found that 8.2% of dogs had antibodies to bratislava. Therefore, a small portion of the bratislava-positive dogs may have been due to a subclinical rather than acute leptospiral infection, causing the “true” infecting serovar to remain unknown. The present study found the serovar autumnalis as often the most strongly reacting serovar; autumnalis has not generally been included in the panel of serovars used for canine MAT in the United States (32,34). Furthermore, it has been suggested that autumnalis is a highly cross-reactive serovar (15,31), and that the high frequency of autumnalis positive dogs reflects cross-reaction, rather than an actual infecting serovar. For example, in our study, of the 209 dogs that had equally high titers to more than 1 serovar, 151 (72.2%) included the serovar autumnalis. It is a characteristic of the sera of dogs with acute leptospirosis that they show extensive cross-reactivity in the MAT (15,31), in part because of the high cross-reactivity of IgM, the immunoglobulin isotype typical of the acute serological responses. Cross-reactions are a common problem with the MAT, so that serologic analysis has little value in identification of the infecting serovar in human infection (35). For example, Kingscote (36) isolated serovar pomona from the kidneys of red foxes in southwestern Ontario but found that the foxes reacted most highly to serovar autumnalis. A recent study of MAT response of dogs to vaccination with serovars grippotyphosa and pomona described a dramatically higher and longer response to serovar autumnalis than to the immunizing serovars (37). It was, however, interesting that the frequency of sera reacting most strongly to serovar pomona was low compared with other studies, 0.75% compared to 40–44% reported by others (1,32). Since highest MAT titers in dogs cannot identify the infecting serovars with confidence, more elaborate diagnostic methods are required. It is misleading to attempt to define the influence of the infecting serogroup on the clinical features of leptospirosis in dogs (38). Although raccoons infected with serovar grippotyphosa are widely believed to be the major source of leptospires for urban dogs, they have been described in the past in Georgia, USA, as a host for serovar autumnalis (39).
Past research regarding risk factors for canine leptospirosis have resulted in varying conclusions. Ward et al (6) found that dogs of the herding, working, and gun-dog breed classes, as well as intact males, were significantly more likely to have leptospirosis than other dogs. These results differ markedly from the present study, which did not identify these as risk factors, and which found, for the study period before 2001, that the sporting breed class was less likely to have leptospirosis. The reason for this time-dependent breed risk is unclear. Although previous research in the USA suggested that large working and hunting dogs were at greater risk for leptospirosis presumably because they spend much of their time outdoors and thus are more exposed to leptospires (10,16,32), the current study suggests that all breed types (except sporting dogs) are at equal risk. This may reflect the greater likelihood of exposure in urban environments. Furthermore the present study could not confirm a predisposition based on gender classification. Although other studies have found various age effects, including an increased risk in older dogs, (16) or dogs aged < 1 y and/or > 8 y (1), this study did not identify age as a risk factor when age was evaluated as a continuous variable or as a categorical variable. Age was therefore left as a categorical variable for consistency with previous studies. In summary, with one apparently minor exception, dogs in Ontario appear to be at risk to leptospirosis independent of breed type, age, or gender.
The investigation of seasonality of canine leptospirosis confirmed a distinctive increased pattern of cases during the summer and especially in the fall (October to December), which is similar to what was observed in previous studies (late summer and fall) (5,15,40), but also indicated that leptospirosis could be diagnosed throughout the year. This is likely a result of environmental conditions favorable for the survival of these fastidious bacteria during the fall, but may also reflect to some extent the increased likelihood of time spent outdoors by dogs, and the possibly increased shedding of leptospiras by young wild animals coinciding with the higher numbers of young raccoons born in the spring and weaned from their mother during late summer. Future analyses could include analyzing the peak season of disease (October to December) in relation to the average rainfall and temperature in northern, southern, western, and eastern Ontario. This would help to determine if there is a correlation between cases of canine leptospirosis and local climate, as well as any differences in disease rates due to climate change over the study period.
This study has described the current situation and trends of canine leptospirosis in Ontario, which show some differences from similar studies in the United States. Dogs of all breeds, ages, and gender are susceptible. The seroprevalence of leptospirosis has increased since the late 1990s although incidence rates may be stabilizing, possibly due to vaccination. It is also apparent that there is a difference between clinics in their suspicion and/or diagnosis of leptospirosis.
Acknowledgments
This work was supported by the Ontario Veterinary College Pet Trust Fund and by Wyeth Animal Health.
We thank Dr. Beverly McEwen for her assistance with the data extraction from the AHL database and Brandon Geer for his work and research with the geographical data files.
References
- 1.Ghneim GS, Viers JH, Chomel BB, Kass PH, Descollonges DA, Johnson ML. Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet Res. 2007;38:37–50. doi: 10.1051/vetres:2006043. [DOI] [PubMed] [Google Scholar]
- 2.Farr RW. Leptospirosis. Clin Infect Dis. 1995;21:1–20. doi: 10.1093/clinids/21.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bharti A, Nally J, Ricaldi J, et al. Leptospirosis: A zoonotic disease of global importance. Lancet. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 4.Levett P. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward MP. Seasonality of canine leptospirosis in the United States and Canada and its association with rainfall. Prev Vet Med. 2002a;56:203–213. doi: 10.1016/s0167-5877(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 6.Ward MP. Clustering of reported cases of leptospirosis among dogs in the United States and Canada. Prev Vet Med. 2002;56:215–226. doi: 10.1016/s0167-5877(02)00160-5. [DOI] [PubMed] [Google Scholar]
- 7.Andre-Fontaine G. Canine Leptospirosis-Do we have a problem? Vet Microbiol. 2006;117:19–24. doi: 10.1016/j.vetmic.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Harkin KR, Gartrell CL. Canine leptospirosis in New Jersey and Michigan: 17 cases (1990–1995) J Am Anim Hosp Assoc. 1996;32:495–501. doi: 10.5326/15473317-32-6-495. [DOI] [PubMed] [Google Scholar]
- 9.Bolin CA. Diagnosis of leptospirosis: A reemerging disease of companion animals. Semin Vet Med Surg (Small Anim) 1996;11:166–171. doi: 10.1016/s1096-2867(96)80029-6. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum N, Barr SC, Center SA, Schermerhorn T, Randolph JF, Simpson KW. Naturally acquired leptospirosis in 36 dogs: Serological and clinicopathological features. J Small Anim Pract. 1998;39:231–236. doi: 10.1111/j.1748-5827.1998.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 11.Ward MP, Guptill L, Prahl A, Wu C. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997–2002) J Am Vet Med Assoc. 2004a;224:1958–1963. doi: 10.2460/javma.2004.224.1958. [DOI] [PubMed] [Google Scholar]
- 12.Ward MP, Guptill L, Wu C. Evaluation of environmental risk factors for leptospirosis in dogs: 36 cases (1997–2002) J Am Vet Med Assoc. 2004b;225:72–77. doi: 10.2460/javma.2004.225.72. [DOI] [PubMed] [Google Scholar]
- 13.Wohl JS. Canine Leptospirosis. Compendium. 1996;18:1215–1222. [Google Scholar]
- 14.Higgins R. Emerging or re-emerging bacterial zoonotic diseases: Bartonellosis, leptospirosis, Lyme borreliosis, plague. Rev Sci Tech Off Int Epiz. 2004;23:569–581. doi: 10.20506/rst.23.2.1503. [DOI] [PubMed] [Google Scholar]
- 15.Prescott JF, McEwen B, Taylor J, Woods P, Abrams-Ogg A, Wilcock B. Resurgence of leptospirosis in dogs in Ontario: Recent findings. Can J Vet Res. 2002;43:995–961. [PMC free article] [PubMed] [Google Scholar]
- 16.Ward MP, Glickman LT, Guptill LF. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998) J Am Vet Med Assoc. 2002;220:53–58. doi: 10.2460/javma.2002.220.53. [DOI] [PubMed] [Google Scholar]
- 17.Prescott JF, Key D, Osuch M. Ontario: Leptospirosis in dogs. Can J Vet Res. 1999;40:430–431. [PMC free article] [PubMed] [Google Scholar]
- 18.Organisation Mondiale de la Sante Animale (OIE), Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Leptospirosis. [Last accessed May 18, 2009];2006 Available from http://www.oie.int/eng/normes/mmanual/A_00043.htm.
- 19.Canadian Kennel Club Inc. [Last accessed May 18, 2009];Breeds and Abbreviations [Web Page] 2007 Available from http://www.ckc.ca/en/ Canadian Kennel Club Home Page.
- 20.Mechanda K, Puderer H. How Postal Codes Map to Geographic Areas. Geography Working Paper Series, Statistics Canada. 2007 Feb;:1–43. Catalogue no. 92F0138MIE. [Google Scholar]
- 21.Armitage P, Berry G, Matthews J. Statistical Methods in Medical Research. 4th ed. Oxford: Blackwell Scientific; 2002. pp. 227–231. [Google Scholar]
- 22.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- 23.Durr P, Gatrell A. GIS and Spatial Analysis in Veterinary Science. 1st ed. Oxfordshire, United Kingdom: CABI; 2004. pp. 76–77.pp. 130pp. 217–218. [Google Scholar]
- 24.Kulldorff M Information Management Services, Inc. SaTScanTM v7.0: Software for the spatial and space-time scan statistics. http://www.satscan.org/2006.
- 25.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2006. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- 26.Langston C, Heuter K. Leptospirosis a re-emerging zoonotic disease. Vet Clin North Am Small Anim Pract. 2003;33:791–807. doi: 10.1016/s0195-5616(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 27.Meites E, Jay M, Deresinski S, et al. Reemerging leptospirosis, California. Emerg Infect Dis. 2004;10:406–412. doi: 10.3201/eid1003.030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride A, Santos B, Queiroz A, et al. Evaluation of four whole-celled Leptospira-based serological tests for diagnosis of urban leptospirosis. Clin Vaccine Immunol. 2007;14:1245–1248. doi: 10.1128/CVI.00217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cumberland P, Everard CO, Wheeler JG, Levett PN. Persistence of anti-leptospiral IgM, IgG and agglutinating antibodies in patients presenting with acute febrile illness in Barbados 1979–1989. Eur J Epidemiol. 2001;17:601–608. doi: 10.1023/a:1015509105668. [DOI] [PubMed] [Google Scholar]
- 30.Broadfoot JD, Rosatte RC, O’Leary DT. Raccoon and skunk population models for urban disease control planning in Ontario, Canada. Ecol Appl. 2001;11:295–303. [Google Scholar]
- 31.Moore GE, Guptill LF, Glickman NW, Caldanaro RJ, Aucoin D, Glickman LT. Canine Leptospirosis, United States, 2002–2004. Emerg Infect Dis. 2006;12:501–503. doi: 10.3201/eid1203.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adin CA, Cowgill LD. Treatment and outcome of dogs with leptospirosis: 36 cases (1990–1998) J Am Vet Med Assoc. 2000;216:3371–3375. doi: 10.2460/javma.2000.216.371. [DOI] [PubMed] [Google Scholar]
- 33.Prescott JF, Ferrier RL, Nicholson VM, Johnston KM, Hoff B. Is canine leptospirosis underdiagnosed in southern Ontario? A case report and serological survey. Can J Vet Res. 1991;32:481–486. [PMC free article] [PubMed] [Google Scholar]
- 34.Rentko VT, Clark N, Ross LA, Schelling SH. Canine leptospirosis: A retrospective study of 17 cases. J Vet Intern Med. 1992;6:235–244. doi: 10.1111/j.1939-1676.1992.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 35.Levett P. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. 2003;36:447–452. doi: 10.1086/346208. [DOI] [PubMed] [Google Scholar]
- 36.Kingscote BF. Leptospirosis in red foxes in Ontario. J Wildlife Dis. 1986;22:475–478. doi: 10.7589/0090-3558-22.4.475. [DOI] [PubMed] [Google Scholar]
- 37.Barr SC, McDonough PL, Scipioni-Ball RL, Starr JK. Serologic responses of dogs given a commercial vaccine against Leptospira interrogans serovar Pomona and Leptospira kirschneri serovar grippotypohsa. Am J Vet Res. 2005;66:1780–1784. doi: 10.2460/ajvr.2005.66.1780. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein RE, Lin RC, Langston CE, Scrivani PV, Erb HN, Barr SC. Influence of infecting serovar on clinical features of leptospirosis in dogs. J Vet Intern Med. 2006;20:489–494. doi: 10.1892/0891-6640(2006)20[489:ioisoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.McKeever S, Gorman GW, Galton MM, Hall AD. The raccoon, Procyon lotor, a natural host of Leptospira autumnalis. Am J Hyg. 1958;68:13–14. doi: 10.1093/oxfordjournals.aje.a119945. [DOI] [PubMed] [Google Scholar]
- 40.Miller RI, Ross SP, Sullivan ND, Perkins NR. Clinical and epide-miological features of canine leptospirosis in North Queensland. Aust Vet J. 2007;85:13–19. doi: 10.1111/j.1751-0813.2006.00089.x. [DOI] [PubMed] [Google Scholar]