Abstract
Sarcocystis neurona is the principal etiologic agent of equine protozoal myeloencephalitis (EPM). An immunodominant protein of S. neurona, SnSAG-1, is expressed by the majority of S. neurona merozoites isolated from spinal tissues of horses diagnosed with EPM and may be a candidate for diagnostic tests and prophylaxis for EPM. Five horses were vaccinated with adjuvanted recombinant SnSAG1 (rSnSAG1) and 5 control (sham vaccinated) horses were vaccinated with adjuvant only. Serum was evaluated pre- and post-vaccination, prior to challenge, for antibodies against rSnSAG1 and inhibitory effects on the infectivity of S. neurona by an in vitro serum neutralization assay. The effect of vaccination with rSnSAG1 on in vivo infection by S. neurona was evaluated by challenging all the horses with S. neurona merozoites. Blinded daily examinations and 4 blinded neurological examinations were used to evaluate the presence of clinical signs of EPM. The 5 vaccinated horses developed serum and cerebrospinal fluid (CSF) titers of SnSAG1, detected by enzyme-linked immunosorbent assay (ELISA), post-vaccination. Post-vaccination serum from vaccinated horses was found to have an inhibitory effect on merozoites, demonstrated by in vitro bioassay. Following the challenge, the 5 control horses displayed clinical signs of EPM, including ataxia. While 4 of the 5 vaccinated horses did not become ataxic. One rSnSAG-1 vaccinated horse showed paresis in 1 limb with muscle atrophy. All horses showed mild, transient, cranial nerve deficits; however, disease did not progress to ataxia in rSnSAG-1 vaccinated horses. The study showed that vaccination with rSnSAG-1 produced antibodies in horses that neutralized merozoites when tested by in vitro culture and significantly reduced clinical signs demonstrated by in vivo challenge.
Résumé
Sarcocystis neurona est le principal agent étiologique de l’encéphalomyélite équine à protozoaire (EPM). Une protéine immunodominante de S. neurona, SnSAG-1, est exprimée par la majorité des mérozoïtes de S. neurona isolés de tissus de la moelle épinière de chevaux avec un diagnostic d’EPM et pourrait être un candidat pour des tests diagnostiques et la prophylaxie de l’EPM. Cinq chevaux ont été vaccinés avec une protéine SnSAG1 recombinante avec adjuvant (rSnSAG1) et 5 chevaux témoins (faux vaccinés) ont été vaccinés avec de l’adjuvant seulement. Des échantillons de sérum prélevés pré-et post-vaccination, avant l’infection, ont été testés pour la présence d’anticorps dirigés contre rSnSAG1 et les effets inhibiteurs sur l’infectivité de S. neurona par une épreuve in vitro de séro-neutralisation. Les effets de la vaccination avec rSNsAG1 sur l’infection in vivo par S. neurona ont été examinés en infectant tous les chevaux avec des mérozoïtes de S. neurona. Des examens quotidiens à l’aveugle et 4 examens neurologiques à l’aveugle ont été effectués pour évaluer la présence de signes cliniques d’EPM. Les 5 chevaux vaccinés ont développé des anticorps sériques et dans le liquide céphalo-rachidien (CSF) post-vaccination contre SnSAG1, détectés par essai immuno-enzymatique (ELISA). Le sérum post-vaccination provenant des chevaux vaccinés avait un effet inhibiteur sur les mérozoïtes tel que démontré par un bio-essai in vitro. Suite à l’infection, les 5 chevaux témoins ont montré des signes cliniques d’EPM, incluant de l’ataxie. Quatre des 5 chevaux vaccinés ne sont pas devenus ataxiques. Un des chevaux vaccinés avec rSnSAG-1 a montré de la parésie à un membre avec atrophie musculaire. Tous les chevaux ont montré des déficits légers et transitoires des nerfs crâniens; toutefois, la condition n’a pas progressé jusqu’à l’ataxie chez les chevaux vaccinés avec rSnSAG-1. Cette étude a démontré que la vaccination des chevaux avec rSnSAG-1 a induit des anticorps qui neutralisaient les mérozoïtes lorsque testés par culture in vitro et a réduit de manière significative les signes cliniques tel que démontré par une infection défi in vivo.
(Traduit par Docteur Serge Messier)
Introduction
There are several pathogenic protozoa associated with neurological disease found in the central nervous system (CNS) of horses, including Sarcocystis, Neospora, and Toxoplasma (1–5). Neosporosis is uncommon in horses (6). Toxoplasmic encephalomyelitis is also, surprisingly, rarely reported since the identification of S. neurona as the etiologic agent of equine protozoal myeloencephalitis (EPM) in horses. Equine protozoal myeloencephalitis is acquired when horses ingest feed material contaminated with fecal matter containing sporocysts shed by opossum (7). Sarcocystis neurona can be isolated from the CNS of afflicted horses and is often diagnosed (8–11). A review of the literature indicated that S. neurona has been recovered from the CNS of horses more than a dozen times, but this probably underestimates the success of organism recovery. The majority of merozoites that were recovered by in vitro culture from the CNS of horses express SnSAG1, as demonstrated by the presence of a 29 to 30 kDa antigen on immunoblot or molecular identification of SnSAG1 gene by sequence identification. However, one atypical S. neurona that was isolated from the CNS of a Missouri horse was determined to lack SnSAG1 (12).
Only the SnSAG1 containing merozoites of S. neurona have been shown to experimentally reproduce EPM in the horse and demonstrate the presence of the organism in neural tissues by in vitro isolation (10). Repeated uses of S. neurona sporocysts derived from opossums have not been successful in producing EPM or locating the organism in the CNS (13–15). Isolation of the organism from the CNS using a sporocyst infection challenge model remains elusive. In one such study, Heskett et al (16) concluded that this experimental challenge may not reliably result in CNS infection. Liang et al (17) proposed serum neutralization assays to demonstrate inhibition of S. neurona entry into host cells in vitro and concluded that 2 low molecular weight proteins may be important in invasion and immunity. It was previously determined that the SnSAG1 protein of S. neurona was an immunodominant surface protein that can be of diagnostic value in naturally occurring cases of EPM (18,19). Therefore, this study examined the effects of increased antibodies against rSnSAG1 in horse serum by in vitro bioassay and in vivo infection challenge using the merozoite model.
Materials and methods
Animals
Ten quarter horse/paint weanlings less than 6 mo old (4 fillies, 6 colts) were selected based on clinically normal neurological examinations and absence of antibodies against S. neurona in both the serum and cerebrospinal fluid (CSF). Animals were housed in a 3-acre grass field and supplemented with a concentrate and hay ration. All animals were pre-conditioned for 2 wk. The animals were Coggins tested and assigned a number. Water was provided ad libitum. Prior to the study all animals were dewormed with ivermectin (Equell; Pfizer, Fort Worth, Texas, USA) or fendbendazole (Panacur; Intervet, Millsboro, Deleware, USA) and vaccinated against eastern and western encephalitis, West Nile virus, influenza, and rhinopneumonitis (Encephalo/West Nile Fort Dodge, Fort Dodge, Iowa, USA; Calvenza Boeringer, St. Joseph, Missouri, USA). Two sentinel animals were housed in a pen in the center of the 3-acre field and 6 animals on an adjacent 3-acre field on the farm remained normal for the duration of the study. Study animals were randomly assigned to a treatment schedule by a statistician who was not involved in conducting clinical evaluations. Blinding of the study was possible since all animals were evaluated by personnel who did not have knowledge of the treatment group. The evaluations were done by veterinarians qualified to examine neurological lameness in horses. The investigators complied with all federal, state, and local laws; ordinances; rules; and regulations pertaining to animal welfare and use of experimental material.
Vaccination
The horses were vaccinated on days 0 and 21 with 1 mL adjuvanted (Polygen; MVP Laboratories, Omaha, Nebraska, USA) rSnSAG1 (50 μg) or 1 mL adjuvant alone by IM injection in the left side of the neck.
Challenge infection
Horses in all groups were challenged on study day 36 with S. neurona merozoites, as previously reported (10). The SnSAG1 merozoite strain was isolated from the spinal tissues of a horse that had been diagnosed with EPM and was maintained in continuous culture since the isolation. Blood was obtained from each animal by jugular venipuncture into an EDTA tube. Each tube was inoculated with 6000 S. neurona culture derived merozoites, the EDTA tubes were vented using the inoculating needle and the tubes placed in a atmosphere overnight. The following 37°C incubator with 5% CO2 day the infected autologous blood was injected via jugular venipuncture into the horses. Horses were challenged for 14 consecutive days.
Clinical pathology data
Serum and CSF samples were collected on days 0, 91, and 119. Serum only was collected on days 35 and 49. Serum chemistry and complete blood (cell) counts (CBC) were evaluated prior to the study on days -14, 49, and 119.
Neurologic examinations
All horses were examined daily for the duration of the study. Beginning on day -14 (2 wk before vaccination) appetite, behavior, lameness, ataxia, and neurologic deficits were monitored. Gait was examined at a walk, trot, and free in the paddock. Scores were assigned for ataxia or paresis and graded (Table I).
Table I.
Definitions for the examination of clinical signs score assignment
| Clinical signs | Description | Weighted score |
|---|---|---|
| 1. eating, drops feed | ≥ 1/4 lb | 1 |
| ≤ 1/2 lb | 2 | |
| ≥ 1/2 lb | 3 | |
| 2. tongue tone decreased | normal mastication | 1 |
| abnormal mastication | 2 | |
| paresis | 3 | |
| 3. abnormal feed prehension | unable to chew | 1 |
| bites food | 2 | |
| 4. head | drools when eating | 1 |
| drools continuously | 2 | |
| 5. lip paresis | perceptible | 1 |
| perceptible continuously | 2 | |
| lip hangs | 3 | |
| 6. facial nerve paresis | perceptible | 1 |
| moderate | 2 | |
| severe | 3 | |
| 7. eyelid paresis | ventrally away from eye | 1 |
| over 1/4 eye | 2 | |
| with corneal lesion | 3 | |
| 8. laryngoscope exam | displaced palate | 1 |
| pharyngeal collapse | 2 | |
| laryngeal hemiplegia | 3 | |
| 9. muscle atrophy | just perceptible | 1 |
| immediately noticeable | 2 | |
| severe atrophy | 3 | |
| 10. cauda equina | holds tail rigid | 1 |
| with dribbling urine | 2 | |
| rectum paretic | 3 | |
| 11. attitude | depressed | 1 |
| aggressive | 2 | |
| somnolent | 3 | |
| 12. weakness | mild | 1 |
| parked out stance | 2 | |
| recumbent 30% of day | 3 | |
| 13. trips | sometimes | 1 |
| often | 2 | |
| often falls to knees | 3 | |
| 14. lameness | just noticed at walk | 1 |
| head bob or hip drop at walk | 2 | |
| reluctant to use limb | 3 | |
| 15. ataxia/paresis, Grade | normal gait | 0 |
| ataxia detected at a walk | 1 | |
| ataxia easily detected at walk, exaggerated by backing, turning in a tight circle, and walking with the head elevated | 2 | |
| prominent ataxia at a normal gait | 3 | |
| stumbling, difficulty maintaing balance, falls or nearly falls at a normal gait | 4 | |
| recumbent, unable to rise | 5 |
Neurologic status was evaluated on days 0, 63, 91, and 119 and documented on a neurologic examination form. These examinations were made by at least 2 blinded veterinarians, the principal investigator (veterinarian A) and a consulting veterinarian (veterinarian B). A 3rd veterinarian (veterinarian C) also observed the horses. To determine progression and severity of disease, other localizing neurological signs, such as lameness, weakness on tail pull, body condition, muscle atrophy, cutaneous sensation, and cranial nerve deficits, were recorded under daily observations and are shown as a cumulative score on the day neurological examinations were conducted. The clinical signs used in the cumulative score were weighted according to the scale shown in Table I. The parameters used to assign grade of ataxia or paresis are shown in row 15 of Table I.
Enzyme-linked immunosorbent assays
The serum and CSF immunoglobulin (Ig)G titers were obtained using rSnSAG1 in an indirect ELISA (19).
Statistical analysis
The horse was included as the experimental unit. Given the small size of this study, results were considered statistically significant when P = 0.10. Wilcoxon’s rank sum test (NPAR1WAY, SAS; SAS Institute, Cary, North Carolina, USA) was used to evaluate the effect of vaccination on the cumulative clinical score (the primary outcome variable). The total daily clinical score post-challenge was calculated (the sum of 15 clinical observations), and then the cumulative clinical score was calculated as the sum of the total daily clinical scores (90 d of total scores). The mitigated fraction was estimated and 90% confidence intervals generated. In addition, the total daily clinical scores were evaluated using computer software (GLIMMIX, SAS; SAS Institute). Vaccine group, day post-challenge, and the interaction between group and day were evaluated. If the interaction was significant (P < 0.10), within day treatment effects were evaluated. The ELISA values were reported on multiple days post-challenge; therefore, a repeated measures analysis replaced the one-way ANOVA. All values were log transformed prior to the statistical analysis. Values reported as < X or ≥ Y were imputed as X or Y. Since all pre-vaccination values were same, no covariate was included in the statistical model. Observations reported on the neurological forms were summarized.
Serum neutralization assay
Pre-vaccination and post-vaccination serum samples were collected, aliquoted, and stored frozen. Serum samples were thawed at room temperature for analysis. Fetal calf serum was used as a control. Quadruplicate sets of serum samples were serially diluted 1:2 in classical cell culture medium described by Roswell Park Memorial Institute (RPMI) without serum in a 96-well plate with a final volume of 100 μL. Sarcocystis neurona merozoites were harvested from 18 d infected bovine cells (ATCC CRL 6053) by scraping the monolayer and centrifuging the infected cells at 6000 rpm in a microfuge. The pellet was resuspended in sterile water and passed through a 22-gauge needle to disrupt host cells. The merozoites were diluted with 50 volumes of RPMI and counted by hemocytometer. Approximately 1000 merozoites/100 μL were added to each well resulting in final serum dilutions from 1:4 to 1:512. The serum/merozoite plates were incubated at 37°C for 60 min. After incubation, 200 μL of 1.4 × 103 irradiated mouse fibroblasts (ATCC 48-X, 3T3) were added to each well. Eight wells with no serum and 8 wells with no parasites on each plate served as controls. The plates were incubated and observed daily for lysis of the monolayer. The last well that showed host cell lysis was recorded as the endpoint and indicated S. neurona infections. A tissue culture infective dose at 50% (TCID50) was recorded as the titer (reciprocal of the last serum dilution) from the average of duplicate wells showing complete lysis of the monolayer.
Results
During the entire observation period prior to challenge, the horses were clinically normal. There was no immediate or delayed hypersensitivity, either local or systemic reactions, post-vaccination. Possible clinical signs post-vaccination included injection site reactions, abscesses, lameness, anorexia, neck stiffness, and depression.
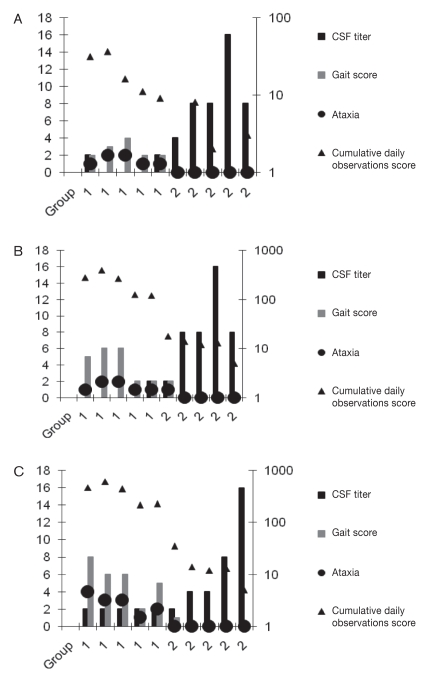
Neurological signs consistent with those of EPM appeared as early as 6 d (day 58) after last day of challenge (day 49). The cumulative daily observations are shown in Figure 1. Cumulative clinical scores were significantly higher in the control group compared to the rSn-SAG1 vaccinated group (Table II, P = 0.0090). The cumulative clinical scores for all 5 vaccinated horses were lower than those for the unvaccinated horses. The algorithm (GLIMMIX, SAS; SAS Institute) used would not converge when the total daily clinical scores were evaluated (Figure 2). Given this result, another more appropriate method (MIXED, SAS; SAS Institute) was used to analyze the total daily clinical scores. The vaccine group by day interaction was statistically significant (P < 0.0001), leading to an evaluation of within day group effects. Vaccinated horses had significantly lower (P < 0.05) total daily clinical scores than the control animals from days 61 to 90.
Figure 1.
Neurologic scores and titers for the control group (1) and rSAG-1 vaccinated group (2) on study day 64 (A), 91 (B), and 125 (C). Note that the cerebrospinal fluid (CSF) titer, gait score, and ataxia are plotted on the left axis; the cumulative daily observations score was plotted on a logarithmic scale, right axis.
Table II.
Summary of the evaluation of the cumulative clinical scores
Cumulative clinical score was calculated as the sum of 15 daily scores summed over 90 d of post-challenge observations.
Wilcoxon’s rank sum test.
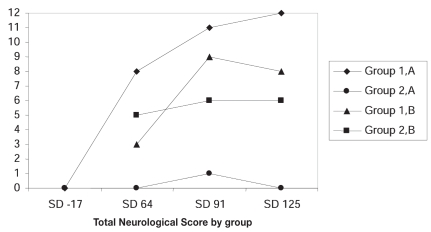
Figure 2.
Graph showing the trend in neurological examination scores in control horses and vaccinated horses — veterinarian A and veterinarian B. Veterinarian B did not evaluate the horses on study day -17.
At day 64, 5 of 5 animals from the control group developed ataxia and signs consistent with EPM. The EPM assessment figure (Figure 1) indicates the highest score for ataxia observed for each horse during the observation period. As days post-challenge increased, the horses in the control group became more ataxic and showed progressive signs of EPM, while the rSnSAG1 vaccinated group had only 1 horse with mild lameness and paresis in 1 limb at day 91 (Figure 1B). The paresis and spasticity in this horse did not progress and began to improve by day 110. By the end of the study, day 125, (Figure 1C) the horse showed mild paresis, no lameness, and some muscle atrophy of the shoulder (scores compared to baseline).
On day 69, horses were observed by veterinarian C. He noted comments on the gait at a walk for each animal. In the vaccinated horses, veterinarian C found the gaits to be normal (n = 5), while ataxia (n = 4) or hypermetria in the rear limbs (n = 1) was present in the control horses. Abnormal mentation was present in 2 horses (animal 5 and 8); both were control horses.
Neurological examinations were conducted by veterinarian B (blinded), after the challenge. Veterinarian B did not evaluate the horses prior to the challenge and only viewed the horses while performing the neurological examinations. Because no baseline was established for gait, the data was used to determine a trend observed by each examiner. For each group the total examination scores were added together and graphed. In the unvaccinated horses, the majority of the horses became progressively worse, while in the vaccinated group, the horses were clinically unchanged (Figure 2).
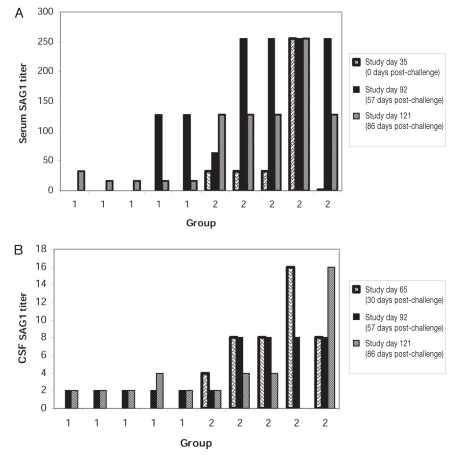
The SnSAG1 ELISA titers are shown in Figure 3. All horses were seronegative and CSF negative for antibodies to rSAG1 prior to vaccination. Serum samples were collected on pre-study day -17, and days 35, 49, 91, and 121. Challenge began at day 36. The CSF samples were collected pre-study day -17, and days 63, 91, and 121. The horses in the control group were seronegative for antibodies to rSnSAG1 on day 49, antibodies were detected at day 91 (55 d post-challenge). Antibodies to S. neurona, measured by SnSAG1 ELISA, were detected in the CSF by day 91 after challenge, in all the horses. The presence of CSF antibodies indicated that all of the control horses were successfully challenged. In the rSnSAG1 vaccinated horses, the CSF SnSAG1 titers were greater than the corresponding SnSAG1 serum titers at day 91 (55 d post-challenge) for 3 horses. Because the titers were higher in the CSF than in the serum, this strongly suggests that there was intrathecal antibody production. If the serum titers had been equivalent or greater than the CSF titers, this would suggest that the antibodies in the CSF had crossed into the CNS from the peripheral circulation. The SnSAG1 ELISA values were reported on multiple days post-challenge; therefore, a repeated measures analysis replaced the one-way ANOVA. Since all pre-vaccination values were the same, no covariate was included in the statistical model. The interaction of vaccine group and study day was not statistically significant (Table III, P = 0.7370) for serum SnSAG1 titers, therefore, the main effect of vaccine was evaluated. The rSnSAG1 vaccinated animals had significantly higher serum titers than controls (Table III, P = 0.0005). The vaccine group by study day interaction was statistically significant for this outcome (Table III, P = 0.0021). Therefore, within study day group effects were evaluated. The rSnSAG-1 vaccinated horses had higher CSF titers on day 63 and day 91 (30 and 56 days post-challenge, respectively), as well as on day 121 (86 days post-challenge) (Table III, P = 0.0926) than controls.
Figure 3.
(A) Serum and (B) cerebrospinal fluid (CSF) SAGI ELISA results for control horses and vaccinated horses. Serum and CSF titers were 0 at selection for horses. The CSF was not measured on study day 35 (day of challenge).
Table III.
(A) Summary of the statistical evaluation of SAG-1 titers (P-values). (B) Summary of the statistical evaluation of geometric means
| A | |||
|---|---|---|---|
| Outcome | Vaccine effect | Study day effect | Vaccine × study day |
| Serum titersa | 0.0005 | 0.0037 | 0.7370 |
| CSF titersa | 0.0006 | 0.5112 | 0.0021 |
| B | |||
| Outcome | Control | Vaccinate | P-value |
| Serum titersb | 7.29 | 92.63 | 0.0005 |
| Cerebrospinal fluid titers | |||
| Post-challenge day 29 | 1.00 | 8.00 | < 0.0001 |
| Post-challenge day 56 | 2.00 | 6.10 | 0.0025 |
| Post-challenge day 85 | 2.30 | 4.00 | 0.0926 |
Titers were log transformed prior to the statistical analysis.
Main effect means (pooled across study days) are presented since the interaction between vaccination group and study day was not statistically significant.
The results of the serum neutralization assays are presented in Table IV. Inhibitory activity for the growth of S. neurona merozoites using irradiated 3T3 cells was observed in the post-vaccination sera, but not in the pre-vaccination sera obtained from the same animal.
Table IV.
Serum neutralization titers pre- and post-vaccination. Pre- and post-vaccine sera were evaluated for the ability to neutralize Sarcocystis neurona infections in vitro. The last dilution of serum showing no parasite growth in 50% of wells containing irradiated mouse fibroblasts was considered the TCID50. The lowest dilution was 1:4 for all sera tested
| Horse ID | Pre-vaccine TCID50 | Post-vaccine TCID50 |
|---|---|---|
| 1 | < 4 | 32 |
| 3 | < 4 | 64 |
| 6 | < 4 | 128 |
| 7 | < 4 | 64 |
| 9 | < 4 | 32 |
Discussion
As previously reported with this challenge model, S. neurona merozoites induce ataxia in 100% of the challenged horses (n = 4) by day 60 post-challenge. Further use of this model has shown that 91.2% of horses (n = 74) develop EPM (data not shown) (10). The theory that the distribution of parasites to the CNS causing disease is via a parasitemia, with the parasite residing in leukocytes, is supported by the equine model used in this study. Interestingly, horses that lack lymphocytes, severe combined immunodeficiency (SCID) foals develop parasitemia without succumbing to the neurological form of sarcocystosis when fed sporocysts (20,21). The merozoite model has been successfully reproduced using the same infection challenge dose, methods, and the same evaluation protocols at Virginia Maryland Regional College of Veterinary Medicine (Witonsky, personal communication 2007). Utilizing this model, we demonstrated that weanlings, which were vaccinated twice with 50 μg of rSnSAG1 vaccine prior to S. neurona challenge, had a reduced incidence of ataxia and paresis while horses from the control group developed ataxia following challenge. As the number of days post-challenge increased, the horses in the control group showed progressive signs of EPM, while the rSnSAG1 vaccinated group had only 1 animal with non-progressive lameness and paresis in 1 limb. Extensive neurological examinations are not exact between veterinarians; however, these observations were corroborated by veterinarian C on day 69. Veterinarian B did not observe the horses prior to the challenge, thus compromising the statistical evaluation of the neurological examinations. The observations by this blinded veterinarian stated that 60% of the rSnSAG1 vaccinated animals observed remained clinically unchanged over the course of this study, while 60% of the control horses had notable progressive disease (Grade 2 or greater ataxia).
Gait abnormalities (often associated with ataxia, paresis, or lameness) are the dominant clinical signs observed in natural cases of EPM and these gait deficits have been used to evaluate experimentally induced disease. However, this model demonstrates that ataxia is not the first indication of infection. There are other observed signs that are potentially important in the evaluation of horses with EPM (22). Therefore, to accurately and quantitatively detect neurologic deficits associated with this disease, other parameters, in addition to the ataxia score, were assessed at intervals throughout the study period. Localizing signs, weighted by degree in order to assess the severity of disease and allow the objective comparison of the horses between days and treatment groups, were used. This average daily score proved useful to monitor disease in individual horses. Some signs, which were present in both treatment groups shortly after challenge, spontaneously resolved in the vaccinated animals. One probable explanation for this is the presence of the organism in the CNS and the inflammatory and immune responses to S. neurona caused these signs. The ability of the vaccine to prevent acute signs of EPM prior to eliciting protective immune mechanisms against S. neurona was not possible using this model. Due to the nature of the challenge model, merozoites are protected until they cross the blood brain barrier. It is expected that natural exposure to S. neurona sporocysts and resulting progression of infection allows sufficient time and exposure of the organism to host immune mechanisms prior to a resulting parasitemia. This may allow an opportunity for the hosts’ protective immunity to quell the infection prior to S. neurona’s entry into the CNS. We hypothesize that in the vaccinated horses the organisms can still rapidly invade the CNS as they are sheltered in host leukocytes initially. However, S. neurona may have been unable to establish infection in the CNS because immune responses by the vaccinated horse controlled disease. The ability of serum from vaccinated horses to neutralize infections in vitro may indicate that antibodies produced in response to rSnSAG1 are involved in disease resolution.
There may be a local inflammatory response associated with the infection. This, combined with the resulting immune response, could produce the observed signs. It is possible that indirect effects of antibody mediated immunity modulated the inflammatory responses to infection. Vaccine effects could include reduction of an exuberant inflammatory response by stimulating a weak response, regulation of inflammatory cytokines, or promotion of opsonins (23). Antibodies that promote or maintain a state of latency have been shown to contribute to protective responses in other apicomplexan infections (23). The unvaccinated horses developed progressive neurologic disease consistent with EPM. In this case, we believe that the CNS signs observed early in the infection could be due to infection and, possibly, the corresponding host immune response. However, as the immune response in an unvaccinated animal did not control the infection, the disease progressed. In 1 horse that received the recombinant vaccine, clinical signs of lameness and paresis with mild muscle atrophy were observed. This animal was scored as ataxic by veterinarian B, but not ataxic by veterinarians A or C. The disease EPM is a result of the interaction between the host and the parasite; it is possible that individual, unknown host factors, such as the genetic background of the horse, contributed to the signs seen in this horse. Investigation of the responses to vaccination followed by challenge in more horses will be required to differentiate the contributions to pathogenesis of disease by host factors, parasite factors, or both.
Sarcocystis neurona is unique in its ability to produce neurologic disease. Sarcocystis neurona may have a predilection for the nervous system or, possibly, EPM is an indirect effect of an exuberant inflammatory response. The mediators of inflammation can be major contributors to host cell damage and it is possible there is an antibody mediated reduction of exuberant inflammatory response in the CNS of rSnSAG1 vaccinated horses. Protective immunity in a natural definitive host after sarcocystis infections has been reported (24). Immune mechanisms stimulated by S. neurona are not yet defined. Because the horse is an unnatural host for S. neurona, the protective immune responses responsible for resolution of disease are not necessarily expected to mimic response to disease in a natural host, which, in sarcocystosis, is generally a non-inflammatory, muscular disease.
The horse is an accidental or aberrant host for S. neurona (25). It is likely horses that are exposed to S. neurona through exposure to sporocyst-infected opossum feces do not always succumb to disease. Sporocyst challenge studies have shown horses do not succumb readily to CNS disease when experimentally challenged by using S. neurona sporocysts orally. In sporocyst challenge models, and in natural disease, there are several confounding factors that should be considered. The first confounding factor in the assessment of protozoa isolated from the CNS of horses is the isolation procedure. The ability to detect S. neurona by isolation in tissue culture is an art that can be further complicated by mixed infections. If a mixed infection from a horse was appreciated, developmental differences in in vitro growth between the strains could complicate interpretation of the isolation results. An example of development differences between 2 species carried by the opossum was published by Lindsay et al (26) in which the comparative development of 2 isolates in cultured cells was reported. Since both parasites can be isolated in several cell lines commonly used for protozoa isolation, it is anticipated that one species will overtake the culture, but this may not necessarily be the virulent species. This phenomenon could account for differences between SN6 at isolation, such as the presence of a 30 kDa protein on immunoblot and pathogenicity for immunodeficient mice, but the absence of these traits when evaluated after continued passage in culture (27,28). It might be possible that there were genotype and consequent phenotype changes with continued culture, but, more likely, is the selection of one organism over the 8 mo culture period. We have maintained the Florida isolate in continuous culture for more than 6 y with no genotype or phenotype changes. It remains highly virulent for horses and expresses SnSAG1.
Howe et al (29) examined isolates of putative S. neurona or neurona-like organisms to assess conservation of the gene family of surface antigens expressed by merozoites of S. neurona. They hypothesize that heterogeneity in the surface antigen composition determined by molecular analysis may extend to other phenotypes, such as strain virulence. Possibly, non-pathogenic species may be present and isolated from the CNS of a horse but not responsible for disease. The ultimate test is to show that the strain lacking SnSAG1 isolated from a horse can induce disease; this has not been shown for in vitro isolated organisms such as Sn4 or SnMu-1. Mixed infections are likely. Antibodies produced against a SnSAG1 containing S. neurona were shown by Hyun et al (30) to react with merozoites from the Missouri horse from which SnMu-1 was isolated and Marsh et al (12) reported that intensity of staining varied in the presence of reducing agents using SnMu-1. Further, the serum from this Missouri horse reacted with S. falcatula suggesting that a multiple infection was possible (12). Until more is known about the ability of SAG-minus or neurona-like organisms to cause EPM by experimental evaluation, biological assays, such as pathogenicity in immunodeficient mice, pathogenicity in budgies, growth in selected host cells, ability to produce EPM in the horse, along with molecular assays, are important to differentiate S. neurona that is pathogenic for horses from at least 3 additional Sarcocystis that use the opossum as a definitive host.
Although this was a small study, the results, the presence of antibodies to an immunodominant surface antigen of virulent S. neurona showed a vaccine response by assessing clinical scores and prevention of ataxia, are encouraging. Further evaluation in a larger group of horses is ongoing.
References
- 1.Beech J, Dodd DC. Toxoplasma-like encephalomyelitis in the horse. Vet Pathol. 1974;11:87–96. doi: 10.1177/030098587401100110. [DOI] [PubMed] [Google Scholar]
- 2.Boy MG, Galligan DT, Divers TJ. Protozoal encephalomyelitis in horses: 82 cases (1972–1986) J Am Vet Med Assoc. 1990;196:632–634. [PubMed] [Google Scholar]
- 3.Dubey JP, Davis GW, Koestner A, et al. Equine encephalomyelitis due to a protozoan parasite resembling Toxoplasma gondii. J Am Vet Med Assoc. 1974;165:249–255. [PubMed] [Google Scholar]
- 4.Dubey JP, Davis SW, Speer CA, et al. Sarcocystis neurona n. sp. (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeloencephalitis. J Parasitol. 1991;77:212–218. [PubMed] [Google Scholar]
- 5.Cheadle M, Lindsay D, Rowe S, et al. Prevalence of antibodies to Neospora sp. in horses from Alabama and characterization of an isolate recovered from a naturally infected horse. Int J Parasitol. 1999;29:1537–1543. doi: 10.1016/s0020-7519(99)00140-x. [DOI] [PubMed] [Google Scholar]
- 6.Hoane JS, Gennari SM, Dubey JP, et al. Prevalence of Sarcocystis neurona and Neospora spp. infection in horses from Brazil based on presence of serum antibodies to parasite surface antigen. Vet Parasitol. 2006;136:155–159. doi: 10.1016/j.vetpar.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Fenger CK, Granstrom DE, Langemeier JL, et al. Identification of opossums (Didelphis virginiana) as the putatuive definitive host of Sarcocystis neurona. J Parasitol. 1995;8:916–919. [PubMed] [Google Scholar]
- 8.Dubey JP, Davis SW, Speer CA, et al. Sarcocystis neurona n. sp. (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeloencephalitis. J Parasitol. 1991;77:212–218. [PubMed] [Google Scholar]
- 9.Bowman DD, Cummings JF, Davis SW, et al. Characterization of Sarcocystis neurona from a thoroughbred with equine protozoal myeloencephalitis. Cornell Vet. 1992;82:41–52. [PubMed] [Google Scholar]
- 10.Ellison SP, Greiner E, Brown K, Kennedy T. Experimental infection of horses with S. neurona merozoites as a model for Equine Protozoal Myeloencephalitis. J App Res Vet Med. 2004;2:79–89. [Google Scholar]
- 11.Granstrom DE, Alvarez JO, Dubey JP, et al. Equine protozoal myelitis in Panamanian horses and isolation of Sarcocystis neurona. J Parasitol. 1992;78:909–912. [PubMed] [Google Scholar]
- 12.Marsh A, Johnson P, Ramos-Vara J, et al. Characterization of a Sarcocystis neurona isolate from a Missouri horse with equine protozoal myeloencephalitis. Vet Parasitol. 2001;95:143–154. doi: 10.1016/s0304-4017(00)00386-1. [DOI] [PubMed] [Google Scholar]
- 13.Saville WJ, Stich RW, Reed SM, et al. Utilization of stress in the development of an equine model for equine protozoal myelo-encephalitis. Vet Parasitol. 2001;95:211–222. doi: 10.1016/s0304-4017(00)00421-0. [DOI] [PubMed] [Google Scholar]
- 14.Sofaly CD, Reed SM, Gordon JC, et al. Experimental induction of equine protozoan myeloencephalitis (EPM) in the horse: effect of Sarcocystis neurona sporocysts inoculation dose on the development of clinical neurologic disease. J Parasitol. 2002;88:1164–1170. doi: 10.1645/0022-3395(2002)088[1164:EIOEPM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Saville WJ, Sofaly CD, Reed SM, et al. An equine protozoal myeloencephalitis challenge model testing a second transport after inoculation with Sarcocystis neurona sporocysts. J Parasitol. 2004;90:1406–1410. doi: 10.1645/GE-128R. [DOI] [PubMed] [Google Scholar]
- 16.Heskett KA, Mackay RJ. 9 Antibody index and specific antibody quotient in horses after intragastric administration of Sarcocystis neurona sporocysts. Am J Vet Res. 2008;69:403. doi: 10.2460/ajvr.69.3.403. [DOI] [PubMed] [Google Scholar]
- 17.Liang F, Granstrom D, Zhao X, et al. Evidence that surface proteins Sn14 and Sn16 of Sarcocystis neurona merozoites are involved in infection and immunity. Infection and Immunity. 1998;66:1834–1838. doi: 10.1128/iai.66.5.1834-1838.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellison SP, Omara-Opyene AL, Yowell CA, Marsh AE, Dame JB. Molecular characterization of a major 29 kDa surface antigen of Sarcocystis neurona. Int J Parasitol. 2002;32:217–225. doi: 10.1016/s0020-7519(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 19.Ellison SP, Kennedy T, Brown K. Development of an ELISA to detect antibodies to rSNSAG1 in the horse. J App Res Vet Med. 2003;1(4):318–327. [Google Scholar]
- 20.Sellon DC, Knowles DP, Greiner EC, et al. Infection of immunodeficient horses with Sarcocystis neurona does not result in neurologic disease. Clin Diagn Lab Immunol. 2004;11:1134–1139. doi: 10.1128/CDLI.11.6.1134-1139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long MT, Mines MT, Knowles DP, et al. Sarcocystis neurona: parasitemia in a severe combined immunodeficient (SCID) horse fed sporocysts. Exp Parasitol. 2002;1003:150–154. doi: 10.1016/s0014-4894(02)00012-7. [DOI] [PubMed] [Google Scholar]
- 22.Ellison SP, Kennedy T, Brown K. Early signs of equine protozoal myeloencephalitis. J App Res Vet Med. 2003;1(4):272–278. [Google Scholar]
- 23.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 24.Fayer R, Dubey JP. Protective immunity against clinical sarcocystosis in cattle. Vet Parasitol. 1984;15:187–201. doi: 10.1016/0304-4017(84)90071-2. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay DS, Thomas NJ, Dubey JP. Biological characterization of Sarcocystis neurona isolated from a Southern sea otter (Enhydra lutris nereis) Int J Parasitol. 2000;30:617–624. doi: 10.1016/s0020-7519(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay D, Dubey J, Horton K, et al. Development of Sarcocystis falcatula in cell cultures demonstrates that it is different from Sarcocystis neurona. Parasitol. 1999;118:227–233. doi: 10.1017/s003118209800376x. [DOI] [PubMed] [Google Scholar]
- 27.Dubey J, Mattson D, Speer C, et al. Characteristics of a recent isolate of Sarcocystis neurona (SN7) from a horse and loss of pathogenicity of isolates SN6 and SN7 by passages in cell culture. Vet Parasitol. 2001;95:155–166. doi: 10.1016/s0304-4017(00)00387-3. [DOI] [PubMed] [Google Scholar]
- 28.Dubey J, Mattson D, Speer C, et al. Characterization of a Sarcocystis neurona isolate (SN6) from a naturally infected horse from Oregon. J Euk Microbiol. 1999;46:500–506. doi: 10.1111/j.1550-7408.1999.tb06067.x. [DOI] [PubMed] [Google Scholar]
- 29.Howe DK, Gaji RY, Marsh AE, et al. Strains of Sarcocystis neurona exhibit differences in their surface antigens, including the absence of the major surface antigen snSNSAG1. Int J Parasitol. 2008;38:623–631. doi: 10.1016/j.ijpara.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Hyun C, Gupta GD, Marsh AE. Sequence comparison of Sarcocystis neurona surface antigen from multiple isolates. Vet Parasitol. 2003;112:11–20. doi: 10.1016/s0304-4017(02)00392-8. [DOI] [PubMed] [Google Scholar]