Abstract
Pasteurella multocida toxin (PMT) is a poor antigen that becomes more immunogenic after its native structure has been destroyed. In contrast, partially truncated PMT proteins, which are predicted to be good antigens when used as a vaccine, might be used to improve the control of atrophic rhinitis in pigs. In this study, 4 truncated PMT fragments were expressed in Escherichia coli, and those 4 fragments were inoculated into mice to produce the polyclonal antibodies. The results of an enzyme-linked immunosorbent assay (ELISA) revealed that #1 and #4 fragments were the most immunogenic. Immunized mice were subsequently challenged intraperitoneally with P. multocida type D. Five of the eight #1 fragment-immunized mice showed some protection against death and bacterial clearance. Pigs immunized with #1 fragment produced no or mild atrophic rhinitis (turbinate conchal score) after challenge, suggesting that this #1 fragment could be a good candidate for a subunit recombinant-type vaccine.
Résumé
La toxine de Pateurella multocida (PMT) est un antigène peu efficace qui devient plus immunogène après que sa structure native ait été détruite. De manière contrastante, des protéines PMT partiellement tronquées qui sont sensées être de bons antigènes lorsque utilisées comme vaccin, peuvent être utilisées pour améliorer la réduction de la rhinite atrophique chez le porc. Dans la présente étude, 4 fragments tronqués de PMT ont été exprimés chez E. coli, et ces 4 fragments ont été inoculés chez des souris afin de produire des anticorps polyclonaux. Les résultats d’une épreuve immuno-enzymatique (ELISA) ont révélé que les fragments 1 et 4 étaient les plus immunogènes. Des souris immunisées ont par la suite été inoculées par voie intra-péritonéale avec P. multocida type D. Cinq des 8 souris inoculées avec le fragment #1 ont montré un certain degré de protection contre la mort et de la clearance bactérienne. Les porcs immunisés avec le fragment #1 n’ont manifesté aucune ou une faible rhinite atrophique (pointage des cornets) après l’infection défi, suggérant ainsi que le fragment #1 pourrait être un bon candidat pour un vaccin recombinant sous-unitaire type.
(Traduit par Docteur Serge Messier)
Introduction
Pasteurella multocida-induced pneumonia and progressive atrophic rhinitis (PAR) are common diseases in grower-finisher pigs, causing growth retardation and a reduction in the efficiency of feed utilization (1,2). The P. multocida toxin (PMT), which is encoded by the toxA gene, is produced by some P. multocida serotype A and D strains (3). Pasteurella multocida toxin is a major virulence factor associated with porcine atrophic rhinitis (AR), which is a respiratory infection that is characterized by the loss of the nasal turbinate bones, a twisting or shortening of the snout, and an inhibition of osteoblast differentiation and bone formation (4–6). Highly toxic to animals, PMT is lethal to mice after intraperitoneal inoculation causing dermonecrotic skin lesions in mice or guinea pigs that have been injected intradermally (7). The intraperitoneal introduction of PMT into pigs causes proliferative changes in the epithelium of the bladder wall and ureter (4,8). A similar effect was also observed after a nasal infection of gnotobiotic pigs with a toxigenic P. multocida strain (9).
Pasteurella multocida toxin is a poor antigen that becomes more immunogenic after its native structure has been destroyed (10). Even PMT has been shown to inhibit the humoral immune response and reduce the antibody level in mice and swine (6,11). In contrast, partial truncated proteins have been predicted to be good antigens (12). In a previous study, vaccination of sows with a mixture of 3 recombinant fragments of the PMT fragments with/without P. multocida bacterin produced high levels of neutralizing antibody and protection of offspring against a PMT challenge (12). However, more detailed studies will be needed to determine the region of PMT essential for the protection. A vaccination using PMT-lacking P. multocida types has not been successful against PMT-harboring P. multocida types, A and D (10). This suggests that the presence of PMT is essential for protection and pathogenicity. Previously, intraperitoneal immunization with a C-terminal recombinant PMT fragment in Freund’s incomplete adjuvant produced high levels of anti-PMT antibodies and protected the mice from an intraperitoneal challenge with PMT (13). However, no detectable antibodies (IgM, IgA, or IgG) to PMT were induced by an intranasal inoculation with the recombinant Bordetella bronchiseptica expressing C-terminal 685 amino acids region of PMT (14). Until recently, however, the immunogenicity of the whole or partial fragments of PMT was not completely understood. This study examined the region of the immunogenic fragment of PMT through the expression of partially truncated fragments for further protection studies. To accomplish this, a mouse and pig protection study was carried out using 4 truncated fragments of PMT.
Materials and methods
Escherichia coli strains and plasmids
The E. coli strains, JM109 and BL21(DE3)pLysS, were purchased from Invitrogen (Carlsbad, California, USA). The pRSET vector (Invitrogen), which is described elsewhere (15), was used to produce the hexahistidine-tagged protein. The pGEM-T Easy vector (Promega, Madison, Wisconsin, USA) was used for PCR cloning. The E. coli manipulations were performed according to the manufacturer’s instructions. The standard DNA and protein manipulations were carried out as described elsewhere (16,17).
Construction of truncated protein expression vectors
The P. multocida type D strain was originally obtained from the National Veterinary Research and Quarantine Service, Korea. The whole toxA gene was amplified by PCR using P. multocida genomic DNA as a template with the 5′-primer containing a BamHI restriction site and a 3′-primer containing a KpnI restriction site (Table I). The amplified DNA product was cloned into pGEM-T Easy, to generate pGEM-toxA. The toxA gene was divided into 4 fragments according to its hydrophilicity. The 1st fragment (toxA #1) encompassing the amino acids 1–390, the 2nd (toxA #2) 391–470, the 3rd (toxA #3) 471–920, and the 4th (toxA #4) 921–1285 were constructed and expressed. Briefly, the coding regions of the #1, #2, #3, and #4 fragments of the toxA gene were amplified using pGEM-toxA, as the template, with the 5′-primers containing a BamHI restriction site and 3′-primers containing a KpnI restriction site (Table I). The PCR products were purified using a PCR purification kit (Qiagen, Hilden, Germany), digested with two restriction enzymes (BamHI and KpnI), and then cloned into BamHI + KpnI digested pRSET to generate pRSET-toxA #1, pRSET-toxA #2, pRSET-toxA #3, and pRSET-toxA #4. The constructs were transformed into JM109 cells, and the plasmids were prepared as described previously. The inserted sequences of each construct were confirmed by DNA sequencing. The constructs were then transformed into the E. coli BL21(DE3) pLysS host cells once the pRSET-toxA #1, #2, #3, and #4 sequences had been verified. Recombinant proteins were expressed by adding IPTG (final conc. 1 mM), and purified using a Probond purification system (Invitrogen) according to the manufacturer’s instructions. The protein concentration was then measured using a protein assay kit (Bio-Rad Protein Assay, Bio-Rad, Hercules, California, USA).
Table I.
Nucleotide sequences of the primers used in this study (GenBank reference number: AF240778)
| Primer | Nucleotide sequence | Restriction enzyme |
|---|---|---|
| ToxA F | 5′-ATATGGATCCaATGAAAACAAAACATTTTTT-3′ | BamHI |
| ToxA R | 5′-TGTGGGTACCTTATAGTGCTCTTGTTAAGC-3′ | KpnI |
| ToxA #1F | 5′-GCGCGGATCCATGAAAACAAAACATTTTTT-3′ | BamHI |
| ToxA #1R | 5′-ATATGGTACCTTAGAGTAATGAAAGAGCATAGT-3′ | KpnI |
| ToxA #2F | 5′-ATAAGGATCCATGGAAACCTTTATTTCACAGTT-3′ | BamHI |
| ToxA #2R | 5′-ATATGGTACCTTATCTAGAGAAAGAAGATAAAA-3′ | KpnI |
| ToxA #3F | 5′-ATAA GGATCCATGACAGAAGAAGATATTCCAGC-3′ | BamHI |
| ToxA #3R | 5′-ATAT GGTACCTTAACTTTTTGAAGAGTTTTGGA-3′ | KpnI |
| ToxA #4F | 5′-ATAAGGATCCATGATTGACTTTTTCCTAAATAA-3′ | BamHI |
| ToxA #4R | 5′-ATATGGTACCTTATAGTGCTCTTGTTAAGC-3′ | KpnI |
Underlined: restriction sites.
Mice immunization
Polyclonal IgG inductivity was interpreted as the immunogenicity of each fragment. The polyclonal antibodies were produced by immunizing eight 8-week-old ICR mice (Charles River, Yokohama, Japan) subcutaneously with each of the purified recombinant proteins (100 μg per mouse in 100 μL PBS with 100 μL complete Freund’s adjuvant). This was followed by 2 more booster injections of incomplete Freund’s adjuvant at 2-week intervals. The control group was immunized with the PBS and adjuvant only. Blood samples were collected from the tail vein 7 days after the final booster inoculation and used for the enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with each of the recombinant proteins (1 μg/well/90 μL). The plates were then blocked with PBS containing 1% skim milk and washed with PBST (PBS + 0.05% Tween-20, pH 7.4). The sera for IgG analysis were prepared at 1:100 dilutions (dilution factor optimized previously). The antibody samples in 90 μL PBST were incubated for 1 h at 37°C, emptied, and washed with PBST 3 times. The bound IgG was detected using goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated (Pierce, Rockford, Illinois, USA) diluted 1:500. The secondary antibody was incubated for 1 h at 37°C, emptied, and washed 3 times with PBST.
The presence of any bound secondary antibody was determined using a substrate solution [100 mM citric acid buffer, pH 4.0, 10 mL; ABTS stock solution (ABTS 100 mg in 4.5 mL DW) 250 μL; H2O2 50 μL]. The plates were developed in the dark at room temperature for 15 min. The absorbance at 405 nm was read using an ELISA reader (Multiskan EX; Thermo LabSystems, Beverly, Massachusetts, USA). The results are expressed as the mean ± standard deviation (s) of the OD values.
Intraperitoneal inoculation of bacteria and bacterial recovery
After evaluating the immunogenicity of each truncated fragment of the PMT, a bacterial challenge study was undertaken to evaluate the protective efficacy of a recombinant protein vaccination. Pasteurella multocida type D was subcultured in the brain heart infusion (BHI) broth and the bacterial suspension was prepared at a concentration of 1 × 108 cells/mL. Eight mice from each of the 5 groups (total of 40 mice), which had been immunized with or without (control) each of the truncated proteins, were inoculated intraperitoneally with volumes of 100 μL (1 × 107 cells/mouse corresponding to LD100) of the bacterial suspension. The mortality rate was recorded over a 7-day period after inoculation. All surviving mice were sacrificed, and the peritoneal fluid was prepared by injecting 500 μL of PBS into the peritoneum and the resulting fluid was collected. The number of bacteria recovered was determined by culturing serially diluted peritoneal fluid on chocolate agar. The data is expressed as the mean of the number of bacteria (±s).
Pig immunization and challenge
The protective efficiency of the recombinant #1 fragment of PMT was also evaluated in pigs. Briefly, fifteen 19- to 20-day-old pigs (weaned, from nonvaccinated sows) were randomly assigned into 5 groups. Before immunization, blood samples were collected to check the presence of maternal anti-PMT antibodies, which could be delivered from maternal colostrum. Pigs in groups 1 and 2 were immunized intramuscularly with PBS, while groups 3 and 4 were immunized with recombinant #1 fragment protein (200 μg per pig in 200 μL PBS with 200 μL complete Freund’s adjuvant). This was followed by two more booster injections of incomplete Freund’s adjuvant at 2-week intervals. Group 5 served as a negative control, which received neither vaccination nor challenge. Blood samples were collected from the ear vein 7 days after the final booster inoculation and used for the ELISA assay as described previously. For this, goat anti-pig IgG HRP-conjugated (Pierce) diluted 1:500 was used as a secondary antibody. After immunization, pigs in group 1 and 3 were challenged with wild-type (W/T) P. multocida bacterial lysate (from 1 × 109 cells) intraperitoneally as described elsewhere (18), while groups 2 and 4 were challenged with live W/T P. multocida (1 × 109 cells per pig). Four weeks later, pigs from all 5 groups were euthanized and all snouts were sectioned transversely in the region of the first premolar teeth. Each scroll of the nasal conchal atrophy was graded from 0 (normal) to 4 (complete atrophy) as described elsewhere (19). Intraperitoneal inspection was also performed to find any pathological changes after challenge.
Statistical analysis
If not indicated separately, the significance of variation among different groups was determined by one-way analysis of variance (ANOVA) analysis and the difference among groups was determined by Dunnett multiple comparisons test using GraphPad Instat Software 3.05 (GraphPad software, La Jolla, California, USA).
Results
ELISA for IgG titration in mice
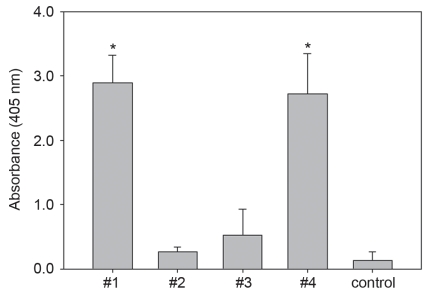
Four recombinant fragments of PMT were successfully produced in E. coli. The molecular weight of each of the 4 fragments was 45, 8, 51, and 40 kDa, respectively (data not shown). The recombinant proteins (100 μg of each of the 4 proteins per mouse) were administered, and the immunogenicity was analyzed by ELISA. An analysis of the IgG levels against each fragment in the sera demonstrated that the mice immunized with the #1 and #4 fragments had significantly higher OD values (5.2- to 11.2-fold) (Figure 1, P < 0.01). The #2 and #3 fragments failed to induce any noticeable IgG, which indicates these 2 fragments to be nonimmunogenic.
Figure 1.
The results of the ELISA study compared with the IgG induction. Mouse sera (1:100 diluted) were used as the primary antibodies from mice immunized with each PMT fragment. Compared with the others, a statistically significant increase in IgG induction was observed in the samples that had been immunized with the toxA #1 and #4 fragment (* P < 0.01), indicating these 2 fragments to be immunogenic.
Mortality and bacterial recovery
The protective efficacy of recombinant protein immunization was examined. Table II shows a lower mortality in the #1 fragment-immunized mice. Five out of 8 (62.5%) mice, which were immunized with the #1 fragment, were protected from the symptoms of the disease and remained alive after being challenged with 1 × 107 of the P. multocida type D cells, whereas the #2, #3, #4, and control mice given the same challenge dose died during the 7-day observation period. The surviving mice were sacrificed, and the amount of bacteria recovered was measured. However, none of the peritoneal fluid obtained from the surviving mice (#1 fragment-immunized) tested positive for P. multocida. This indicates that the inoculated bacteria were cleared and a neutralizing antibody had been produced.
Table II.
Effects of the partial truncated protein vaccination against an experimental infection of mice with wild-type Pasteurella multocida type D
| Groups | Number of mice | Mortality (%) | P. multocida recovery |
|---|---|---|---|
| #1 fragment-immunized | 8 | 37.5* | 0/5a |
| #2 fragment-immunized | 8 | 100 | N/Ab |
| #3 fragment-immunized | 8 | 100 | N/Ab |
| #4 fragment-immunized | 8 | 100 | N/Ab |
| Control | 8 | 100 | N/Ab |
Survived.
Not available.
Two-sided P-value is 0.4267, which is not significant on Fisher’s exact test.
Pig immunization and challenge
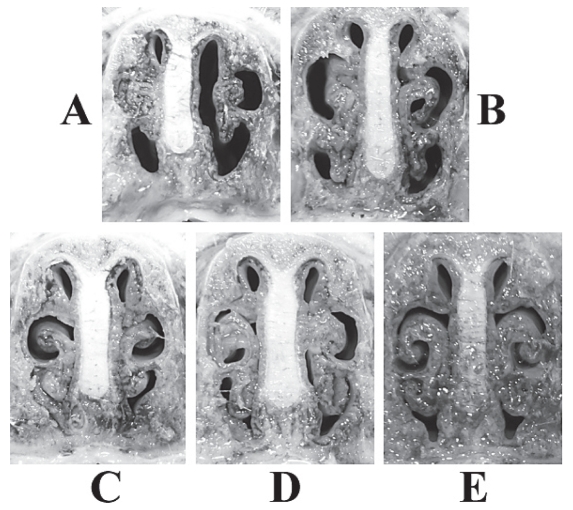
Blood samples from piglets taken before immunization showed no significantly high levels of maternal anti-PMT antibodies (mean OD = 0.23, s = 0.03). An analysis of the IgG levels against #1 fragment in the sera demonstrated that pigs immunized with the #1 fragment successfully produced antibodies (mean OD = 2.66, s = 0.21), while the PBS control did not (mean OD = 0.352, s = 0.29) (P < 0.01). In the nasal conchal gross examination, there were low levels of turbinate atrophy with average scores ranging from 0.23 to 1.0 in the pigs vaccinated with the #1 fragment (groups 3 and 4). In contrast, the 2 control groups showed mild to severe turbinate atrophy with average scores of 1.56 and 2.5, respectively (Figure 2), which were significantly different from vaccinated groups (P < 0.01). However, in neither the vaccinated nor the unvaccinated groups, were any intraperitoneal pathological changes found.
Figure 2.
Representative photographs of the turbinate conchae of pigs in groups unvaccinated (A and B) and vaccinated (C and D), at 4 weeks after W/T P. multocida culture (A and C) or lysate (B and D) challenge. Pigs neither immunized nor challenged served as the negative control (E).
Discussion
Pasteurella multocida is a part of the commensal flora in the upper respiratory tract of pigs. The bacterium causes pneumonia in grower and finisher pigs, and is usually a secondary pathogen invading the lungs of pigs injured by other bacteria or viruses. A subset of P. multocida isolates is a critical agent in upper respiratory disease, PAR (1,3,5,7). These isolates synthesize a 146 kDa toxin that is encoded by the chromosomal toxA gene (20). Pasteurella multocida toxin has been cloned and expressed from the chromosomal DNA of P. multocida (21). However, Pullinger et al (3) suggested that PMT is encoded within a lysogenic bacteriophage. Bacteriophages play an important role in the evolution of many bacterial pathogens (22). The toxins from a number of gram-positive and -negative pathogens are encoded in the genomes of temperate bacteriophages. For example, the structural genes encoding the diphtheria toxin, cholera toxin, and Shiga toxins 1 and 2 are all phage-encoded (23,24). Pullinger et al (3) reported that mitogenic PMT is an example of a toxin encoded in the genome of an inducible prophage. This suggests that PMT is produced by the mitogenic P. multocida for the propagation of the toxA-bearing bacteriophage, and not for the bacterium itself. Moreover, the mitogenic and/or dermonecrotic effects to the host animal cells can be explained as a part of the side effects of PMT. This might explain why the PMT itself is not a strong immunogen to the host animals. Pasteurella multocida toxin is an essential virulence factor for PAR. Isolates of either the common capsule types, A or D, might be toxigenic. The toxin induces turbinate atrophy as well as poor weight gain in pigs (20). Pasteurella multicoda toxin is also a potent mitogen, which at picomolar concentrations induces DNA synthesis and the proliferation of fibroblasts and osteoblastic cells (25). The functional region responsible for the mitogenic effect of PMT was found at the C terminus [amino acids 681–1285 in Pullinger et al (26) and 581–1285 in Busch et al (11)], while the N terminus [amino acids 1–506 in Pullinger et al (26)] is believed to bind to the cells. In particular, a residue in the C-terminal portion of the PMT (Cys1165) has been reported to be essential for its toxic activity (11,27,28). However, it was reported that the biological activity of PMT is localized to the N-terminal part of the holotoxin (29). Antibodies directed against the N-terminal peptide of PMT inhibited the toxin-induced response in Xenopus oocytes but the antibodies against a C-terminal peptide had no effect. Only the full-length protein without the His tag had any activity on Vero cells. The full-length PMT and N-terminal fragments containing the first 500 residues elicited a response in oocytes but the C-terminal 780 amino acid fragment did not. From these results, Wilson et al (29) suggested that the intracellular activity domain of PMT is localized to the N-terminal 500 amino acids of the protein, and that the C terminus is essential for it to enter the cells.
The nature of the immune response to P. multocida in previous vaccine studies was poorly understood (30). Moreover, there is no report clarifying the immunogenic region of PMT. In addition, there are no safe and effective vaccines against pasteurellosis (7). Previously, the vaccination of pigs through exposure to an aerosol of a live temperature-sensitive mutant strain of P. multocida did not produce any significant changes in the serum IgG levels compared with the unvaccinated controls. However, the vaccination produced a significant increase in the levels of the IgA and IgG antibodies in lung lavage fluid (31). Recently, a genetically modified PMT toxin induced protective immunity (28). This suggests that the induction of protective immunity (IgG and/or IgA) against pasteurellosis can be achieved using the appropriate immunogens and administration routes. Despite the availability of the current bacterin vaccines with measurable efficacy, there is a need for more effective and safer vaccines. The aim of this study was to investigate the immunogenicity of 4 truncated fragments of PMT as well as the effectiveness of immunization using those 4 fragments against a subsequent challenge with P. multocida. Mice, which had been immunized with the #1 and/or #4 fragments, showed significantly higher (P < 0.01) levels of IgG induction indicating that these fragments can provide protection against the disease. On the other hand, the #2 and #3 fragments failed to induce any detectable IgG. Similarly, the middle region of PMT (corresponding to the #3 fragment) in one recent study (12) could not elicit high antibody titers. When sows were vaccinated with a mixture of three subunit fragments with/without bacterin, the offspring were successfully protected against a PMT challenge (improved mean weight gain and turbinate conchal score). However, more detailed studies are needed to identify the critical region of PMT responsible for the protection.
In this study, 5 of the 8 mice immunized with the #1 fragment were protected, whereas none of the others including the control group survived. The #1 fragment-immunization offered some protection against death. None of the peritoneal fluids from the surviving (#1 fragment-immunized) mice tested positive for P. multocida, indicating that the inoculated bacteria had been cleared and a neutralizing antibody had been produced. Previously, Nair (13) reported that a C-terminal recombinant PMT fragment, which induced C-terminal-specific antibodies, protected the mice from a lethal challenge with PMT. However, although it produced significantly higher levels of IgG induction, only the N-terminal fragment-immunized (toxA #1 but toxA #4) mice (62.5%) survived the challenge with live P. multocida. It is difficult to understand why the protective efficacy was somewhat different because the same portion of the C-terminal fragment was not used in the present study or in the study reported by Nair (13). This study used live P. multocida for the lethal challenge, whereas Nair (13) used PMT. Therefore, it is possible that there are other lethal virulence factors (not only PMT) that affect the mortality rate. These virulence (pathological) factors need to be examined and included in the formulation of a vaccine to produce better protection against a P. multocida infection. Higher immune responses and protective efficacy might be obtained in pigs given optimal doses of antigen/adjuvant and alternative immunization routes (for example, intranasal and/or tracheal).
Among several types of vaccine preparations, a purified formalin-inactivated PMT toxoid delivered as a single vaccine has been effective for control of PAR (8). However, PMT constitutes very small part (less than 0.6%) of the total cellular proteins of P. multocida, making it necessary to culture a large quantity of bacteria to obtain sufficient amount of PMT for use on a commercial scale. Purification procedures further increase the cost of vaccine production (12). The PMT gene (toxA) has been cloned and a number of nontoxic PMT derivatives were expressed in E. coli. Petersen et al (21) have reported that atoxic PMT derivatives with a short deletion at the N-terminus could induce effective protection against the clinical, pathological and production-limiting effects of PAR in gilts. In order to produce large amount of atoxic PMT, Liao et al (12) expressed several different recombinant PMT derivatives in E. coli via the highly efficient pET expression system. They immunized sows with some mixtures of recombinant PMT fragments to elicit the immune responses of sows to deliver maternal antibodies to offspring, which was quite successful.
In our study, piglets were immunized directly, not sows. Immunized piglets successfully produced IgG against #1 fragment of PMT. Liao et al (12) reported that the short fragments of PMT can elicit a specific immune response against authentic PMT toxin that was superior to that induced by a conventional PAR-toxoid vaccine. Among the fragments, the N-terminal (Tox1) and C-terminal (Tox7) fragments of PMT induced a better humoral immune response than the fragment that was derived from the middle region (Tox2), which was similar to the results herein. There is a difference between the sizes of 2 N-terminal fragments, aa 1–390 in our study and aa 1–487 in Liao et al (12), which contains our #2 fragment (aa 391–470). As discussed, our #2 fragment failed to induce IgG, which means this region is not involved in the immunogenic component. The N-terminal portion of amino acid residues 1–506 within PMT has been suggested to contain a cell binding domain (10) and a putative translocation domain in residues 402–457 (3). Despite the absence of this putative translocation domain, the antibody against our #1 fragment showed a strong immune response and protective effect. It therefore seems that the main immune response region within the N-terminus is restricted to aa 1 to aa 390, at the best. In vivo, as proposed in Liao et al (12), the antibodies against #1 fragment might prevent PMT binding to the target cells and inhibit translocation of toxin. As a result, the PMT toxin activity could be blocked in the nasal cavity of swine.
Before vaccination, the piglets used in this study showed very low anti-PMT IgG levels. Nevertheless, piglets vaccinated with the #1 fragment exhibited a low level of turbinate conchal atrophy after being challenged with W/T P. multocida culture or lysate. This indicated that vaccination with this #1 fragment can protect piglets with/without the maternal antibodies.
Vaccines supplemented with PMT showed significantly better efficacy than those lacking the toxin (21). Although vaccination with individual recombinant subunit proteins can induce protective immune responses, other virulence factors of toxigenic strains of P. multocida should be considered. This study identified the most immunogenic region of PMT. Vaccination with #1 fragment of PMT resulted in high IgG induction and exhibited a low level of turbinate conchal atrophy. This result can be used as the basic information for the development of new vaccines that will improve the effect of a P. multocida vaccination. In conclusion, protection against PAR is possible if the protective level of the antibodies against PMT can be increased around the time of weaning by applying the most immunogenic truncated recombinant protein as a vaccine candidate.
Table III.
Mean turbinate conchal score of the pigs after 4 weeks challenged with W/T bacterial lysate (group 1 and 3) and bacterial culture (group 2 and 4)
| Group | Vaccine composition | Challenged with | Mean score of turbinate conchal atrophya |
|---|---|---|---|
| 1 | PBS | Lysate | 2.5 ± 1.0* |
| 2 | PBS | Culture | 1.56 ± 0.55** |
| 3 | #1 fragment | Lysate | 1.0 ± 0.2 |
| 4 | #1 fragment | Culture | 0.23 ± 0.25 |
| 5 | None | None | 0 ± 0 |
The degrees of turbinate conchal atrophy ranged from 0 (normal) to 4 (complete atrophy).
P < 0.01,
P < 0.05.
Acknowledgment
The authors thank Ms. Myeonghwa Kim (College of Veterinary Medicine, Chonnam National University) for her excellent technical assistance.
References
- 1.de Jong MF. Progressive and nonprogressive atrophic rhinitis. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Ames, Iowa: Iowa State Univ Pr; 1999. pp. 355–384. [Google Scholar]
- 2.Pijoan C. Pneumonic pasteurellosis. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Ames, Iowa: Iowa State Univ Pr; 1999. pp. 511–520. [Google Scholar]
- 3.Pullinger GD, Bevir T, Lax AJ. The Pasteurella multocida toxin is encoded within a lysogenic bacteriophage. Mol Microbiol. 2004;51:255–269. doi: 10.1046/j.1365-2958.2003.03829.x. [DOI] [PubMed] [Google Scholar]
- 4.Rutter JM, Mackenzie A. Pathogenesis of atrophic rhinitis in pigs, a new perspective. Vet Rec. 1984;114:89–90. doi: 10.1136/vr.114.4.89. [DOI] [PubMed] [Google Scholar]
- 5.Chanter N, Rutter JM, Mackenzie A. Partial purification of an osteolytic toxin from Pasteurella multocida. J Gen Biol. 1986;132:1089–1097. doi: 10.1099/00221287-132-4-1089. [DOI] [PubMed] [Google Scholar]
- 6.Mullan PB, Lax AJ. Pasteurella multocida toxin is a mitogen for bone cells in primary culture. Infect Immun. 1996;64:959–965. doi: 10.1128/iai.64.3.959-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt ML, Adler B, Townsend KM. The molecular biology of Pasteurella multocida. Vet Microbiol. 2000;72:3–25. doi: 10.1016/s0378-1135(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 8.Lax AJ, Chanter N, Pullinger GD, et al. Sequence analysis of the potent mitogenic toxin of Pasteurella multocida. FEBS Lett. 1990;277:59–64. doi: 10.1016/0014-5793(90)80809-w. [DOI] [PubMed] [Google Scholar]
- 9.Hoskins IC, Thomas LH, Lax AJ. Nasal infection with Pasteurella multocida causes proliferation of bladder epithelium in gnotobiotic pigs. Vet Rec. 1997;140:22. doi: 10.1136/vr.140.1.22. [DOI] [PubMed] [Google Scholar]
- 10.van Diemen PM, de Vries Reilingh G, Parmentier HK. Immune response of piglets to Pasteurella multocida toxin and toxoid. Vet Immunol Immunopathol. 1994;41:307–321. doi: 10.1016/0165-2427(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 11.Busch C, Orth J, Djouder N, Aktories K. Biological activity of a C-terminal fragment of Pasteurella multocida toxin. Infect Immun. 2001;69:3628–3634. doi: 10.1128/IAI.69.6.3628-3634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao CM, Huang C, Hsuan SL, et al. Immunogenicity and efficacy of three recombinant subunit Pasteurella multocida toxin vaccines against progressive atrophic rhinitis in pigs. Vaccine. 2006;24:27–35. doi: 10.1016/j.vaccine.2005.07.079. [DOI] [PubMed] [Google Scholar]
- 13.Nair RV. Knoxville, Tennessee: University of Tennessee; 2001. Construction of recombinant Pasteurella multocida toxin fragments and evaluation of their immunogenicity and protective efficacy in mice. [PhD dissertation] [Google Scholar]
- 14.Rajeev S, Nair RV, Kania SA, Bemis DA. Expression of a truncated Pasteurella multocida toxin antigen in Bordetella bronchiseptica. Vet Microbiol. 2003;94:313–323. doi: 10.1016/s0378-1135(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 15.Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 16.Ausubel FM, Brent R, Kingston RE, et al. Short Protocols in Molecular Biology. New York, New York: John Wiley & Sons; 2002. [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Lab Pr; 2001. 2001. [Google Scholar]
- 18.Kim TJ, Lee JI, Lee BJ. Development of a toxA gene knock-out mutant of Pasteurella multocida and assessment of its protective effects. J Microbiol. 2006;44:320–326. [PubMed] [Google Scholar]
- 19.Nagy LK, MacKenzie T, Scarnell J. Barcelona, Spain: IPVS 9th Congress; 1986. Age related susceptibility of swine to experimental infection either with Bordetella bronchiseptica- Pasteurella multocida or with P. multocida alone; p. 238. [Google Scholar]
- 20.Lichtensteiger CA, Steenbergen SM, Lee RM, et al. Direct PCR analysis for toxigenic Pasteurella multocida. J Clin Microbiol. 1996;34:3035–3039. doi: 10.1128/jcm.34.12.3035-3039.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen SK, Foged NT. Cloning and expression of Pasteurella multocida Toxin gene, toxA, in Escherichia coli. Infect Immun. 1989;57:3907–3913. doi: 10.1128/iai.57.12.3907-3913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheetham BF, Katz ME. A role for bacteriophages in the evolution and transfer of bacterial virulence determinants. Mol Microbiol. 1995;18:201–208. doi: 10.1111/j.1365-2958.1995.mmi_18020201.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang A, Friesen J, Brunton JL. Characterization of a bacteriophage that carries the genes for production of Shiga-like toxin 1 in Escherichia coli. J Bacteriol. 1987;169:4308–4312. doi: 10.1128/jb.169.9.4308-4312.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 25.Rozengurt E, Higgins T, Chanter N, et al. Pasteurella multocida toxin: Potent mitogen for cultured fibroblasts. Proc Natl Acad Sci USA. 1990;87:123–127. doi: 10.1073/pnas.87.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pullinger GD, Sowdhamini R, Lax AJ. Localization of functional domains of the mitogenic toxin of Pasteurella multocida. Infect Immun. 2001;69:7839–7850. doi: 10.1128/IAI.69.12.7839-7850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward PN, Miles AJ, Sumner IG, et al. Activity of the mitogenic Pasteurella multocida toxin requires an essential C-terminal residue. Infect Immun. 1998;66:5636–5642. doi: 10.1128/iai.66.12.5636-5642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.To H, Someno S, Nagai S. Development of a genetically modified nontoxigenic Pasteurella multocida toxin as a candidate for use in vaccines against progressive atrophic rhinitis in pigs. Am J Vet Res. 2005;66:113–118. doi: 10.2460/ajvr.2005.66.113. [DOI] [PubMed] [Google Scholar]
- 29.Wilson BA, Ponferrada VG, Vallance JE, Ho M. Localization of the intracellular activity domain of Pasteurella multocida toxin to the N terminus. Infect Immun. 1999;67:80–87. doi: 10.1128/iai.67.1.80-87.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgson JC, Finucane A, Dagleish MP, et al. Efficacy of vaccination of calves against hemorrhagic septicemia with a live aroA derivative of Pasteurella multocida B:2 by two different routes of administration. Infect Immun. 2005;73:1475–1481. doi: 10.1128/IAI.73.3.1475-1481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller G, Köhler H, Diller R, Erler W. Antibody reactions after aerogenous or subcutaneous immunization of pigs with Pasteurella multocida antigens. Vaccine. 2000;19:751–757. doi: 10.1016/s0264-410x(00)00263-2. [DOI] [PubMed] [Google Scholar]