Abstract
AIM: To determine clinical characteristics and treatment outcome of gastric lymphoma after chemotherapy and immuno-chemotherapy.
METHODS: Thirty four patients with primary gastric mucosa associated lymphoid tissue (MALT) lymphoma (Ann Arbor stages I to IV) were enrolled. All had upper gastric endoscopy, abdominal ultrasonography, CT and H pylori status assessment (histology and serology). After anti-H pylori treatment and initial chemotherapy, patients were re-examined every 4 mo.
RESULTS: Histological regression of the lymphoma was complete in 22/34 (64.7%) and partial in 9 (26.5%) patients. Median follow up time for these 31 responders was 60 mo (range 48-120). No regression was noted in 3 patients. Among the 25 (73.5%) H pylori positive patients, the eradication rate was 100%.
CONCLUSION: Using univariate analysis, predictive factors for overall survival were international prognostic index (IPI) score, hemoglobin level, erythrocyte sedimentation rate (ESR), and platelet numbers (P < 0.005). In addition to this, Cox proportion hazard model differentiate IPI score, ESR, and platelets as predictors of survival.
Keywords: MALT lymphoma, Prognostic factors, Clinical features, Treatment
INTRODUCTION
Gastric low grade B cell lymphomas arising from mucosa associated lymphoid tissue (MALT) are the most frequent lymphomas among those located in the primary digestive tract. Approximately 40% of all non-Hodgkin’s lymphomas occur in extranodal locations. The majority is of the diffuse large B cell type, but extranodal marginal zone B cell lymphoma of MALT is the commonest extranodal small B cell non-Hodgkin’s lymphoma. Approximately 37% of extranodal lymphomas occur in the gastrointestinal tract and esophagus. The most common site is the stomach (23% of extranodal lymphomas) followed by the small intestine (7.5%) and the colorectum (5.5%). The initiating step in the pathogenesis of MALT lymphoma at all sites is the acquisition of organized lymphoid tissue. This will have the characteristic features of MALT with a germinal centre, mantle and marginal zone, plasma cell differentiation, and an associated T cell component. B cells may infiltrate epithelial structures, if present, to mimic a lymphoepithelium similar to that seen in native MALT in Peyer’s patches[1]. The B cells of MALT lymphoma share the immunophenotype of reactive marginal zone B cells (CD20+, CD21+, CD35+, IgM+, and IgD-)[2]. The analysis of the rearranged variable region of the immunoglobulin heavy chain gene by means of polymerase chain reaction (PCR) shows a monoclonal proliferation of neoplastic B cells[3].
Gastric MALT lymphoma seems to develop along 2 major molecular pathways that emerge from the oncogenic inflammatory milieu of the stomach, one dependent on the presence of t (11; 18) and the other associated with a methylator-prone phenotype (CIMP). Four disparate chromosomal translocations are associated with extranodal MALT lymphoma, including t (11; 18) (q21; q21), t (1; 14) (p22; q32), t (14; 18) (q32; q21), and t (3; 14) (p14; q32)[4]. They most frequently affect the MALT1 gene, including both the t (11; 18) (q21; q21) and t (14; 18) (q32; q21), and interestingly, are mutually exclusive cytogenetic events. However, 3 of the translocations appear to involve a common pathogenic mechanism, leading to constitutive activation of NF-κB signaling. It has been reported that lymphomas responding to H pylori therapy are t (11; 18) negative[5], thus this translocation may be associated with more advanced disease. In the majority of cases in the stomach (but not all) the stimulus for acquisition of MALT is H pylori infection. Indeed, H pylori provides the antigenic stimulus which is mediated by mucosal T cells for sustaining growth of gastric MALT lymphoma. H pylori tumor cells show somatic hypermutation in the immunoglobulin genes that are characteristic of antigen selection[6]. MALT lymphoma cells resemble memory B cells still responsive to differentiation signals, such as CD40 costimulation and cytokines produced by antigen-stimulated helper T cells, and dependent for their growth on stimulation by H pylori-specific T cells. Thus, H pylori stimulation of lymphoma B cells is not direct, but occurs through tumor-infiltrating T cells, involving both CD40 and CD40L costimulatory molecules. The surface immunoglobulin on gastric MALT lymphoma B cells does not recognize H pylori, but instead recognizes various autoantigens, suggesting that malignant cells are transformed from autoreactive B cells. Initially, MALT lymphoma is sustained by H pylori-induced T cell help, remains localized, and subsequently regresses[7]. Since the first cases of gastric lymphoma (GL) regression after such eradication were reported in 1993, various remission rates of 41%-100% have been published for several low grade GL series[8].
In contrast to primary gastric NHL, secondary involvement of the GI tract by nodal NHL, which occurs in 20% to 60% of newly diagnosed cases, reflects disseminated disease that necessitates systemic treatment strategies[9,10]. Studies analyzing the incidence of secondary gastric NHL revealed a great discrepancy between the frequencies of GI involvement diagnosed before treatment as opposed to postmortem findings. The aim of this study was, therefore, to determine, in consecutive patients with gastric MALT lymphoma, the predictive factors of lymphoma regression in concordance with the course of the disease.
MATERIALS AND METHODS
Patients and diagnostic criteria
From July, 1997 to September, 2003, thirty four patients with primary gastric MALT lymphomas were enrolled in the study. Informed consent was obtained from all patients and the study was approved by the local Ethics Scientific Committee.
All patients underwent baseline endoscopy and gastric mucosal biopsies of the stomach. Lymphomas were staged clinically by the Ann Arbor system modified by Musshoff and Schmidt-Vollmer and by Rohatiner[11,12]. Staging procedures included recording of the patients’ physical examination, ileocolonoscopy, together with upper gastrointestinal tract endoscopy, small bowel and chest radiography, abdominal computed tomography (CT) scan, Waldeyer's ring examination with endoscopy and biopsies or CT scan, and bone marrow biopsy.
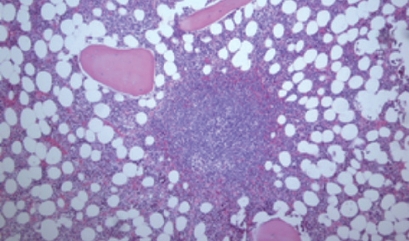
The histopathological diagnosis was based on the REAL classification. Histological specimens were stained with hematoxylin eosin (HE) and Giemsa (Figure 1). Immunohistochemical analyses (Dako, Glostrup, Denmark) were performed on all paraffin embedded biopsies, and MALT lymphoma cells, like marginal zone B lymphocytes, had phenotypic profile: CD20+, CD21+, CD35+, IgM+, CD5-, CD10-, IgD-, BCL-10+ (Figure 2).
Figure 1.
Bone marrow pathohistology in gastric MALT lymphoma patients (HE, × 100). Paratrabecular nodal lymphoid BM infiltration with neoplastic cells.
Figure 2.
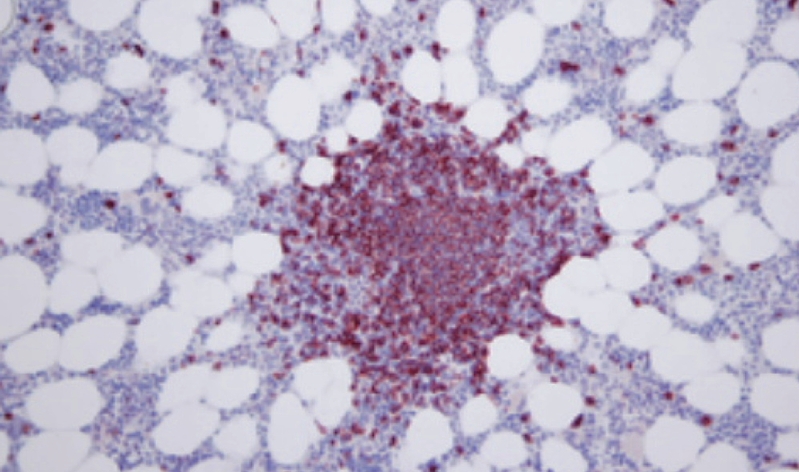
Immunohistochemistry of CD20 antigen expression in bone marrow biopsy (× 200). Nodal lymphoid BM infiltration with CD20+ in lymphoma cells.
All patients had HIV negative status (detected by ELISA testing-Axym, Abbott, USA, and Vitros, Ortho USA). H pylori was systematically looked for using the Giemsa stain method. Also, for culture, tissue biopsies were grounded, plated on three media (two selective and one non-selective), and incubated in a microaerobic atmosphere at 37°C for 7-10 d. H pylori was identified on the basis of positive oxidase, catalase, and urease tests.
Treatment
All patients with gastric MALT lymphoma received poly- or monochemotherapy (Cyclophosphamide, Doxorubicine, Vincristine, Prednisolone-CHOP; Chlorambucil, Vincristine, Procarbazine, Prednisolone-LOPP; or Chlorambucil) or combined immuno-chemotherapy (Rituximab-CHOP) as the first line treatment. Four patients were operated (subtotal gastrectomy). Patients who were H pylori positive, received triple anti-H pylori treatment combined with chemotherapy or immuno-chemotherapy as first line management.
Statistical analysis
Overall survival was calculated from the date of diagnosis until death or last follow-up. The actuarial survival curves were estimated using the Kaplan-Meier method. Student t-test was used to evaluate the difference in the values of clinical parameters. The log-rank test evaluated association between overall survival (OS) and clinical characteristics. The use of the Cox proportional hazards model determined independent prognostic factors which influenced OS, while logistic regression detected prognostic factors overall survival significant for outcome.
RESULTS
A total of 34 (18 male, 16 female, median age 62 years, range 34-82 years) patients with gastric MALT lymphoma were included in the study. Median follow up was 72 mo (range 48-120). Accordingly to clinical stage (Table 1), all patients were treated with chemotherapy (23/34 i.e. 67.6%), monochemotherapy only in patients with IE2 clinical stage, or immuno-chemotherapy (11/34 i.e. 34.4%). The reason for giving chemotherapy or immuno-chemotherapy to all patients as first line treatment was the BCL-10 positivity of all patients with clinical stage I + II. There were no differences in outcome of patients between the groups with mono and polychemotherapy. Also, all patients who were H pylori positive (25/34 i.e. 73.5%) received on d 0, the same anti-H pylori treatment for 14 d: 40 mg Omeprazole twice daily, 1 g amoxicillin twice daily, and 500 mg Clarithromycin twice daily. The first endoscopy follow up examination was performed 30 d after the end of treatment to check both the effectiveness of H pylori eradication, as assessed by histology, tissue culture, and PCR, and the absence of macroscopic tumor progression. Those patients with persistent H pylori were tested for susceptibility to Metronidazole and given a second line treatment. Patients with stable and progressive disease, and one patient with partial remission were operated (subtotal gastrectomy) after restaging 4 mo after the starting initial systemic therapy.
Table 1.
Modified Ann-Arbor clinical staging system
| Clinical stage | Number of patients (n) | |
| IE1 | Mucosa + submucosa | 0 |
| IE2 | Muscularis propria + subserosa | 8 |
| IIE1 | Perigastric lymph nodes | 2 |
| IIE2 | Regional lymph nodes | 8 |
| IIIE | Lymph nodes on both sides of the diaphragm | 4 |
| IVE | Visceral metastases or second extranodal site | 12 |
Various clinical characteristics of patients with MALT lymphoma, and data relating to therapy response are summarized in Table 2. Distribution of IPI value showed that the most number of patients, nine, had an IPI score of 3 (Figure 3).
Table 2.
Clinical data and response to treatment
| Clinical characteristics | n (%) |
| Dominant site of gastric lesion | |
| Fundus | 10 (29.4) |
| Corpus | 18 (53.0) |
| Antrum | 4 (17.6) |
| Anemia (Hb < 110 g/L) | 21 (61.7) |
| Thrombocytopenia (PLT < 100 × 109/L) | 9 (26.5) |
| Elevated ESR (> 10/1 h) | 16 (47.1) |
| Bone marrow infiltration | 8 (23.5) |
| Constitutional “B” symptoms | 28 (82.4) |
| Answer to therapy | |
| Complete remission | 22 (64.7) |
| Partial remission | 9 (26.5) |
| Stable disease | 1 (2.9) |
| Progressive disease | 2 (5.9) |
Figure 3.
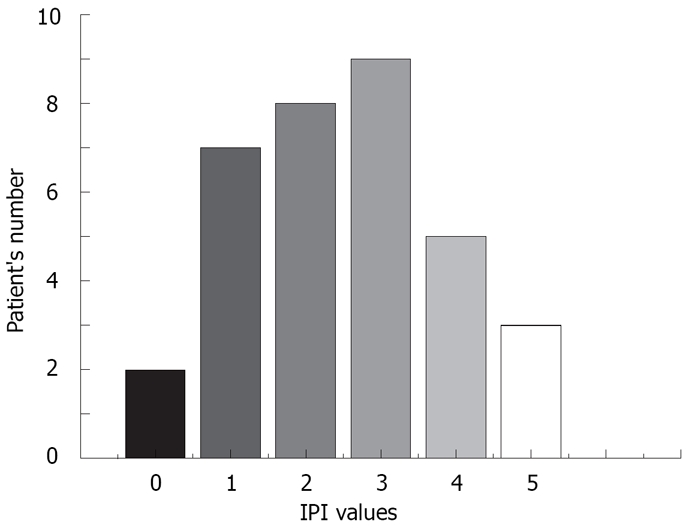
Distribution of IPI values in gastric MALT lymphoma patients. IPI score was a highly significant (P < 0.01) prognostic factor for overall survival of patients.
Patients had high remission rate to first-line chemotherapy or immunochemotherapy combined with anti-H pylori treatment. Remission was obtained in 91.2%, CR in 64.7% and PR in 26.5% of patients. After the end of the follow up period 28 (82.4%) patients were still alive. The cause of death in all patients was disease progression.
The actuarial survival curve estimated high survival rate (83%) in the first 12 mo of the following period (Figure 4).
Figure 4.
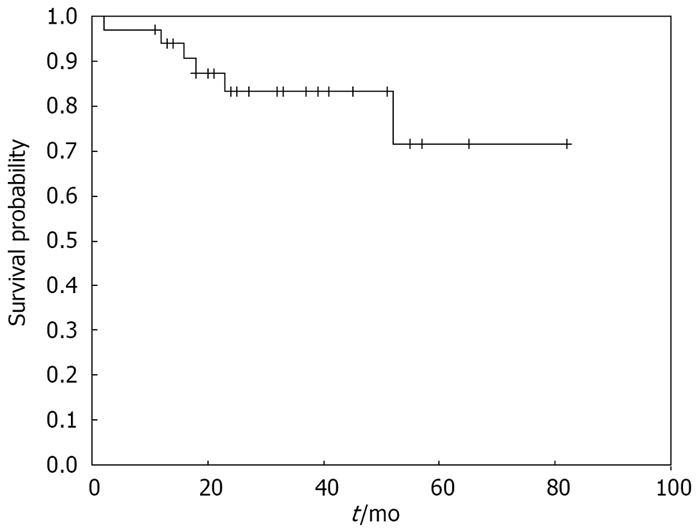
Kaplan-Meir curve of cumulative survival probability in gastric MALT lymphoma patients. Cumulative survival probability was high in the following period with low rate of death.
The use of the Cox proportional hazard model determined negative independent prognostic predictive factors for overall survival (OS): high IPI score, elevated ESR (erythrocyte sedimentation rate) and low platelets (χ2 = 13.397, df = 3, P = 0.0039).
Multivariate logistic regression showed the significance of elevated CS and low value of hemoglobin as independent prognostic factors that had negative influence for the outcome (dead or alive) of the disease (df = 1, P = 0.0049), whilst the elevated ESR had negative impact for achieving CR (df = 1, P = 0.0107).
DISCUSSION
We have described the clinical characteristics of primary gastric MALT lymphoma in 34 patients. Gastric MALT lymphoma offers a paradigm of infection-associated malignant disease. Most of them are multifocal and involve the antrum or distal body, but may occur in any part of the stomach[13].
Classically, MALT lymphomas reveal a histological triad composed of reactive lymphoid follicles, diffuse infiltration of small irregular lymphocytes (centrocytes), and lymphoepithelial lesions (LELs). The histological features closely simulate those of Peyer’s patches[14].
The lymphoma infiltrates, around reactive follicles in the region corresponding to the marginal zone, were found to be spreading diffusely into the surrounding mucosa. The tumor cells typically resemble reactive marginal zone B cells, having moderate amounts of pale clear cytoplasm. However, the cytological appearance in individual cases was varied. A characteristic feature of MALT lymphomas is the presence of LELs formed by the invasion of glands by neoplastic cells.
We have focused on the patients treated with chemotherapy (67.6%) or immuno-chemotherapy (34.4%) as first line treatment, combined with triple way therapy against H pylori in Helicobacter positive patients. The reason for this approach was the fact that all patients in I and II CS expressed BCL-10, and also had constitutional “B” symptoms, so we identified them as high risk patients. In about 25% of cases, resistance of gastric MALT lymphoma to H pylori eradication seems to be caused by chromosomal translocation involving the BCL-10 locus, such as t (1; 14) (p22; q32) and t (1;2) (p22; p12)[15]. They are typically found at advanced stages and are unlikely to respond to H pylori eradication. GL bearing these translocations can be detected immunohistochemically by strong BCL-10 nuclear expression[16].
In concordance with this is fact that patients who have t (11; 18) do not respond to anti-H pylori treatment. This translocation was described in 1989, and occurs specifically in MALT lymphoma, but not in other non-Hodgkin’s lymphomas, including its absence in cases of nodal and splenic marginal zone lymphoma.
The translocation is absent from H pylori gastritis and other premalignant diseases[17]. The t (1; 14) (p22; q32) and t (1; 2) (p22; p12) were described in MALT lymphoma in 1990 and 2000, respectively[18,19]. They have been reported exclusively in MALT lymphoma and represent approximately 3% of gastric MALT lymphomas[20].
The translocation t (11; 18) seems to favorably influence the response to H pylori eradication whereas its expression and the presence of BCL-10 mutations appear to be associated with failure to respond to antibiotics and with a more aggressive disease behavior[21,22].
Under normal circumstances, signaling through the antigen receptor facilitates the interaction of BCL-10 and MALT1, which synergize to activate NF-κB. BCL-10 forms a complex with MALT1 and the 2 molecules synergize in the activation of NF-κB. In cases with t (11; 18) (q32; q21), the API2-MALT1 fusion protein activates the NF-κB pathway through self-oligomerization. MALT lymphomas with these translocations show strong to moderate nuclear BCL-10 expression, and give anti-apoptotic signal to neoplastic lymphoid cells[20–22].
There is some evidence that a few factors may influence a better chance of response. One factor may be limited extension of the disease, affecting only superficial layers of the stomach, low grade histopathology with low proportion of blast cells and clusters of blast cells[23].
Other factors may be the specific gastric localization (with improved chances for gastric distal localization). The favorable response to H pylori eradication is rather exceptional and does not mean that all patients with localized (stage I) high grade gastric MALT lymphoma should be treated exclusively with eradication treatment. Rather it suggests that after eradication therapy as the initial treatment in patients with limited superficial disease, no infiltration of deeper layers of the gastric wall, and limited areas of high grade lymphoma, and most of all, when these patients can be closely monitored, chemotherapy may be postponed until follow up indicates whether or not further treatment is necessary. A prospective study of larger numbers of such patients may help to detect the factors that may predict a good response to H pylori eradication to avoid the use of an unnecessary and toxic treatment such as chemotherapy in patients that may already have been cured[24,25].
In our group of patients, we have a large proportion with advanced CS, III and IV (16 i.e. 47%), but the diagnosis was clear that it was primary gastric lymphoma. Obvious differences between primary and secondary gastric lymphoma became evident with regard to tumor localization and growth pattern. Secondary gastric NHL was seen more frequently as multifocal disease involving the gastric fundus and duodenum; both sites are rarely affected in primary gastric NHL like in our patients. In contrast to secondary gastric NHL, unifocal growth pattern is the most important endoscopic finding in primary gastric NHL. A unifocal growth pattern facilitates local radical treatment strategies, such as surgery, which may be associated with prolonged remission and a more favorable prognosis[10,26].
As the part of diagnostic procedures, we used abdominal CT in concordance to modified Ann Arbor staging system. According to CS and findings that none of the patients had CS IE1 and these with CS IE2, CS IIE1 and more advanced staged were BCL-10+ with “B” symptoms, we made decision to aggressively treat them with or without anti-H pylori treatment (in dependence of H pylori status). The most recent studies presented the role of endoscopic ultrasound (EUS) as procedure that should be included as part of staging for the diagnosis and treatment of gastric MALT lymphoma. The presence or absence of deep submucosal invasion as assessed by EUS was the most critical factor for pretreatment assessment. Therefore, EUS has a much higher sensitivity in distinguishing stage IE from stage IIE1 lymphoma, compared with CT scan. Furthermore, with analysis of the response data according to depth of infiltration, the overall response rate was highest for mucosa stage IE1 and then decreased with the depth of gastric wall infiltration[23–29].
Among all examined prognostic factors, the IPI score was the most powerful prognostic factor for OS, together with ESR and platelet number in the Cox hazard regression model (P = 0.0039). The distribution of IPI score showed that only two patients had IPI = 0, and the highest number of patients (9) had IPI of intermediate-high risk (IPI = 3). About one quarter of patients had thrombocytopenia, due to BM infiltration, which correlated with shorter survival. The standard biochemistry parameter ESR was elevated in one half of patients, and the acute phase reactant had prognostic influence on survival. In addition, elevated ESR had a negative influence on achieving CR by using multivariate logistic regression. Anemia, as a result of erythrocytopoiesis suppression in malignant disease and due to BM infiltration, had a negative influence on disease outcome as well as high Ann Arbor modified clinical stage. Our findings are in agreement with prognostic factor analysis in described literature data[29,30].
In contrary to persistence of disease, the basic goal would be prevention of MALT lymphoma appearance. Even though, H pylori is a tumor-inducing pathogen, it does not induce malignant diseases in the vast majority of infected hosts. Therefore, vaccination strategies must acknowledge this co-evolution of H pylori with its human host in which many of the host-pathogen interactions that occur could prove potentially beneficial[31].
In conclusion, these data show that adequate patient selection for treatment option offers them a good chance for long-term survival. Using novel diagnostic procedures, such as EUS, differentiate low risk patient candidates for only antibiotic approach and avoids an aggressive treatment option. As a final point, murine models using infection with several gastric Helicobacter species provided a distinctive experimental method to examine the progression of the MALT lymphoma from early immune responses to fully developed malignant disease. These systems offer the model of gastric lymphoma development through a series of molecularly events. Analysis of these steps using gene expression profiling gave some insights into the mechanisms involved in the lymphogenesis and can be prove in large prospective studies[32].
COMMENTS
Background
Gastric low grade B cell lymphomas arising from mucosa associated lymphoid tissue (MALT) are the most frequent lymphomas among those located in the primary digestive tract. The translocation t (11; 18) seems to favorably influence the response to H pylori eradication whereas its expression and the presence of BCL-10 mutations appear to be associated with failure to respond to antibiotics and with a more aggressive disease behavior. In about 25% of cases, resistance of gastric MALT lymphoma to Helicobacter pylori eradication seems to be caused by chromosomal translocation involving the BCL-10 locus, such as t (1; 14) (p22; q32) and t (1; 2) (p22; p12). They are typically found at advanced stages and are unlikely to respond to H pylori eradication. Besides, few factors may influence a better chance of response. One factor may be limited extension of the disease, distal gastric localization, affecting only superficial layers of the stomach, low grade histopathology with low proportion of blast cells and clusters of blast cells.
Research frontiers
The favorable response to H pylori eradication is rather exceptional and does not mean that all patients with localized (stage I) high grade gastric MALT lymphoma should be treated exclusively with eradication treatment. Rather it suggests that after eradication therapy as the initial treatment in patients with limited superficial disease, no infiltration of deeper layers of the gastric wall, and limited areas of high grade lymphoma, and most of all, when these patients can be closely monitored, chemotherapy may be postponed until follow up indicates whether or not further treatment is necessary.
Innovations and breakthroughs
We have focused on the patients treated with chemotherapy (67.6%) or immuno-chemotherapy (34.4%) as first line treatment, combined with triple way therapy against H pylori in Helicobacter positive patients. The reason for this approach was the fact that all patients in I and II CS expressed BCL-10, and also they had constitutional “B” symptoms, so we identified them as high risk patients. Patients had high remission rate (91.2%) to first-line chemotherapy or immuno-chemotherapy combined with anti-H pylori treatment. Among all examined prognostic factors, the IPI score was a powerful prognostic factor for OS, together with ESR and platelet number in the Cox hazard regression model (P = 0.0039).
Applications
These data show that adequate patient selection for treatment option offers them good chance for long-term survival.
Peer review
The manuscript is an interesting, generally well written series of gastric MALTOMA patients, reflecting on the impact of clinical factors upon outcome with current treatment.
Supported by Ministry of Science, Project No. 145061
Peer reviewer: Marc Basson, MD, PhD, MBA, Chief of Surgery, John D. Dingell VA Medical Center, 4646 John R. Street, Detroit, MI 48301, United States
S- Editor Sun YL L- Editor Rippe RA E- Editor Yin DH
References
- 1.Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology. 1992;20:29–34. doi: 10.1111/j.1365-2559.1992.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 2.Manson SD. Mucosa-associated lymphoid tissue (MALT) lymphoma. Semin Oncol Nurs. 2006;22:73–79. doi: 10.1016/j.soncn.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Spencer J, Finn T, Pulford KA, Mason DY, Isaacson PG. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol. 1985;62:607–612. [PMC free article] [PubMed] [Google Scholar]
- 4.Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652–658. doi: 10.1038/sj.leu.2403644. [DOI] [PubMed] [Google Scholar]
- 5.Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Goodlad J, Lavergne-Slove A, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–1018. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- 6.Du M, Diss TC, Xu C, Peng H, Isaacson PG, Pan L. Ongoing mutation in MALT lymphoma immunoglobulin gene suggests that antigen stimulation plays a role in the clonal expansion. Leukemia. 1996;10:1190–1197. [PubMed] [Google Scholar]
- 7.Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, Megraud F, Palazzo L, de Mascarel A, Molina T, Rambaud JL. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297–303. doi: 10.1136/gut.48.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong SS, Jung HY, Choi KD, Song HJ, Lee GH, Oh TH, Jo JY, Kim KJ, Byeon JS, Myung SJ, et al. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter. 2006;11:569–573. doi: 10.1111/j.1523-5378.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 10.Kolve M, Fischbach W, Greiner A, Wilms K. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-Hodgkin's lymphoma. German Gastrointestinal Lymphoma Study Group. Gastrointest Endosc. 1999;49:307–315. doi: 10.1016/s0016-5107(99)70006-4. [DOI] [PubMed] [Google Scholar]
- 11.Musshoff K, Schmidt-Vollmer H. Proceedings: Prognosis of non-Hodgkin's lymphomas with special emphasis on the staging classification. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1975;83:323–341. doi: 10.1007/BF00573019. [DOI] [PubMed] [Google Scholar]
- 12.Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397–400. doi: 10.1093/oxfordjournals.annonc.a058869. [DOI] [PubMed] [Google Scholar]
- 13.Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology. 1992;20:29–34. doi: 10.1111/j.1365-2559.1992.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 14.Isaacson PG, Wotherspoon AC, Diss T, Pan LX. Follicular colonization in B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Surg Pathol. 1991;15:819–828. doi: 10.1097/00000478-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Isaacson PG, Du MQ. Gastric lymphomas: genetics and resistance to H. pylori eradication. Verh Dtsch Ges Pathol. 2003;87:116–122. [PubMed] [Google Scholar]
- 16.Ye H, Dogan A, Karran L, Willis TG, Chen L, Wlodarska I, Dyer MJ, Isaacson PG, Du MQ. BCL10 expression in normal and neoplastic lymphoid tissue. Nuclear localization in MALT lymphoma. Am J Pathol. 2000;157:1147–1154. doi: 10.1016/S0002-9440(10)64630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine EG, Arthur DC, Machnicki J, Frizzera G, Hurd D, Peterson B, Gajl-Peczalska KJ, Bloomfield CD. Four new recurring translocations in non-Hodgkin lymphoma. Blood. 1989;74:1796–1800. [PubMed] [Google Scholar]
- 18.Wotherspoon AC, Soosay GN, Diss TC, Isaacson PG. Low-grade primary B-cell lymphoma of the lung. An immunohistochemical, molecular, and cytogenetic study of a single case. Am J Clin Pathol. 1990;94:655–660. doi: 10.1093/ajcp/94.5.655. [DOI] [PubMed] [Google Scholar]
- 19.Achuthan R, Bell SM, Leek JP, Roberts P, Horgan K, Markham AF, Selby PJ, MacLennan KA. Novel translocation of the BCL10 gene in a case of mucosa associated lymphoid tissue lymphoma. Genes Chromosomes Cancer. 2000;29:347–349. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1048>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Ye H, Gong L, Liu H, Hamoudi RA, Shirali S, Ho L, Chott A, Streubel B, Siebert R, Gesk S, et al. MALT lymphoma with t(14;18)(q32;q21)/IGH-MALT1 is characterized by strong cytoplasmic MALT1 and BCL10 expression. J Pathol. 2005;205:293–301. doi: 10.1002/path.1715. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Garces J, Dong HY. Detection of the t(11;18) API2/MALT1 translocation associated with gastric MALT lymphoma in routine formalin-fixed, paraffin-embedded small endoscopic biopsy specimens by robust real-time RT-PCR. Am J Clin Pathol. 2006;126:931–940. doi: 10.1309/unxjvamv77jam4fm. [DOI] [PubMed] [Google Scholar]
- 22.Ohshima K, Muta H, Kawasaki C, Muta K, Deyev V, Kanda M, Kumano Y, Podack ER, Kikuchi M. Bcl10 expression, rearrangement and mutation in MALT lymphoma: correlation with expression of nuclear factor-kappaB. Int J Oncol. 2001;19:283–289. [PubMed] [Google Scholar]
- 23.Flieger D, Fischbach W. MALT-lymphoma. Praxis (Bern 1994) 2006;95:1163–1168. doi: 10.1024/0369-8394.95.31.1163. [DOI] [PubMed] [Google Scholar]
- 24.Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdorffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. doi: 10.1016/s0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 25.Montalban C, Santon A, Boixeda D, Bellas C. Regression of gastric high grade mucosa associated lymphoid tissue (MALT) lymphoma after Helicobacter pylori eradication. Gut. 2001;49:584–587. doi: 10.1136/gut.49.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streubel B, Seitz G, Stolte M, Birner P, Chott A, Raderer M. MALT lymphoma associated genetic aberrations occur at different frequencies in primary and secondary intestinal MALT lymphomas. Gut. 2006;55:1581–1585. doi: 10.1136/gut.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgner A, Thiede C, Bayerdörffer E, Alpen B, Wündisch T, Neubauer A, Stolte M. Long-term follow-up of gastric MALT lymphoma after H. pylori eradication. Curr Gastroenterol Rep. 2001;3:516–522. doi: 10.1007/s11894-001-0073-9. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454–460. doi: 10.1136/gut.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisbert JP, Aguado B, Luna M, Nistal S, Asenjo LM, Reina T, Acevedo A, Arranz R. Gastric MALT lymphoma: clinical characteristics and prevalence of H. pylori infection in a series of 37 cases. Rev Esp Enferm Dig. 2006;98:655–665. doi: 10.4321/s1130-01082006000900003. [DOI] [PubMed] [Google Scholar]
- 30.Castrillo JM, Montalbán C, Abraira V, Carrion R, Cruz MA, Laraña JG, Menarguez J, Bellas C, Piris MA, Gomez-Marcos F, et al. Evaluation of the international index in the prognosis of high grade gastric malt lymphoma. Leuk Lymphoma. 1996;24:159–163. doi: 10.3109/10428199609045724. [DOI] [PubMed] [Google Scholar]
- 31.Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 32.O'Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of 'Helicobacter heilmannii' infection. J Pathol. 2004;203:896–903. doi: 10.1002/path.1593. [DOI] [PubMed] [Google Scholar]