Abstract
The main objective of this study was to evaluate a recumbent left flank approach to bilateral ovariectomy in prepubertal heifers and to develop an optimal surgical technique for this procedure. Both ovaries were removed from 6 Nelore heifers by left flank approach without any complications, except in 1 heifer, which was believed to have had only 1 ovary based on ultrasound and exploration during surgery, but was later found to have a remaining functional ovary. Ovariectomy via left flank approach in recumbent prepubertal heifers is feasible and technically easy. This procedure does not involve special instrumentation and, despite the invasive approach, it allows optimal visualization of the ovaries and uterus.
Résumé
L’objectif principal de cette étude était d’évaluer une approche par le flanc gauche pour exécuter l’ovariectomie bilatéral des génisses pré-pubères et pour développer une technique chirurgicale optimale pour ce procédé. Les deux ovaires de six génisses de la race Nelore ont été prelevés par moyen de l’approche par le flanc gauche sans aucune complication sauf celle d’une génisse qui était censée avoir eu seulement un ovaire basés sur l’ultrasonographie et sur l’exploration pendant la chirurgie mais plus tard on a constaté qu’elle avait un ovaire fonctionnel restant. L’ovariectomie de l’approche gauche de flanc chez les génisses pré-pubère est faisable et techniquement facile. Ce procédé ne comporte pas d’instrumentation spéciale, et en dépit de l’approche invahissante il permet la visualisation optimale des ovaires et de l’utérus.
(Traduit par Dr. Peiró)
The practice of spaying heifers was first used in the early 1930s to simplify the management of these animals (1). Oophorectomy not only prevents pregnancy, but also reduces social problems related to the estrus cycle, improves average daily weight gain (2,3), and can be used to treat ovarian pathology in adult cows or to prepare teaser cows for artificial insemination programs (2). Because ovariectomized heifers have reduced live-weight gains and carcass weights compared with intact heifers (4), spaying did not become widely accepted (1). Recently, spaying of heifers has become popular and profitable once again, due to new surgical and technical developments (1,4).
Spay techniques for heifers have been widely studied in an attempt to investigate the reproductive, endrocrine, and physiologic aspects (4–8). Within these studies, heifers have traditionally been flank-spayed; however, 2 vaginal spay methods have been used: Kimberlin-Rupp and dropped-ovary (8). More recently, standing laparoscopic ovariectomy has been used in cows, mares, and ponies (3,9,10). Ovariectomy via a flank or ventral abdominal approach has also been described in heifers, in which the vagina is too narrow for colpotomy, and in cows with large ovarian tumors and adhesions involving the ovary (11).
The advantages of vaginal spay methods compared with a flank approach are the reduced times needed for surgical preparation. When properly performed, oophorectomy has minimal unfavorable sequelae and can be done using low epidural anesthesia (2). Disadvantages are that heifers must be sufficiently large to allow the surgeon’s arm to extend into the rectum; above average-sized ovaries are difficult to remove and often leave remnants with signs of estrus and possible pregnancy, laceration of intestines during surgery by an inexperienced surgeon, and postoperative peritonitis if the surgical site or instruments are unclean (2). In contrast, in young heifers with low body weight, vaginal spaying techniques are not feasible, mainly due to the size and anatomical position of the uterus and ovaries.
Based on these facts, this study examines a left paralumbar fossa approach in prepubertal heifers with low body weight. The study and experimental design were approved by the Committee for Animal Experimentation, São Paulo State University. Six healthy Nelore heifers, born within the same month, aged 180 d and weighing 130 kg were used. All animals were kept on Tanzania grass (Panicum maximum, J. cv. Tanzania) pasture, supplemented with hydrolyzed sugarcane bagasse and corn and soybean flour to allow 0.4 kg weight gain/day. They received commercial mineral salt (Matsuda Fós 20S; Matsuda, Álvares Machado, São Paulo, Brazil) and water ad libitum. All heifers were transrectally palpated and the abdominal cavity was examined by ultrasound to evaluate the presence and size of ovaries.
Food and water were withheld, 24 h and 12 h, respectively, from the patients prior to surgery. Intramuscular sedation was achieved using xylazine (Coopazine; Coopers Brasil, São Paulo, São Paulo, Brazil) 0.2 mg/kg BW. Animals underwent surgery in right lateral recumbency with their legs in an extended position. The hair over the left mid-paralumbar fossa was trimmed and the skin was scrubbed with povidone iodine followed by alcohol. An infiltrative local anesthesia with 2% lidocaine (Xylestesin; AstraZeneca do Brasil, Cotia, São Paulo, Brazil) was administered in the left mid-paralumbar fossa, using the needle cannula of a 14-G catheter (BD Insyte, Becton, Dickinson Indústrias Cirúrgicas, Juiz de Fora, Minas Gerais, Brazil) to reach the more profound planes, subcutaneous tissue, and musculature. The mean volume of lidocaine was 40 mL.
A vertical skin incision with a number 23 scalpel blade was made over the left mid-paralumbar fossa extending long enough (30 cm) to permit the insertion of the surgeon’s arms. All muscular layers were incised with the scalpel until reaching the peritoneum, which was held with forceps and punctured with the scalpel. The incision was extended with scissors proximally and distally. Hemorrhage was controlled using Halsted forceps and ligature with 2-0 chromic catgut. Farabeuf retractors were used to optimize visualization of the abdominal cavity (Figure 1). The surgeon dislocated the rumen cranially and grasped the uterus and the ovaries located 5 cm cranial to the pelvic wall. Due to the small opening of the surgical site, a 2nd surgeon kept the rumen in cranial position while the 1st surgeon made an extracorporeal knot with 2 chromic catgut. Lidocaine (2%) was sprayed over the left ovarian pedicle; it was then ligated by the preformed knot. Following this, the left ovary was removed using curved Mayo scissors and the ovarian pedicle was inspected for hemorrhage. The extremities of the suture were cut and the procedure was repeated for the right ovary.
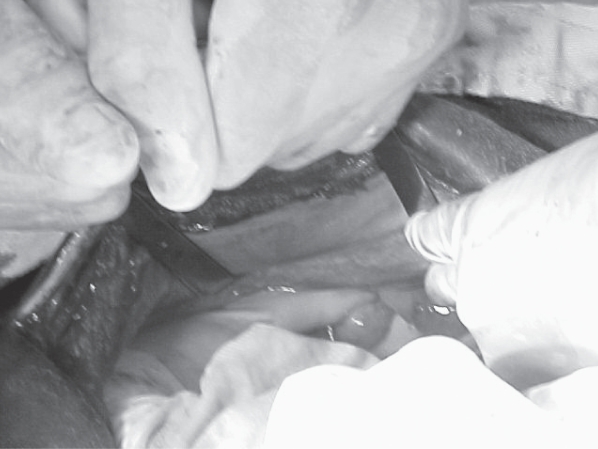
Figure 1.
Left ovary is being held between the surgeon’s left thumb and index finger.
After removal of the ovaries, the peritoneum and the abdominal transverse muscle were sutured together and a 2nd layer of sutures was used to close the internal and external abdominal oblique muscles. Both layers were sutured with 3 chromic catgut in a Sultan pattern. The subcutaneous tissue was closed with 2-0 chromic catgut in Cushing pattern. Skin was closed using nylon in Ford-interlocking pattern. All heifers were returned to pasture. Over the following 10 d, heifers were observed twice daily for complications; dosed with a long-acting oxytetracycline antibiotic (Cyamicina LA 20%, Fort Dodge, Campinas, São Paulo, Brazil), 20 mg/kg, BW, IM, SID q48h, totaling 5 administrations; and provided with wound care consisting of 10% povidone iodine (Riodeíne tópico a 10%, Indústria Farmacêutica Rioquímica, São José do Rio Preto, São Paulo, Brazil) and carbamate powder (Facthal pó, Minerthal, São Paulo, São Paulo, Brazil), q12h, to control for flies. Skin sutures were removed 10 d post-surgery.
The approach from the left side allowed the oophorectomy to be performed in all animals. Ovaries had a mean diameter of 1 cm. The mean time for the entire procedure (anesthesia included) was 30 min (range: 30 to 40 min). No discomfort or complications (peritonitis, wound infection) were observed after surgery. No other problems, such as stiffness, straining, or lack of appetite, were observed. There was no morbidity or mortality associated with the ovariectomy in these heifers.
In one of the heifers (#133), it was not possible to locate the right ovary in the abdominal cavity by ultrasonography or rectal palpation before surgery. During surgery, the left ovary was excised and the uterine ligament was palpated without any problems. A transrectal ultrasonography was performed during surgery and the right ovary could not be identified. The uterine ligament was palpated again and a 2-mm firm mass was found on the ligament. As this small structure could not be ligated and ressected, it was crushed with a Kelly forcep in an attempt to destroy this tissue we believed to be the right ovary. Following the surgery, the animal was evaluated by monthly rectal palpation and ultrasound and a structure similar to an ovary was detected.
The plasma luteinizing hormone (LH) concentration, as previously described (12,13), was observed in these animals over 9 mo to compare the levels between intact and ovariectomized heifers and to confirm that the spay was successful. The LH concentration was also used to determine if heifer #133 was ovariectomized or intact. The sensitivity of the LH assay was 0.05 ng/mL, and intra- and interassay coefficients of variation were 14.4% and 18.3%, respectively. The age at onset of puberty was identified when plasma progesterone concentration (RIA) was > 1 ng/mL and maintained for at least 2 consecutive samples (collected every 4 d). The mean monthly LH concentration (ng/mL) for each group was compared with heifer #133 (t-test for one mean), using computer software (GraphPad Instat 3.0 for Windows; GraphPad Software, San Diego, California, USA). Data are presented as mean and 95% confidence intervals (upper and lower).
Before ovariectomy (6 mo), average plasma LH concentrations were similar in both intact and ovariectomized heifers. From 8 mo of age on, secretion of LH was greater in ovariectomized heifers than in intact heifers. Gonadal steroids have a strong inhibitory influence on LH secretion, as evidenced by low circulating concentrations of LH in the intact group and in ovariectomized heifers before surgery. Also, 17β-estradiol (E2) negative feedback effects on the hypothalamic-pituitary axis in regulation of LH secretion decreases during peripuberty in Bos indicus heifers (14). This decreased negative feedback could have contributed to stimulate the secretion of LH in ovariectomized heifers. Heifer #133 manifested a distinct pattern of LH secretion. Its hypoplastic ovary could have secreted a higher concentration of LH in comparison with intact heifers or the ovary tissue could have produced small quantities of gonadal steroids that were sufficient to induce a negative feedback effect upon the synthesis and secretion of LH. Average LH secretion increased over time in all heifers, decreasing the negative feedback effects of estradiol prior to the first ovulation (15). In a previous study, the physiological LH concentration increase in intact prepubertal Nelore heifers was also influenced by age (16).
Small body size often limits the surgeon’s ability to ovariectomize heifers (17). Due to the difficulty in accessing the ovaries in animals under 1 y of age by the left flank in a standing position, we decided to perform surgery with the heifers in right lateral recumbency to provide better comfort for the animal and the surgeon. This recumbency allows for the localization and removal of both ovaries quickly (5 to 10 min total time), depending on the position (in the pelvic inlet or in the abdominal cavity) and the size of the ovary. Also, recumbency allows for increased length of incision, thus allowing the hands of the surgeon to reach the uterus and ovaries. This manipulation, however, is sometimes uncomfortable for animals in the standing position and stimulates them to try to move or jump from the stocks, increasing the probability of contamination of the surgical site.
During excision of the ovaries, a hand was positioned around them to protect the bowel from the scissors. Compared with the vaginal approach, the left approach oophorectomy increases the time needed for surgical preparation, the anesthetic procedure is minimally more expensive (low caudal epidural versus mid-paralumbar fossa anesthesia), but allowed for good visualization and removal of both ovaries, with the exception of heifer #133, which had a hypoplastic ovary.
The left flank approach is also preferred by others (3) because the risk of organ trauma is reduced and visualization of the ovaries, uterus, and hemorrhage control is improved when compared with the right-flank approach. Some other advantages when performing the ovariectomy via a flank approach include a reduced risk of inadvertent damage to viscera and it does not require special equipment that surgeons would not already have (17,18). Also, the duration of the procedure was considerably lower (30 min versus 120 to 150 min) when compared to a study using laparoscopy in standing cows (3). In animals suspected of a hypoplastic ovary, we suggest that a ligature involving the uterine ligament around the ovary be performed before resection of this tissue, since this ovary can further develop after the comtralateral ovary is excised and the animal will begin cycling and could become pregnant if bred.
Our results indicate that the left flank ovariectomy is an efficient, low risk technique for prepubertal heifers when they are intended for reproductive studies.
Table I.
Plasma luteinizing hormone (LH) concentrations (ng/mL) from intact heifers, ovariectomized heifers, and heifer #133 sampled every 4 d from 6 to 15 mo of age. Results are reported as mean and 95% confidence intervals (upper and lower)
| Age (months) | Intact (n = 9) | Ovariectomized (n = 5) | #133 (with 1 ovary) |
|---|---|---|---|
| 6 | 0.47 (0.31, 0.63)a | 0.60 (0.35, 0.84)a | 1.00 |
| 7 | 0.67 (0.60, 0.74)a | 0.99 (0.75, 1.24) | 0.93 |
| 8 | 0.58 (0.49, 0.68)a | 1.51 (1.30, 1.72)a | 1.20 |
| 9 | 0.57 (0.50, 0.65)a | 1.59 (1.33, 1.85)a | 0.94 |
| 10 | 0.63 (0.59, 0.66)a | 1.72 (1.48, 1.95)a | 0.85 |
| 11 | 0.70 (0.62, 0.77)a | 2.20 (1.84, 2.56)a | 0.98 |
| 12 | 0.69 (0.62, 0.76)a | 2.22 (1.70, 2.75)a | 0.90 |
| 13 | 0.62 (0.57, 0.67)a | 2.13 (1.81, 2.45)a | 1.29 |
| 14 | 0.77 (0.67, 0.86)a | 2.20 (1.99, 2.41)a | 1.03 |
| 15 | 0.65 (0.59, 0.71)a | 2.40 (1.85, 2.95)a | 1.58 |
Significantly different from heifer #133 (P ≤ 0.05).
Acknowledgments
This study was supported by the State of São Paulo Research Foundation (FAPESP: process no. 99/10446-7). The authors thank CFM Group São Francisco Farm (Magda, São Paulo, Brazil) for providing the heifers for this study and Mrs. Tereza Forato for revision of the French résumé.
References
- 1.Cain DV, Jr, Jones AL, Milliken G. Do different spay techniques and growth implant frequencies affect weight gain in heifers? Vet Med. 1986;81:464–468. [Google Scholar]
- 2.Noordsy JL. Oophorectomy in cattle. Comp Cont Edu Pract Vet. 1997;19:1392–1394. [Google Scholar]
- 3.Bleul U, Hollenstein K, Kähn W. Laparoscopic ovariectomy in standing cows. Anim Reprod Sci. 2005;90:193–200. doi: 10.1016/j.anireprosci.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Jubb TF, Fordyce G, Bolam MJ, et al. Trial introduction of the Willis dropped ovary technique for spaying cattle in northern Australia. Aust Vet J. 2003;81:66–70. doi: 10.1111/j.1751-0813.2003.tb11436.x. [DOI] [PubMed] [Google Scholar]
- 5.Richards MW, Wettemann RP, Spicer LJ, Morgan GL. Nutritional anestrus in beef cows: Effects of body condition and ovariectomy on serum luteinizing and insulin-like growth factor-I. Biol Reprod. 1991;44:961–966. doi: 10.1095/biolreprod44.6.961. [DOI] [PubMed] [Google Scholar]
- 6.Spicer LJ, Hanrahan JP, Zavy MT, Enright WJ. Relationship between ovulation rate and concentrations of insulin-like growth factor-1 in plasma during the oestrous cycle in various genotypes of sheep. J Reprod Fertil. 1993;97:403–409. doi: 10.1530/jrf.0.0970403. [DOI] [PubMed] [Google Scholar]
- 7.Enright WJ, Spicer LJ, Prendiville DJ, Murphy MG, Campbell RM. Interaction between dietary intake and ovariectomy on concentrations of insulin-like growth factor-I, GH and LH in plasma of Heifers. Theriogenol. 1994;41:1231–1240. doi: 10.1016/0093-691x(94)90480-7. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Hudson D, Clanton D, Johnson J. Evaluation of spaying techniques for heifers. Mod Vet Prac. 1987;68:98–101. [Google Scholar]
- 9.Rodgerson DH, Belknap JK, Wilson DA. Laparoscopic ovariectomy using sequential electrocoagulation and sharp transection of the equine mesovarium. Vet Surg. 2001;30:572–579. doi: 10.1053/jvet.2001.28435. [DOI] [PubMed] [Google Scholar]
- 10.Shoemaker RW, Read EK, Duke T. In situ coagulation and transection of the ovarian pedicle an alternative to laparoscopic ovariectomy in juvenile horses. Can J Vet Res. 2004;68:27–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmeyr CFB. The female genitalia. In: Hofmeyr CFB, editor. Ruminant Urogenital Surgery Ames. Iowa: Iowa State Univer Pr; 1987. pp. 122–147. [Google Scholar]
- 12.Bolt DJ, Rollins R. Development and application of a radioimmunoassay for bovine follicle-stimulating hormone. J Anim Sci. 1983;56:146–154. doi: 10.2527/jas1983.561146x. [DOI] [PubMed] [Google Scholar]
- 13.Bolt DJ, Scott V, Kiracofe GH. Plasma LH and FSH after estradiol, norgestomet and Gn-RH treatment in ovariectomized beef heifers. Anim Reprod Sci. 1990;23:263–271. [Google Scholar]
- 14.Rodrigues HD, Kinder JE, Fitzpatrick LA. Estradiol regulation of luteinizing hormone secretion in heifers of two breed types that reach puberty at different ages. Biol Reprod. 2002;66:603–609. doi: 10.1095/biolreprod66.3.603. [DOI] [PubMed] [Google Scholar]
- 15.Gasser CL, Bridges GA, Mussard ML, Grum DE, Kinder JE, Day ML. Induction of precocious puberty in heifers III: Hastened reduction of estradiol negative feedback on secretion of luteinizing hormone. J Anim Sci. 2006;84:2050–2056. doi: 10.2527/jas.2005-638. [DOI] [PubMed] [Google Scholar]
- 16.Nogueira GP, Oliveira DJC. Gaba inhibitor stimulates LH secretion in pre-pubertal nelore heifers. Proc Int Cong Anim Reprod. 2004;122 [Google Scholar]
- 17.Habermehl NL. Heifer ovariectomy using the Willis spay instrument: Technique, morbidity, and mortality. Can J Vet Res. 1993;34:664–667. [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JR. Ovariectomizing heifers. Mod Vet Pract. 1984;65:13–15. [PubMed] [Google Scholar]