Abstract
Infectious bronchitis (IB) is an economically important viral disease with worldwide distribution. Every country with an intensive poultry industry has infectious bronchitis virus (IBV). The virus rapidly spreads from bird to bird through horizontal transmission by aerosol or ingestion. Sentinel bird studies were carried out in southern Ontario and IBV has been isolated from layer flocks. Genetic analysis of the S1 region of the strains showed that they were not vaccine related. The pathogenicity of selected Ontario variants of IBV isolates was studied and the subsequent work was to determine the degree of protection against field isolates provided by a commonly used vaccine MILDVAC-Ma5 in Ontario. The protection was evaluated by challenging immunized chickens with the respiratory (IBV-ON1) and nephropathogenic (IBV-ON4) viruses. The mean vaccine efficacy for IBV-ON1 was 66.7% indicating that a Massachusetts serotype vaccine would provide some protection against IBV field isolates.
Résumé
La bronchite infectieuse (IB) est une maladie virale économiquement importante ayant une distribution mondiale. Le virus de la bronchite infectieuse (IBV) est retrouvé dans tous les pays où il se fait de l’élevage industriel de poulet. Le virus se répand rapidement d’oiseau à oiseau via une transmission horizontale par aérosol ou ingestion. Des études avec oiseaux sentinelles ont été effectuées dans le sud de l’Ontario et l’IBV a été isolé dans des troupeaux de poules pondeuses. Des analyses génétiques de la région S1 des souches ont montré qu’elles n’étaient pas reliées au vaccin. La pathogénicité de variants ontariens sélectionnés d’isolats d’IBV a été étudiée et le travail subséquent était de déterminer le degré de protection conféré par le vaccin MILDVAC-Ma5 couramment utilisé en Ontario contre des isolats de champs. La protection a été évaluée par infection défi de poulets immunisés avec les virus respiratoire (IBV-ON1) et néphropathogène (IBV-ON4). L’efficacité moyenne du vaccin pour IBV-ON1 était de 66,7 % indiquant qu’un vaccin avec le sérotype Massachusetts procurerait une certaine protection contre des isolats de champs d’IBV.
(Traduit par Docteur Serge Messier)
Introduction
Infectious bronchitis (IB), an important viral disease of commercial poultry, was first described by Shalk and Hawn (1). In 1941, an infectious bronchitis virus (IBV) was isolated in Massachusetts and this strain, now known as M41 became the prototype of the Massachusetts (Mass) serotype group. A serologically different virus (IBV 4) was isolated from a case of mild respiratory disease in Connecticut by Jungherr et al (2), thus giving the name to the other major serological group, “Conn.” Since then, IB has been reported in almost all poultry-raising areas of the world. Although some distinctly different strains of IBV belonging to a variety of serotypes were documented in North America, Europe, Asia, and Australia, the Mass serotype viruses remained the dominant ones in many countries with the exception of Australia and New Zealand (3,4).
In the 1950s, attenuated Mass type viruses, named H strains obtained by serial passages in embryonated eggs were introduced as live vaccines (5,6) and they are still widely used. The earlier passage (51st passage) of the virus, which was originally isolated from broilers with respiratory disease, although it has lost some virulence, is only suitable for the vaccination of older birds that already have some immunity. Further egg passages reduced the virulence of the virus and the 120th passage virus, as the H120 vaccine, has been successfully used worldwide as a primary vaccine for broilers and for initial vaccination of breeders and layers. Moreover, the H strains have the ability to induce at least some cross-protection against heterologous serotype viruses, providing a broader range of immunity. Inactivated oil-emulsion IBV vaccines have also been developed that give long-lasting immunity to laying hens to prevent drops in egg production (7,8). However, in spite of good vaccines and rigorous vaccination programs IB remains a problem (9,10). Genetic and antigenic variants of IBV are continuously emerging and causing disease in flocks, which have only partial protection against the new viruses (11,12). Stachowiak et al (13) conducted a survey and sentinel bird study to gain insight into the IB situation of layers in Ontario. Stachowiak et al (13) and Grgić et al (14) partially characterized several IBV isolates and studied the pathogenicity of 5 selected viruses. The objective of this study was to determine if the commonly used vaccine(s) would provide adequate protection against pathogenic field variants of IBV isolated from Ontario layers.
Materials and methods
IBV challenge isolates
The isolates IBV-ON1, IBV-ON4, and IBV-ONM were chosen as the challenge viruses based on the restriction fragment length polymorphisms of the PCR products of the S1 genes and sequence analyses. Isolated by Stachowiak et al (13) from sentinel birds placed among layers in Ontario, IBV-ON1 and IBV-ON4 were designated as respiratory and nephropathogenic isolates, respectively. A field isolate (IBV-ONM) from an IB outbreak in Ontario was obtained from the Animal Health Laboratory (AHL), University of Guelph, and because of its sequence identity with the Massachusetts serotype, it was considered as a positive control challenge virus in the study.
The viruses were propagated and titrated as previously described (14,15). Briefly, the procedures were carried out in specific pathogen free (SPF) embryonated eggs and the titers were determined by the method of Reed and Muench (16). The viruses were administered by the oculo-nasal route giving 1 droplet with a volume of 0.05 mL to each eye and nostril, with a total of 0.2 mL virus per bird. The titers of all 3 viruses were 105.5EID50/mL.
IBV vaccine
A commercial product, MILDVAC-Ma5 of the Massachusetts serotype vaccine was administered by the ocular route as recommended by manufacturer (Intervet, Millsboro, Delaware, USA).
Study design
Two hundred and thirty one-day-old White Leghorn chickens were obtained from Bonny’s Chick Hatchery Ltd. Elmira, Ontario. The birds were housed in the Isolation Facility of the University of Guelph under standard conditions and provided with feed (21% protein unmedicated starter ration) and water ad libitum. Chickens were identified by wing bands and randomly divided into 16 groups comprising 15 birds per group with the exception of the 2 unvaccinated, unchallenged groups that contained 10 birds per group. Eight groups were part of the “clinical signs” and pathology studies, and 8 groups were monitored for weight gains.
The vaccinated groups were vaccinated twice at 1 day of age and 15 days of age. The unvaccinated groups were housed separately until challenge. Birds were challenged at 25 days of age with 1 of the 3 Ontario IBV isolates: IBV-ON1, IBV-ON4, or IBV-ONM. Two groups of vaccinated unchallenged, and another 2 groups unvaccinated, unchallenged served as negative controls. The outcomes monitored included clinical signs, gross and histological lesions, and weight gains over time. The birds were observed for clinical signs for 10 d post-challenge and any sick or injured bird was euthanized by CO2 inhalation according to The Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care (CCAC). Three chickens from challenged groups and 2 chickens from control groups were drawn randomly for necropsy at 2, 4, 6, 8, and 10 d post-challenge as described in the pathogenicity study (14), and examined for the presence of gross lesions in the trachea, lung, and kidney. Lesion scoring was performed as described by Grgić et al (14). Each organ was sectioned into 2 portions. The anterior part of each trachea, left lung, and kidney were placed into buffered formalin (10%) for histological examination. The rest of each organ was stored at −70°C. Samples for histopathological evaluation were processed, embedded in paraffin, sectioned, and stained with hematoxylin and eosine (H&E) (17) by the AHL. The assessment of histological lesions was done blindly and lesions in the trachea were scored using the protocol outlined by Grgić et al (14).
Statistical analysis of vaccine trial
Descriptive and inferential statistical analyses of the data from the trial were performed as described earlier (14). Scores of histological lesions in individual birds, and proportion of birds expressing any clinical signs in a cage among treatment groups (combination of inoculation with field isolates and a vaccination status) were evaluated using the Kruskal-Wallis test. Following statistically significant results, scores and proportions were compared between unvaccinated birds and negative control, and between vaccinated and unvaccinated birds that were challenged with the same isolate. In addition, vaccine efficacy for each isolate that was used in the trial was calculated for diseased and severely diseased animals using EpiInfo 6.04d (Center for Disease Control, Atlanta, Georgia, USA). An animal was considered diseased if it had a histological score > 1. An animal was considered severely diseased if it had a histological score > 3. Vaccine efficacy was calculated using a method for cohort studies, which is based on formula VE = [(attack rate in the unvaccinated − attack rate in the vaccinated)/attack rate in the unvaccinated] × 100. It is interpreted as the proportion of animals in the unvaccinated group that could have been protected had the vaccine been applied in that group. The term attack rate is used to describe the proportion of animals that develop the disease when the risk period is short (18).
Analysis of body weight throughout the study period (33 d) was done using linear mixed effect model (Proc mixed) with intercept and time at the bird level, and with the effect of gender and the initial weight included. Effect of virus isolate on the weight gain of birds was assessed as the interaction between the virus and time, and evaluated by the F-test. Effect of vaccine on the weight gain of birds was assessed as the interaction between the vaccine and time, and evaluated by the F-test. In addition, the difference in average daily gain in the first 7 and the first 14 d post-challenge among the isolates was evaluated in an analysis of covariance (ANCOVA; Proc mixed) using the F-test.
Results
Assessment of protection provided by the commonly used Mass type MILDVAC-Ma5 vaccine against challenge by recent IBV isolates of Ontario was based on several criteria such as clinical signs, gross and histological lesions, and weight gain.
Clinical signs and pathology
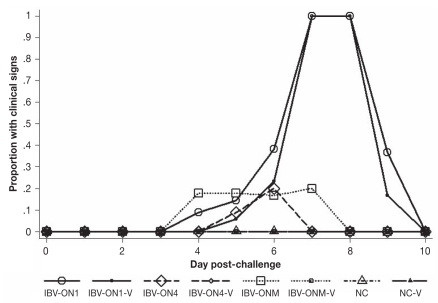
The summary of the clinical examination is presented in Table I. Briefly, it shows the results of descriptive statistical analysis of proportion of birds in each group of the trial that was challenged and the control groups. P-values obtained from comparison of proportions between unvaccinated birds and negative control, and between vaccinated and unvaccinated birds that were challenged with the same isolate are reported in Table II. Figure 1 depicts the mean proportions of birds that showed any clinical signs after challenge. Difference in proportion of birds exhibiting any clinical signs among the treatment groups was statistically significant (Kruskal-Wallis; P < 0.01). The clinical signs were very mild for all 3 viruses. The disease was of short duration; in chickens challenged with IBV-ON1 isolate 2 of the birds in the unvaccinated group developed sneezing as early as 4 d post-challenge. The following 24 h, sneezing started among the vaccinated chickens as well. Sneezing was noticed among all chickens (vaccinated and unvaccinated) challenged with IBV-ON1 isolate and lasted for about 48 h, but it did not influence feed and water consumption and did not change the behavior of the animals. Tracheal rales were absent during the observation period. The clinical signs ceased by day 10 post-challenge.
Table I.
Description of proportion of birds that showed any clinical signs during the first 10 days of vaccine trial after challenge with different IBV isolates
| Isolate | Na | Mean | Median | Minimum | Maximum | Standard deviation |
|---|---|---|---|---|---|---|
| IBV-ON1b | 21 | 0.28 | 0.07 | 0.00 | 1.00 | 0.40 |
| IBV-ON1-Vc | 21 | 0.23 | 0.00 | 0.00 | 1.00 | 0.39 |
| IBV-ON4 | 21 | 0.03 | 0.00 | 0.00 | 0.33 | 0.08 |
| IBV-ON4-V | 21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| IBV-ONM | 21 | 0.07 | 0.00 | 0.00 | 0.33 | 0.11 |
| IBV-ONM-V | 21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NCd | 21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NC-V | 21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Represents the number of cages evaluated throughout the study period in each treatment group. Each treatment group comprised 2 cages, which were observed twice during 10 days post-challenge. On day 11 there was 1 observation and birds were then euthanized.
Unvaccinated and challenged.
V = vaccinated and challenged.
Negative control.
Table II.
The Wilcoxon P-values obtained from comparison of challenged birds and negative control birds, and from comparison of unvaccinated and vaccinated birds inoculated with the same isolate
| Group 1 | Group 2 | P-value |
|---|---|---|
| IBV-ON1 | Negative control | < 0.01 |
| IBV-ON1 | IBV-ON1-Va | 0.42 |
| IBV-ON4 | Negative control | 0.05 |
| IBV-ON4 | IBV-ON4-V | 0.05 |
| IBV-ONM | Negative control | 0.01 |
| IBV-ONM | IBV-ONM-V | 0.01 |
V = vaccinated.
Figure 1.
Mean proportion of chickens exhibiting any clinical signs of infectious bronchitis through the study period. IBV-ON1, IBV-ON4 and IBV-ONM were unvaccinated challenged groups; IBV-ON1-V, IBV-ON4-V and IBV-ONM-V were vaccinated challenged groups. IBV-ONM and IBV-ONM-V represented the positive control groups because IBV-ONM was a Massachusetts serotype field isolate. Groups NC and NC-V were the negative control groups.
The challenge virus (IBV-ON4) caused transient clinical signs and only in 10% of chickens in the unvaccinated group (Figure 1). The occasional sneezing lasted up to 48 h. In groups challenged with IBV-ONM, sneezing was first observed at day 4 post-challenge and was seen in 4 unvaccinated chickens. No clinical disease was seen by day 8 post-challenge (Figure 1).
No gross lesions were observed in the trachea, lung, and kidney in any of the challenged chickens.
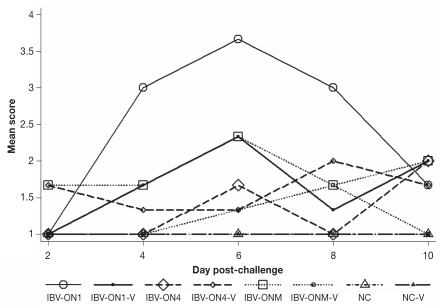
Table III shows the results of the descriptive statistical analysis of scores of histological lesions of birds in each group that was challenged and the control groups. Figure 2 depicts the mean scores of histological lesions during the first 10 d of the study period. Difference in scores of histological lesions among the treatment groups was statistically significant (Kruskal-Wallis; P < 0.01). P-values obtained from comparison of scores of histological lesions between unvaccinated birds and negative control, and between vaccinated and unvaccinated birds that were challenged with the same isolate are reported in Table IV. In addition, Table III summarizes the number of birds that were classified as diseased with any or diseased with medium or severe histological lesions, and were used to calculate the efficacy of the vaccine. The mean vaccine efficacy for IBV-ON1 was 36.4% (95% CI: −18.4%, 65.8%) for birds affected with any histological lesions, and 66.7% (95% CI: 0.5%, 88.8%) for birds affected with medium and severe histological lesions. The mean vaccine efficacy for IBV-ON4 was not calculated because the number of birds affected was numerically higher in the vaccinated group than in the unvaccinated group (Table III). Hence, the mean vaccine efficacy for IBV-ON4 was below zero. The mean vaccine efficacy for IBV-ONM strain was 62.5% (95% CI: −14.6%, 87.7%) for birds affected with any histological lesions, and 100% (95% CI: 100%, 100%) for birds affected with medium and severe histological lesions.
Table III.
Description of scores of histological lesions during the first 10 days of vaccine trial after challenge with different IBV field isolates and frequency of birds that were classified as diseased on the basis of any histological lesions, or lesions that were medium or severe
| Isolate | Challenged (N) | Mean | Standard deviation | Diseaseda (Any; N) | Diseased (medium and severe; N) |
|---|---|---|---|---|---|
| IBV-ON1 | 15 | 2.47 | 1.06 | 11 | 9 |
| IBV-ON1-Vb | 15 | 1.67 | 0.82 | 7 | 3 |
| IBV-ON4 | 15 | 1.33 | 0.62 | 4 | 1 |
| IBV-ON4-V | 15 | 1.60 | 0.91 | 6 | 2 |
| IBV-ONM | 15 | 1.87 | 0.92 | 8 | 5 |
| IBV-ONM-V | 15 | 1.20 | 0.41 | 3 | 0 |
| NCc | 10 | 1.00 | 0.00 | 0 | 0 |
| NC-V | 10 | 1.00 | 0.00 | 0 | 0 |
The frequencies were used to calculate vaccine efficacy.
V = vaccinated.
Negative control.
Figure 2.
Mean scores of histological lesions of chickens in the vaccine efficacy trial for the first 10 days after challenge.
Table IV.
The Wilcoxon P-values obtained from comparison of scores of histological lesions in challenged birds and negative control birds, and from comparison of non-vaccinated and vaccinated birds challenged with the same isolate
| Group 1 | Group 2 | P-value |
|---|---|---|
| IBV-ON1 | Negative control | < 0.01 |
| IBV-ON1 | IBV-ON1-Va | 0.05 |
| IBV-ON4 | Negative control | 0.10 |
| IBV-ON4 | IBV-ON4-V | 0.43 |
| IBV-ONM | Negative control | 0.01 |
| IBV-ONM | IB V-ON-V | 0.04 |
V = vaccinated.
Weight gain
There was no difference in average daily weight gain between vaccinated and unvaccinated birds throughout the entire study period (P = 0.14) in the first 7 d of study period (P = 0.53), and in the first 14 d of study period (P = 0.20). In addition, there was no difference in average daily weight gain of birds challenged with different isolates throughout the entire study period (P = 0.38), and in the first 7 d of the study period (P = 0.12), but these differences were statistically significant in the first 14 d of the study period (P < 0.01). Birds challenged with IBV-ON1 had 1.67 g lower average weight gain in the first 14 d than birds in the negative control group (P < 0.01). Birds challenged with IBV-ON4 had 1.17 g lower average daily weight gain in the first 14 d than birds in the negative control group (P = 0.01). Birds inoculated with IBV-ONM had 1.94 g lower average daily weight gain in the first 14 d than birds in the negative control group (P < 0.01).
Discussion
The MILDVAC-Ma5 of the Massachusetts serotype (Intervet) vaccine was chosen because it is the most commonly used vaccine in Ontario layer flocks. The 2 selected challenge viruses, IBV-ON1 and IBV-ON4, do not have high nucleotide similarity to that of the live Mass and Conn vaccine strains. Pair-wise comparisons at the nucleotide and amino acid level revealed that IBV-ON1 has 70.6%, while IBV-ON4 has only 57.2% homology with Ma5 vaccine virus, and the Ontario isolates did not evolve from the Ark and Ark DPI vaccines strains (14).
Comparison of the clinical signs and histological lesions in the trachea of unvaccinated-challenged and vaccinated-challenged birds indicated that the vaccine-induced immunity provided partial, but not full protection against challenge with IBV-ON1. These findings were similar to those of Gelb et al (19), who reported that when the amino acid identity of the S1 protein of the field isolate is less than 85% compared with the vaccine virus, then the protection is limited. The mean vaccine efficacy for IBV-ON1 was 66.7% for birds scored with medium and severe histological lesion. However, this estimate could be even lower in the field when husbandry and management conditions are not optimal, and additional viral and bacterial infections contribute to the severity of disease. Moreover, in this trial the vaccine was delivered individually, which is not the routine application in large operations, so the immunity among chickens may not be uniform. When a commonly applied single serotype vaccine does not offer solid protection, and when an increase of emergence of new variants is documented, 2 antigenically different IB vaccines could be used. Therefore, bivalent vaccines might provide better protection in such conditions. As it has been reported by Cook et al (20), protective immunity was ensured after application of the Ma5 vaccine at 1 day of age followed by the 4/91 vaccine 2 wk later, while Ma5 alone was less effective. Another study conducted by Gelb et al (21) showed that a vaccination program based on Mass vaccine alone ensures much lower protection than a program based on a combination of Mass and Ark. This program, however, which was effective in the Delmarva Peninsula region, probably would not be equally effective somewhere else.
The vaccine efficacy for IBV-ON4 was not calculated, because there was no significant difference between vaccinated and unvaccinated groups, and also the group of unvaccinated birds was not significantly different from the uninfected control group. During the vaccine trial the weight gain was also monitored, which was not significantly affected in unvaccinated birds when compared with vaccinated and uninfected control groups for any of the viruses.
In conclusion, a vaccination program such as the one used in this experiment may not ensure full protection against challenge with every IBV variant, especially not against the emerging ones. However, the data presented here do show that a Massachusetts serotype vaccine, like the MILDVAC-Ma5 vaccine would provide some protection against field variants.
Acknowledgments
This work was supported by the Egg Farmers of Ontario and the Ontario Ministry of Agriculture, Food and Rural Affairs. This study was part of the MSc thesis of Dr. Helena Grgić. The authors thank the dedicated help of the animal technicians Tony Cengija and Barbara Mitchell.
References
- 1.Shalk AF, Hawn MC. An apparently new respiratory disease of baby chicks. Am Vet Med Assoc. 1931;78:413. [Google Scholar]
- 2.Jungherr EL, Chomiak TW, Luginbuhl RE. Immunologic differences in strains of infectious bronchitis virus. Proc 60th Ann Meet US Livestock San Assoc. 1956:203–209. [Google Scholar]
- 3.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 4.Ignjatovic J, Gould G, Sapats S. Isolation of a variant infectious bronchitis virus in Australia that further illustrates diversity among emerging strains. Arch Virol. 2006;151:1567–1585. doi: 10.1007/s00705-006-0726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijlenga G. The infectious bronchitis virus of chicks detected in the Netherlands by means of egg inoculation, animal experiments, and serum neutralisation test. Tijdschrift voor Diergeneeskunde. 1956;81:43–55. [Google Scholar]
- 6.Bijlenga G, Cook JK, Gelb J, Jr, de Wit JJ. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj GD, Jones RC. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh D, Naqi SA. Diseases of Poultry. 11th ed. Ames: Iowa State Univ Pr; 2003. pp. 163–199. [Google Scholar]
- 9.Jackwood MW, Hilt DA, Lee CW, et al. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49:614–618. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- 10.Smati R, Silim A, Guertin C, et al. Molecular characterization of three new avian infectious bronchitis virus (IBV) strains isolated in Quebec. Virus Genes. 2002;25:85–93. doi: 10.1023/A:1020178326531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MP, Wakenell PS, Woolcock P, O’Connor B. Evaluation of the effectiveness of two infectious bronchitis virus vaccine programs for preventing disease caused by a California IBV field isolate. Avian Dis. 2007;51:584–589. doi: 10.1637/0005-2086(2007)51[584:EOTEOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Cavanagh D, Casais R, Armesto M, et al. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins. Vaccine. 2007;25:5558–5562. doi: 10.1016/j.vaccine.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stachowiak B, Key DW, Hunton P, Gillingham S, Nagy É. Infectious bronchitis virus surveillance in Ontario commercial layer flocks. J Appl Poult Res. 2005;14:141–146. [Google Scholar]
- 14.Grgić H, Hunter DB, Hunton P, Nagy É. Pathogenicity of infectious bronchitis virus isolates from Ontario chickens. Can J Vet Res. 2008;72:403–410. [PMC free article] [PubMed] [Google Scholar]
- 15.Gelb J., Jr . Infectious bronchitis virus. In: Purchase, et al., editors. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. 4th ed. The American Association of Avian Pathologists, Dubuque, Iowa: Kendall/Hunt Publ; 1998. pp. 169–175. [Google Scholar]
- 16.Reed LJ, Muench H. A simple method for estimation fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 17.Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory Methods in Histotechnology . Washington, DC: American Registry of Pathology; 1992. pp. 53–58. [Google Scholar]
- 18.Thrusfield M. Veterinary Epidemiology. 2nd ed. Ames, Iowa: Blackwell Science; 1995. pp. 37–59. [Google Scholar]
- 19.Gelb J, Jr, Weisman Y, Ladman BS, Meir R. S1 gene characteristic and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) Avian Pathol. 2005;34:194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- 20.Cook JKA, Orbell SJ, Woods MA, Huggins MB. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28:477–485. doi: 10.1080/03079459994506. [DOI] [PubMed] [Google Scholar]
- 21.Gelb J, Jr, Rosenberger K, Fries PA, et al. Protection afforded infectious bronchitis virus-vaccinated sentinel chickens raised in a commercial environment. Avian Dis. 1989;33:764–769. [PubMed] [Google Scholar]