Abstract
AIM: To examine the molecular mechanism of exocytosis in the Brunner’s gland acinar cell.
METHODS: We used a submucosal preparation of guinea pig duodenal Brunner’s gland acini to visualize the dilation of the ductal lumen in response to cholinergic stimulus. We correlated this to electron microscopy to determine the extent of exocytosis of the mucin-filled vesicles. We then examined the behavior of SNARE and interacting Munc18 proteins by confocal microscopy.
RESULTS: One and 6 μmol/L carbachol evoked a dose-dependent dilation of Brunner’s gland acini lumen, which correlated to the massive exocytosis of mucin. Munc18c and its cognate SNARE proteins Syntaxin-4 and SNAP-23 were localized to the apical plasma membrane, and upon cholinergic stimulation, Munc18c was displaced into the cytosol leaving Syntaxin-4 and SNAP-23 intact.
CONCLUSION: Physiologic cholinergic stimulation induces Munc18c displacement from the Brunner’s gland acinar apical plasma membrane, which enables apical membrane Syntaxin-4 and SNAP-23 to form a SNARE complex with mucin-filled vesicle SNARE proteins to affect exocytosis.
Keywords: Apical exocytosis, Brunner’s gland acini, Munc18c, Syntaxin-4, Carbachol
INTRODUCTION
The Brunner’s glands in the duodenum contribute to the discharge of mucin[1,2] and also the secretion of a number of other products, including immunoglobulin, lysozyme, epidermal growth factor, and trefoil peptides, which collectively contribute to mucosal protection[2–5]. Brunner’s glands also secrete bicarbonate that contributes to the neutralization of the massive amount of acid coming from the stomach[2], and duodenal alkalinization is critical for pancreatic enzyme survival. The Brunner’s gland is, therefore, a most versatile exocrine microorgan, but despite its importance in gastrointestinal health, relatively little is known about its secretory biology and regulation, in part because they exist as very small micro-organs buried within the duodenal mucosal epithelium.
To better understand Brunner’s physiology, we previously reported the development of an in vitro model that permitted the examination of Brunner’s gland secretion by video microscopy, which recorded real time changes in diameter of the dilating lumen of Brunner’s gland acini that corresponded to the extent of mucin exocytotic emptying[6,7]. Using this model, we demonstrated cholinergic stimulation of compound exocytosis of mucin into the ductal lumen, which was confirmed by electron microscopy and histology[6]. We then went on to examine vagal neural cholinergic innervation[6], and its coupling to potassium channel current, which regulated the acinar cell membrane excitability leading to secretion[7].
Almost nothing is known about the molecular mechanisms regulating exocytosis per se in Brunner’s gland acini. In contrast, there has been much insight into molecular mechanism of exocytosis in the pancreatic acinar cell[8,9]. It is very likely that similar exocytotic molecules in the pancreatic acinar cell would be conserved in Brunner’s gland acini to mediate exocytosis of mucin. This led us to begin to explore for such exocytotic molecules, including SNARE (soluble NSF attachment protein receptor) proteins and associated Munc18 proteins, which regulate SNARE complex assembly[10]. Munc18c binds Syntaxin-4 on the basolateral plasma membrane of the pancreatic acinar cell[11]. Upon supramaximal cholinergic (or CCK) stimulation, Munc18c becomes phosphorylated causing its displacement from Syntaxin-4 into the cytosol, which activates Syntaxin-4 to bind SNAP-23, rendering the basolateral plasma membrane receptive to exocytosis by zymogen granules[11]. We recently demonstrated this to be a contributing mechanism to supramaximal secretagogue-induced pancreatitis as well as alcoholic pancreatitis[11–15].
In this work, we also found Munc18c, Syntaxin-4 and SNAP-23 to be present in Brunner’s gland acini. Unlike pancreatic acini[11–15], these exocytotic molecules are concentrated on the apical plasma membrane. Upon physiologic cholinergic stimulation, Munc18c behavior mimicked that of pancreatic acini[11–15], becoming displaced from the apical membrane into the cytosol, which correlated to massive exocytosis of mucin into the dilating Brunner’s gland acinar lumen.
MATERIALS AND METHODS
Antibodies and reagents
Antibodies used include those generated against Munc18c (a gift from Y Tamori, Kobe University, Japan), Syntaxin-4 (a gift from J Pessin, Stony Brook University, NY, USA), SNAP-23 (generated by us), and Mucin 5AC-clone 45M1 from Lab Vision (Fremont, CA, USA). Fluorochrome-conjugated secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA, USA). All reagents were from Sigma Chemical Co. (St. Louis, MO, USA).
Brunner’s gland preparation and stimulation by carbachol
In vitro submucosal preparations containing Brunner’s glands were dissected from the duodenum of guinea pigs (150-200 g) of either sex, as previously described[1,6,7]. Briefly, animals were anesthetized with isofluorane and killed by decapitation. The duodenum was opened along the mesenteric border and pinned flat with the mucosa side up in Sylgaard-lined petri dishes. The mucosa was dissected off and the underlying submucosa containing sheets of Brunner’s glands dissected free from the circular muscle. Submucosal preparations were cut about 1 cm2 and stored at room temperature (maximum time of 2 h) in physiological Krebs solution containing (in mmol/L): 126 NaCl, 2.5 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 5 KCl, 25 NaHCO3 and 11 glucose, equilibrated with 95% O2-5% CO2. Experiments were approved by the Queen’s University and University of Toronto Animal Care Committees and met the guidelines of the Canadian Council of Animal Care.
In all studies, preparations were initially pinned in small organ baths (1 mL), and superfused with Kreb’s solution at 37°C for a 10 min equilibration period. They were then superfused for 3 min with Kreb’s solution (control) or carbachol (1 μmol/L or 6 μmol/L). Tissues were then fixed by one of two means (see below) and coded to enable measurements to be performed in a blinded fashion.
Examination of exocytosis by transmission electron microscopy
Following superfusion with carbachol or control solution (Kreb’s), preparations were fixed in 2% glutaraldehyde (pH 7.0) for 2 h and washed in sodium phosphate buffer. The fixed tissue was sectioned into segments about 2 mm2 and immersed in 1% osmium tetroxide for 1 h. Tissue blocks were embedded in Eponar aldite. Brunner’s glands were identified in semi-thin sections (0.5-1.5 μmol/L), which were cut perpendicular to the surface of the submucosal preparation and stained with toluidine blue. Plastic blocks were then trimmed to areas of about 0.5 mm2 and ultrathin sections were cut and mounted on copper grids. These were stained with uranyl acetate and lead citrate and sections were viewed using a Zeiss EM 10 CR transmission electron microscope.
Confocal immunofluorescence microscopy
This was performed as similarly described in our previous reports[11–15]. Frozen tissues were treated with Tissue-Tek OCT mounting compound from Tedpella (Redding, CA, USA), and then cut at 5 μm-thin sections. Frozen tissue sections were thaw-mounted onto VistaVision HistoBond adhesive slides from VWR (Mississauga, ON, Canada), fixed with 4% paraformaldehyde (1 h), rinsed in PBS and then treated with 100 mmol/L glycine (10 min). The tissues were then permeabilized with 0.1% Triton-X (in PBS), blocked with 5% albumin (overnight, 4°C) and then incubated with the primary antibody (1:50, 5 h). After extensive washing, fluorescently labeled secondary antibodies (1:1000) were added for 1 h, followed by washing, and treatment with Fluorescence Mounting Medium (DAKO Diagnostics, Mississauga, ON). The tissue samples were examined by a laser scanning confocal imaging system (Zeiss LSM510), equipped with LSM software version 5.00 (Carl Zeiss, Oberkochen, Germany).
Measurement of Brunner’s gland dilation
Concurrent to obtaining the immunofluorescence images, DIC (differential interference contrast) images from frozen tissue sections were taken using the DIC optics of the confocal microscope. The apical lumens of the acini were measured using Image J software (NIH) by two independent observers in a blinded fashion. Significant differences between the two independent observers were not found and, therefore, the data were averaged for each measurement.
Statistical analysis
Slides were coded to avoid observer bias and measurements were performed in a blinded manner by two independent observers and their results averaged. All data are presented as mean ± SE. Statistical analysis was done by Student’s t test. Significance was assumed at a P value of less than 0.05.
RESULTS
Carbachol evokes a dose-dependent dilatation of Brunner’s gland acinar lumens
Brunner’s gland acinar cells contain mucin granules in the apical cytoplasm and previous studies have shown that stimulation-evoked dilation of Brunner’s gland apical lumen is associated with the deposition of mucin in this space[1]. In the current study, carbachol (1 μmol/L and 6 μmol/L) evoked a dose-dependent dilation of the lumen (Figure 1A-C). Compared to controls (1.0 ± 0.2), carbachol 1 μmol/L caused 2.5-fold increase in luminal surface area (2.5 ± 0.5, P < 0.05) and 6 μmol/L caused a 5-fold increase (5.1 ± 0.7, P < 0.001; Figure 1D).
Figure 1.
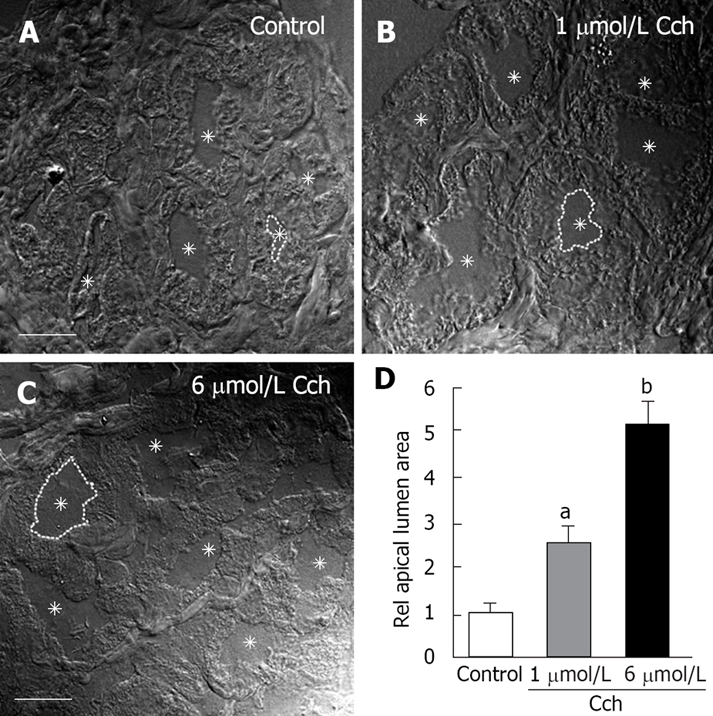
Carbachol-evoked dilatation of Brunner’s gland acinar lumens. A-C: Representative differential interference contrast (DIC) images of control (A), 1 μmol/L Carbachol (B), and 6 μmol/L Carbachol (C) showing a dose-dependent dilation of the acinar lumen. In each panel, the lumens are indicated by asterisks and one of the lumens is highlighted with a broken line. D: Statistical analysis of apical lumen area relative to control. For each condition 50 glands from 3 different sets of animals were measured (n = 150). Only those glands where the complete acinus was contained in the section were studied. Scale bar = 10 μm. aP < 0.05, bP < 0.01.
Carbachol evokes a dose-dependent mucin granule exocytosis into Brunner’s gland acinar lumens
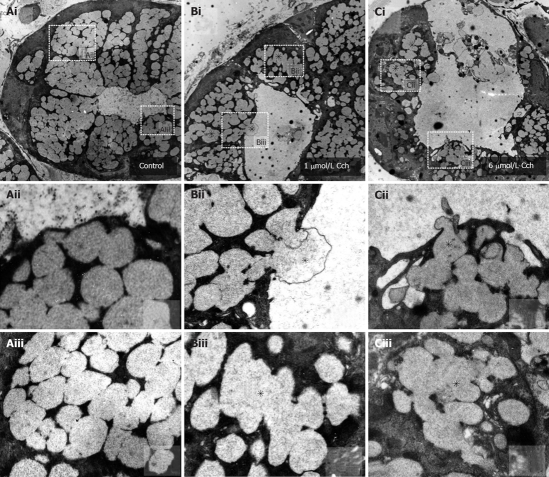
Transmission E.M. was employed to observe the effects of carbachol stimulation on the location and size of the mucin granules (Figure 2). In unstimulated preparations (Figure 2A), membrane-bound, electron-lucent vesicles were packed into the apical cytoplasm. Carbachol stimulation (1 μmol/L and 6 μmol/L in Figure 2B and C, respectively) caused fusion of the mucin vesicles deeper in the cytoplasm (indicated by asterisks, shown clearly in image blowups in Biii and Ciii), and migration and fusion of the vesicles with the apical membrane. Apical exocytosis included compound exocytosis (indicated by asterisks, shown clearly in image blowups in Bii and Cii) which enabled very efficient extrusion of the mucin cargo into the lumen resulting in complete collapse of the vesicles and progressive luminal dilatation, which was massive with the 6 μmol/L carbachol stimulation. These findings, together with the measurements of acinar lumen, directly corroborate that carbachol activated compound exocytosis in these tissues.
Figure 2.
Carbachol-evoked mucin granule exocytosis into Brunner’s gland acinar lumens. Representative transmission electron micrographs of Brunner’s glands in unstimulated control (A), 1 μmol/L (B) and 6 μmol/L carbachol (C) stimulated tissue. A: Unstimulated Brunner’s acinus (× 6250). Aii and Aiii are blowups of indicated regions in Ai, showing small separate vesicles; B: One μmol/L carbachol-stimulated Brunner’s acinus showing a larger apical lumen with reduced number of electron-lucent mucin vesicles within the apical poles, with some vesicles undergoing vesicle-vesicle fusions (indicated by asterisk, × 6250). Bii and Biii are blowups of indicated regions in Bi, showing compound exocytosis and vesicle-vesicle fusion (indicated by asterisks), respectively; C: Six μmol/L carbachol stimulated Brunner’s acinus. There is much less remaining vesicles some of which are undergoing vesicle-vesicle fusion (indicated by asterisk, × 5000). Cii and Ciii are blowups of indicated regions in Ci, showing compound exocytosis and vesicle-vesicle fusion (indicated by asterisks), respectively.
Carbachol displaces Munc18c from the apical plasma membrane of Brunner’s gland acinar cells
The distinct exocytotic events of Brunner’s gland acini observed in Figures 1 and 2 are reminiscent of those in pancreatic acini[8,9], and which we and others had reported to involve distinct SNARE and Munc18 proteins[11–24]. We, therefore, probed the Brunner’s gland acini with antibodies against Syntaxin (-2, -3 and -4), SNAP-23, VAMP (-2 and -8) and Munc18 (b and c), with the expectation that these exocytotic proteins would be differentially distributed in a manner reported in the rat pancreatic acinar cells[8,9,11–24]. We also probed for mucin to verify its retention when unstimulated and its absence indicating exocytosis after stimulation. Importantly, these studies were conducted on the same tissues where carbachol-evoked dilation of the acinar lumen was demonstrated (see above), providing parallel functional evidence of exocytosis.
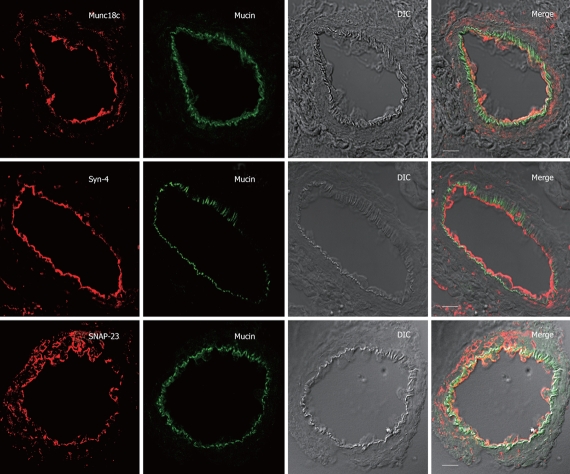
Of all these exocytotic protein antibodies, only Syntaxin-4, SNAP-23 and Munc18c antibodies showed consistent positive signals in the confocal immunofluorescence studies (Figures 3, 4, 5). Of note, all of these antibodies were raised against rat sequences and, therefore, the lack of detectable signals suggest that the antibodies may not recognize the guinea pig sequences or that those protein levels in guinea pig acini were not sufficiently abundant. In unstimulated acini, Syntaxin-4, SNAP-23 and Munc18c were abundant on the apical plasma membrane (Figure 3). This is surprisingly different from what had been demonstrated in pancreatic acini wherein these proteins were most abundant along the basolateral plasma membrane, and in fact, undetectable in the apical plasma membrane[11–17]. Mucin lodged just beneath these exocytotic proteins, indicating their location in vesicles docked onto the apical pole of the acini. SNAP-23 signal seems to be also present in intracellular structures and the basal plasma membrane.
Figure 3.
Apical SNARE localization in unstimulated Brunner’s gland acini. Confocal images of unstimulated Brunner’s gland acini labeled with anti-mucin antibody (in green) and double labeled with anti-Munc18c, Syntaxin-4 or SNAP-23 antibodies (in red), and an overlay (merge) of the two with the DIC, which is also separately shown. Note the apical plasma membrane staining of Munc18c (and SNAP-23 and Syntaxin-4) and mucin accumulation beneath it. Scale bars correspond to 10 μm.
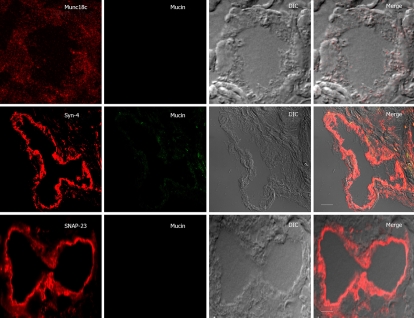
Figure 4.
SNARE localization after low carbachol (1 μmol/L) stimulation of Brunner’s gland acini. Confocal images of 1 μmol/L carbachol-stimulated Brunner’s gland acini labeled with anti-mucin antibody (in green) and double labeled with anti-Munc18c, Syntaxin-4 or SNAP-23 antibodies (in red), and an overlay (merge) of the two with the DIC, which is also separately shown. Note the loss of apical plasma membrane staining of Munc18c with some staining in the cytosol, and much reduced mucin staining. Scale bars correspond to 10 μm.
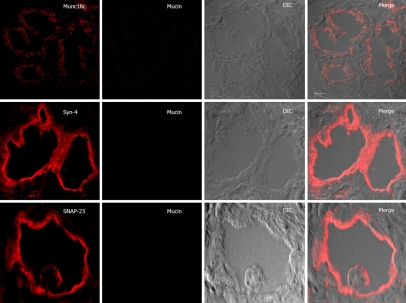
Figure 5.
SNARE localization after high carbachol (6 μmol/L) stimulation of Brunner’s gland acini. Confocal images of 6 μmol/L carbachol-stimulated Brunner’s gland acini labeled with anti-mucin antibody (in green) and double labeled with anti-Munc18c, Syntaxin-4 or SNAP-23 antibodies (in red), and an overlay (Merge) of the two with the DIC, which is also separately shown. Note the further reduced apical plasma membrane staining of Munc18c (vs Figure 4) and the absence of mucin staining in the cells. DIC images exhibit dilated lumens. Scale bars correspond to 10 μm.
Upon 1 μmol/L (Figure 4) and 6 μmol/L carbachol (Figure 5) stimulation, Munc18c staining of the apical plasma membrane was progressively reduced. With 1 μmol/L carbachol stimulation (Figure 4), there was much cytosolic staining of Munc18c, but which was no longer present with 6 μmol/L carbachol (Figure 5). The latter is likely due to greater cytosolic proteolysis of Munc18c, which we had previously observed in rat pancreatic acini with supramaximal cholinergic stimulation[11–14]. Our acini preparation would not permit us to obtain a large pure population of Brunner’s gland acini required to perform biochemical analysis (Western blots) to detect Munc18c proteolytic products, or for the presence of the other exocytotic SNARE and Munc18b proteins. In contrast to Munc18c, Syntaxin-4 and SNAP-23 signals on the apical plasma membrane after carbachol stimulation were as strong as unstimulated acini. As we had previously suggested in pancreatic acini, Munc18c displacement from the plasma membrane would enable Syntaxin-4 binding to SNAP-23 rendering the membrane receptive to exocytosis[11–15]. Indeed, the mucin staining of the apical poles of the acini was very much reduced after 1 μmol/L carbachol stimulation (Figure 4) and completely absent after 6 μmol/L carbachol stimulation (Figure 5). This reduction or disappearance of acini mucin would correlate to the E.M. results (Figure 3) of the loss of electron-lucent mucin vesicles. The absence of mucin staining in the lumen, which would have been an indication of exocytosed mucin, is likely due to dilution of mucin within the lumen as to render the mucin signal below detection.
DISCUSSION
Using frozen sections of carbachol-stimulated submucosal preparation of Brunner’s gland from guinea pig duodenum, we demonstrated the dilatation of acini lumen as an index of activation of secretion. These results are very similar to our previous reports using video-microscopy real-time recording of the real time changes of dilatation of acini lumen to track secretion[1,6,7]. In those reports, we showed the range of cholinergic stimulation was between 0.1 μmol/L to 10 μmol/L, with EC50 of 2 μmol/L. Here, we used carbachol concentrations on the upstroke of the dose-response curve, that is 1 and 6 μmol/L, to demonstrate mild and near maximal dilation of the ductal lumen. At 1 μmol/L carbachol stimulation, E.M. performed on the same preparation demonstrated some reduction of electron-lucent mucin containing vesicles and a mildly dilated ductal lumen. Here, many of the vesicles have undergone homotypic fusion and these prefused vesicles would surface to the apical plasma membrane to undergo compound exocytosis. These events were even more efficient at 6 μmol/L carbachol stimulation, where we noted near total collapse and emptying of these mucin vesicles, leaving fewer apical vesicles. Here, the ductal lumen was massively dilated by the exocytosed mucin, the latter confirmed on our earlier report by PAS staining where upon stimulation the PAS positive material is found between acinar cells rather than within the apical cytoplasm as observed in unstimulated preparations[1]. Due to anatomical reasons the best species to isolate Brunner’s gland acini is the guinea pig as both the quantity and quality of the acini obtained are superior to that rendered by rat or mouse. This is also an excellent model to examine both hormonal regulation and neural innervation (which is intact in the preparation)[1,6,7]; however, this preparation has some disadvantages. We were not able to perform biochemical studies that require large homogeneous populations of acini, which was not possible to obtain with this preparation. This limitation was a problem in this study as all of antibodies used were against rat and mouse proteins, most of which were not appropriate for immunofluorescence studies of guinea pig acini, but would have been at least slightly positive on western blotting of purified membrane fractions of acini of rat and mouse.
It appears that the molecular machinery for exocytosis is conserved in Brunner’s gland acini to mediate exocytosis. This machinery includes distinct combinations of syntaxins, SNAP-25 and VAMP proteins on cognate membrane compartments that would assemble into SNARE complex inducing fusion of the membranes[11–14]. Munc18 proteins bind Syntaxins to prevent their interactions with the other SNARE proteins[10]. Agonist stimulation of cells induces PKC mediated phosphorylation of Munc18 proteins, which activates the Syntaxins into open conformations capable of binding SNAP-25 and VAMPs[10]. We and others have previously demonstrated the distinct localization of homologs of these Munc18 (b and c) and SNARE proteins in rat pancreatic acini[11–24]. Specifically, Syntaxin-2 is on the pancreatic acinar apical plasma membrane, Syntaxin-3 on the zymogen granules and Syntaxin-4 on the basolateral membrane. Munc18b was on the apical membrane and Munc18c on the basolateral membrane. We had shown that supramaximal cholinergic (or cholecystokinin) stimulation redirected apical exocytosis to the basolateral plasma membrane, a process requiring Munc18c displacement from Syntaxin-4[11–15]. We postulated that Munc18c displacement activates Syntaxin-4 to form the exocytotic Syntaxin-4/SNAP-23/VAMP SNARE complex[11–15]. In this work, we find that this molecular exocytotic machinery is also operational in Brunner’s gland acini. However, it was surprising to see that this exocytotic machinery (Munc18c, Syntaxin-4 and SNAP-23) was localized to the apical plasma membrane of Brunner’s gland acini to mediate apical exocytosis. Of note, this exocytotic machinery also mediated apical exocytosis in rat parotid acinar cells[25–27]. In contrast, rabbit lacrimal acini seem to follow a similar distribution for these exocytotic proteins as those described in rat pancreatic acini[28]. In fact, like pancreatic acini[11–14], apical exocytosis in rabbit lacrimal acini could also be redirected to the basolateral membrane surface by prolactin[29]. The distinct localization of these exocytotic proteins within the family of exocrine tissues is of fundamental importance. Conventional thinking indicates that the localization of these proteins is conferred by signal sequences within these proteins[30,31], which does not seem to be the case for the family of exocrine tissues. Additional cues likely from the membrane compartments themselves or from cytosolic chaperoning proteins that may be different between exocrine glands also seem to be required in determining the compartmental specific targeting of these exocytotic proteins. This notion is supported by our very recent report of a novel protein called Cab45b that is required to chaperone Munc18b to its targeted compartments in pancreatic acinar cells[19].
The behavior of activated Munc18c seems to be cell-context specific. In exocrine tissues (Brunner’s gland, pancreatic and parotid acini), agonist stimulation resulted in Munc18c displacement from the plasma membrane into the cytosol as a prerequisite for Syntaxin-4 activation[11–15,26]. This is, however, not the case for GLUT4 exocytosis in adipose tissues and skeletal muscles, which also use this set of exocytotic proteins[32]. More recent reports suggest that Munc18c facilitates and actually stabilizes SNARE complex assembly[33,34], a process that induces Syntaxin-4 into its open conformation[35]. Although this process is associated with reduced Munc18c affinity to Syntaxin-4, the actual displacement of Munc18c from Syntaxin-4 is not required[33,34]. Perhaps in acinar tissues, changes in Munc18c conformation after phosphorylation may render the 65 kDa Munc18c protein susceptible to proteolytic degradation[11,14], and such cytosolic proteases may not be present or activated in adipose tissues and muscles. This possibility is supported by our reports in rat pancreatic acini demonstrating cytosolic Munc18c to be mostly a degraded 35 kDa product[11–14].
While apical exocytosis in Brunner’s gland acini is mediated by Munc18c/Syntaxin-4/SNAP-23, vesicle-vesicle fusions should be mediated by another exocytic machinery, likely involving Syntaxin-2 or -3 (and Munc18b) as is the case in pancreatic acini[8,9,16,21]. As for vesicle VAMPs, this likely includes VAMP-2 and/or VAMP-8[18,22,24,36]. Unfortunately, immunofluorescence studies we had carried out with a battery of antibodies directed against rat sequences (Munc18b, Syntaxin-2 and -3, VAMP-2 and -8) could not detect these proteins in the guinea pig Brunner’s gland acini. Whether other secretory products (immunoglobulin, lysozyme, growth factors, trefoil peptides) of Brunner’s gland acini[3–5] are contained in the same mucin vesicles or distinct vesicle populations involving distinct sets of exocytotic proteins is particularly intriguing and would require further investigation.
COMMENTS
Background
Brunner’s glands are small group of exocrine cells clustered together in small islands located in the duodenum where most peptic ulcers occur. These glands secrete bicarbonate and mucus (packaged in small vesicles) to protect the surrounding mucosa from injury that could be caused by the massive acid coming from the stomach.
Research frontiers
Exocytosis requires of membranes fusion. The SNARE Hypothesis implies minimal machinery for membrane fusion, including a cognate set of vesicle and target SNAREs on opposing membranes. Additionally, this hypothesis proposes that these SNARE proteins are prevented from spontaneous assembly by clamping proteins, here represented by Munc18c.
Innovations and breakthroughs
Brunner’s glands have been extremely difficult to study until recently when we developed a duodenal submucosal preparation from guinea pigs. Using this preparation, we have now started to examine the molecules responsible for stimulated fusion of the mucus-containing vesicles with the plasma membrane, called exocytosis.
Applications
Here, we have determined the involvement of a group of intimately-interacting molecules (Munc18c, Syntaxin 4, SNAP-23) in the exocytosis of mucus. This set of molecules is very similar to that which mediates similar exocytotic processes in other exocrine glands in the body, including pancreatic (secreting digestive enzymes) and salivary glands (secreting digestive enzymes and mucus), which are important for food digestion and lubrication. This would indicate that these molecules are conserved in all exocrine glands to regulate secretions important for intestinal protection and food digestion, and which could be targeted for novel drugs to improve these important functions in the treatment of related disorders (peptic ulcer disease, pancreatic insufficiency, salivary gland disorders).
Peer review
The authors evaluated mechanisms to mediate exocytosis of Brunner’s gland acini. Although many previous studies about exocytosis of pancreatic acinar cell were reported, as the authors mentioned, few were known about Brunner’s gland acini. They demonstrated with sophisticated technique that Munc18c and SNARE proteins including Syntaxin-4 and SNAP-23 act like they do in the pancreatic acini, and speculated that the mechanisms are similar to those of pancreatic acini. Since a model of Brunner’s gland in guinea pig duodenum is previously reported, the novelty of this report is to define the localization of Munc18c and SNARE proteins before and after cholinergic stimulus.
Acknowledgments
The authors thank Ms. Margaret O’Reilly for her excellent technical assistance.
Supported by Grants to H.Y.G. from the U.S. National Institute of Health, R21 AA015579-01A1 and to S.V. form the Canadian Institute of Health Research
Peer reviewer: Takafumi Ando, MD, PhD, Department of Gastroenterology, Nagoya University Graduate School of Medicine, Therapeutic Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 4668550, Japan
S- Editor Zhong XY L- Editor Rippe RA E- Editor Yin DH
References
- 1.Moore BA, Morris GP, Vanner S. A novel in vitro model of Brunner's gland secretion in the guinea pig duodenum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G477–G485. doi: 10.1152/ajpgi.2000.278.3.G477. [DOI] [PubMed] [Google Scholar]
- 2.Flemstrom G, Kivilaakso E. Demonstration of a pH gradient at the luminal surface of rat duodenum in vivo and its dependence on mucosal alkaline secretion. Gastroenterology. 1983;84:787–794. [PubMed] [Google Scholar]
- 3.Coutinho HB, Robalinho TI, Coutinho VB, Amorin AM, Almeida JR, Filho JT, Walker E, King G, Sewell HF, Wakelin D. Immunocytochemical demonstration that human duodenal Brunner's glands may participate in intestinal defence. J Anat. 1996;189(Pt 1):193–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen PS, Kirkegaard P, Poulsen SS, Nexo E. Effect of secretin and somatostatin on secretion of epidermal growth factor from Brunner's glands in the rat. Dig Dis Sci. 1994;39:2186–2190. doi: 10.1007/BF02090369. [DOI] [PubMed] [Google Scholar]
- 5.Khulusi S, Hanby AM, Marrero JM, Patel P, Mendall MA, Badve S, Poulsom R, Elia G, Wright NA, Northfield TC. Expression of trefoil peptides pS2 and human spasmolytic polypeptide in gastric metaplasia at the margin of duodenal ulcers. Gut. 1995;37:205–209. doi: 10.1136/gut.37.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore BA, Kim D, Vanner S. Neural pathways regulating Brunner's gland secretion in guinea pig duodenum in vitro. Am J Physiol Gastrointest Liver Physiol. 2000;279:G910–G917. doi: 10.1152/ajpgi.2000.279.5.G910. [DOI] [PubMed] [Google Scholar]
- 7.Kovac J, Moore B, Vanner S. Potassium currents regulating secretion from Brunner's glands in guinea pig duodenum. Am J Physiol Gastrointest Liver Physiol. 2004;286:G377–G384. doi: 10.1152/ajpgi.00153.2003. [DOI] [PubMed] [Google Scholar]
- 8.Gaisano HY. A hypothesis: SNARE-ing the mechanisms of regulated exocytosis and pathologic membrane fusions in the pancreatic acinar cell. Pancreas. 2000;20:217–226. doi: 10.1097/00006676-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Pickett JA, Edwardson JM. Compound exocytosis: mechanisms and functional significance. Traffic. 2006;7:109–116. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 10.Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 11.Gaisano HY, Lutz MP, Leser J, Sheu L, Lynch G, Tang L, Tamori Y, Trimble WS, Salapatek AM. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–1611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam PP, Cosen Binker LI, Lugea A, Pandol SJ, Gaisano HY. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic. 2007;8:605–617. doi: 10.1111/j.1600-0854.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 13.Cosen-Binker LI, Lam PP, Binker MG, Gaisano HY. Alcohol-induced protein kinase Calpha phosphorylation of Munc18c in carbachol-stimulated acini causes basolateral exocytosis. Gastroenterology. 2007;132:1527–1545. doi: 10.1053/j.gastro.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Cosen-Binker LI, Lam PP, Binker MG, Reeve J, Pandol S, Gaisano HY. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem. 2007;282:13047–13058. doi: 10.1074/jbc.M611132200. [DOI] [PubMed] [Google Scholar]
- 15.Gaisano HY, Sheu L, Whitcomb D. Alcoholic chronic pancreatitis involves displacement of Munc18c from the pancreatic acinar basal membrane surface. Pancreas. 2004;28:395–400. doi: 10.1097/00006676-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Sheu L, Tamori Y, Trimble WS, Gaisano HY. Cholecystokinin-regulated exocytosis in rat pancreatic acinar cells is inhibited by a C-terminus truncated mutant of SNAP-23. Pancreas. 2001;23:125–133. doi: 10.1097/00006676-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Gaisano HY, Sheu L, Foskett JK, Trimble WS. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. J Biol Chem. 1994;269:17062–17066. [PubMed] [Google Scholar]
- 19.Lam PP, Hyvarinen K, Kauppi M, Cosen-Binker L, Laitinen S, Keranen S, Gaisano HY, Olkkonen VM. A cytosolic splice variant of Cab45 interacts with Munc18b and impacts on amylase secretion by pancreatic acini. Mol Biol Cell. 2007;18:2473–2780. doi: 10.1091/mbc.E06-10-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen NJ, Antonin W, Edwardson JM. Identification of SNAREs involved in regulated exocytosis in the pancreatic acinar cell. J Biol Chem. 1999;274:22871–22876. doi: 10.1074/jbc.274.32.22871. [DOI] [PubMed] [Google Scholar]
- 21.Pickett JA, Thorn P, Edwardson JM. The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J Biol Chem. 2005;280:1506–1511. doi: 10.1074/jbc.M411967200. [DOI] [PubMed] [Google Scholar]
- 22.Pickett JA, Campos-Toimil M, Thomas P, Edwardson JM. Identification of SNAREs that mediate zymogen granule exocytosis. Biochem Biophys Res Commun. 2007;359:599–603. doi: 10.1016/j.bbrc.2007.05.128. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J Biol Chem. 2007;282:9635–9645. doi: 10.1074/jbc.M611108200. [DOI] [PubMed] [Google Scholar]
- 25.Takuma T, Arakawa T, Tajima Y. Interaction of SNARE proteins in rat parotid acinar cells. Arch Oral Biol. 2000;45:369–375. doi: 10.1016/s0003-9969(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 26.Imai A, Nashida T, Shimomura H. Roles of Munc18-3 in amylase release from rat parotid acinar cells. Arch Biochem Biophys. 2004;422:175–182. doi: 10.1016/j.abb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Imai A, Nashida T, Yoshie S, Shimomura H. Intracellular localisation of SNARE proteins in rat parotid acinar cells: SNARE complexes on the apical plasma membrane. Arch Oral Biol. 2003;48:597–604. doi: 10.1016/s0003-9969(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Jerdeva GV, da Costa SR, Sou E, Schechter JE, Hamm-Alvarez SF. Molecular mechanisms of lacrimal acinar secretory vesicle exocytosis. Exp Eye Res. 2006;83:84–96. doi: 10.1016/j.exer.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Chiu CT, Nakamura T, Walker AM, Petridou B, Trousdale MD, Hamm-Alvarez SF, Schechter JE, Mircheff AK. Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am J Physiol Endocrinol Metab. 2007;292:E1122–E1134. doi: 10.1152/ajpendo.00381.2006. [DOI] [PubMed] [Google Scholar]
- 30.Parlati F, McNew JA, Fukuda R, Miller R, Sollner TH, Rothman JE. Topological restriction of SNARE-dependent membrane fusion. Nature. 2000;407:194–198. doi: 10.1038/35025076. [DOI] [PubMed] [Google Scholar]
- 31.ter Beest MB, Chapin SJ, Avrahami D, Mostov KE. The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol Biol Cell. 2005;16:5784–5792. doi: 10.1091/mbc.E05-07-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurmond DC, Pessin JE. Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane (review) Mol Membr Biol. 2001;18:237–245. doi: 10.1080/09687680110082400. [DOI] [PubMed] [Google Scholar]
- 33.Latham CF, Lopez JA, Hu SH, Gee CL, Westbury E, Blair DH, Armishaw CJ, Alewood PF, Bryant NJ, James DE, et al. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 34.Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Andrea-Merrins M, Chang L, Lam AD, Ernst SA, Stuenkel EL. Munc18c interaction with syntaxin 4 monomers and SNARE complex intermediates in GLUT4 vesicle trafficking. J Biol Chem. 2007;282:16553–16566. doi: 10.1074/jbc.M610818200. [DOI] [PubMed] [Google Scholar]
- 36.Wang CC, Shi H, Guo K, Ng CP, Li J, Gan BQ, Chien Liew H, Leinonen J, Rajaniemi H, Zhou ZH, et al. VAMP8/endobrevin as a general vesicular SNARE for regulated exocytosis of the exocrine system. Mol Biol Cell. 2007;18:1056–1063. doi: 10.1091/mbc.E06-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]