Abstract
AIM: To investigate the correlation between the changes of pancreatic enzyme, the biochemical markers and the clinical results according to the Balthazar computer tomography (CT) grade.
METHODS: Between July 2004 and July 2005, we reviewed the charts of 119 patients who were admitted to our hospital with acute pancreatitis.
RESULTS: Eighty-three patients (69.7%) were male, and the mean age of the patients was 57 ± 15.7 years. The biliary pancreatitis patients had an older mean age. Forty-nine patients (41.1%) had biliary pancreatitis and forty-six (38.6%) had alcoholic pancreatitis. Group 3 patients had a longer duration of pain (2.51 ± 1.16 vs 3.17 ± 1.30 vs 6.56 ± 6.13, P < 0.001), a longer period of fasting (7.49 ± 4.65 vs 10.65 ± 5.54 vs 21.88 ± 13.81, P < 0.001) and a longer hospital stay (9.17 ± 5.34 vs 14.63 ± 8.65 vs 24.47 ± 15.52, P < 0.001) than the other groups. On the univariate analysis, the factors that affected the radiological grade were the leukocyte count at admission (P = 0.048), the hemoglobin (P = 0.016) and total bilirubin concentrations (P = 0.023), serum lipase (P = 0.009), the APACH II scores at admission (P = 0.017), the APACH II scores after 24 h (P = 0.031), the C-reactive protein (CRP) titer (P = 0.0001) and the follow up CRP titer (P = 0.003). But the CRP level (P = 0.001) and follow up CRP titer (P = 0.004) were only correlated with the radiological grade on multivariate analysis. According to the ROC curve, when we set the CRP cut off value at 83 mg/L, the likelihood ratio for a positive test was 3.84 and the likelihood ratio for a negative test was 0.26 in group 3.
CONCLUSION: In conclusion, our study suggests that the CRP with the radiological severity may be used to estimate the severity of acute pancreatitis.
Keywords: Acute pancreatitis, Computed tomography, C-reactive protein
INTRODUCTION
Acute pancreatitis is major cause of acute abdominal pain, and it is caused by alcohol ingestion, biliary stone, idiopathic causes and therapeutic endoscopy[1,2]. The clinical presentation of the acute pancreatitis is variable. Most of these patients recover without specific complications, but some patients display severe complication such as pancreatic ascites and pancreatic necrosis; these patients show high mortality[3,4].
The diagnostic markers (pancreatic enzymes such as amylase and lipase) and the risk factors that affect the clinical outcome are not well established by the previous clinical studies[5–7]. However, we have reservations about any correlation of various diagnostic markers, including pancreatic enzymes and the clinical features, to the outcome of acute pancreatitis. Further, the recent development of radiological diagnostic instruments allows for a more accurate diagnosis and easier follow up[8–10]. So in this study, we determined the correlation between the levels of pancreatic enzyme and the radiological severity of acute pancreatitis.
MATERIALS AND METHODS
Between July 2004 and July 2005, we reviewed the records of 119 patients who were admitted to Chung Nam National University Hospital with acute pancreatitis. The diagnosis of acute pancreatitis was based on typical symptoms, including acute abdominal pain and a serum amylase level that was three times higher than the normal limit. After diagnosis is established, computed tomography (CT) scanning was performed to determine the findings and grade of disease. We excluded cases which the CT scan was not performed. We reclassified the CT grade into three groups. Group 1 was CT grade A + B, group 2 was CT grade C and group 3 was CT grade D + E. Serum amylase and lipase levels, the equivalent series resistance (ESR), and C-reactive protein (CRP) were tested and measured at admission, and again at 24, 48 and 72 h after admission. Also, the Ranson score and APACHE II score were calculated at the same time. The above markers were also measured when the patients started their oral diet. Abdominal CT was performed weekly to evaluate the change of severity of pancreatitis before starting an oral diet. We evaluated the correlation of these various factors and the clinical severity of acute pancreatitis.
Statistical analysis
Each result was calculated as a mean value and standard error. We analyzed the data using the SPSS 13.0 for windows. Chi-square tests, Student t-test, One-Way ANOVA test and MANOVA tests were used. For the different scoring systems, the sensitivity, specificity and overall correctness of prediction, the positive and negative predictive values and the likelihood ratios of the positive and negative tests were determined. Each score value obtained from the different scoring systems was used to calculate the different true positive (sensitivity) and false positive (1-specificity) rates to create the ROC curves. A P value less than 0.05 was considered a statically significant result.
RESULTS
A total of 119 acute pancreatitis patients were included in this study. The characteristics of the patients are shown in Table 1. Eighty-three patients (69.7%) were male, and the mean age of the patients was 57 ± 15.7 years. The biliary pancreatitis patients mean age was older than that of the alcoholic pancreatitis. Forty-nine patients (41.1%) showed biliary pancreatitis and there were forty-six (38.6%) alcoholic pancreatitis patients. Forty-six patients suffered with drug-associated pancreatitis and 21 patients suffered with idiopathic pancreatitis. Various autoantibodies were checked for rule out autoimmune pancreatitis for the idiopathic pancreatitis patients. According to radiological severity, there were 41 (34.5%) patients in-group 1, 46 (38.6%) patients were in-group 2 and 32 (26.8%) patients were in-group 3. The CT grade was severe in the alcoholic pancreatitis patients. The serum pancreatic enzyme level at admission was higher in the biliary pancreatitis patients than in the alcoholic pancreatitis patients, but statical significance was not present (Table 2).
Table 1.
Characteristics of each group (%)
| Total | G-1 | G-2 | G-3 | |
| Sex | ||||
| Male | 83 (69.7) | 8 (23.5) | 30 (25.2) | 25 (21.0) |
| Female | 36 (30.3) | 13 (10.9) | 16 (13.4) | 7 (6.0) |
| Mean age (yr) | 55.7 ± 15.7 | 51.2 ± 15.3 | 62.4 ± 14.6 | 51.9 ± 14.8 |
| Etiology | ||||
| Biliary | 49 | 21 (42.9) | 21 (42.9) | 7 (14.2) |
| Alcoholic | 46 | 8 (17.0) | 19 (41.3) | 19 (41.3) |
| Drug | 3 | 2 (67.0) | 0 ( 0.0) | 1 (33.3) |
| Idiopathic | 21 | 10 (47.6) | 6 (28.6) | 5 (23.8) |
| Radiologic grade | 41 (34.3) | 46 (38.6) | 32(26.8) |
G-1: Balthazar CT grade A, B; G-2: Balthazar CT grade C; G-3: Balthazar CT grade D, E.
Table 2.
Serum pancreatic enzyme (at initial) according to etiology
| Amylase | Lipase | L/A ratio | |
| Alcohol | 614 ± 466.9 | 2227.2 ± 1988.8 | 4.3 ± 2.4 |
| Biliary | 1107.6 ± 1185.9 | 3561.6 ± 3023.8 | 3.9 ± 2.0 |
| Drug | 427 ± 273.5 | 1320.3 ± 811.7 | 3.1 ± 0.1 |
| Idiopathic | 893 ± 1026.9 | 2481.9 ± 2983.9 | 3.6 ± 2.5 |
Reference values: Total amylase 13-65 IU/L, lipase 0-200 IU/L.
Early elevation of serum pancreatic enzyme showed a statically significant result for the severe pancreatitis group. In this group, 3 of the patients’ initial serum lipase concentrations were higher than that of the other groups and this decreased more rapidly during 24 h (P = 0.008) after admission. The serum albumin level, BMI and Ranson score are known to be predictive risk factors for acute pancreatitis, but in our study, they did not correlate with the CT grade.
What factors were associated with radiological severity grade in our study? On univariate analysis, old age, the initial leukocyte count, the initial hemoglobin level, the initial APACH II score and the APACH II score after 24 h, the initial serum lipase concentration and initial CRP titer were all correlated with the CT grade (Table 3). But, the initial CRP level and follow up CRP titer were only correlated with the radiological grade on multivariate analysis (Table 4). Between the CRP, APACH II score and the Ranson score (Table 5), the CRP titer is more predictive and this diagnostic test result will raise the pre-test probability for prediction of the radiological severity. Group 3 patients had a longer duration of pain (2.51 ± 1.16 vs 3.17 ± 1.30 vs 6.56 ± 6.13, P < 0.001), a longer fasting period without an oral diet (7.49 ± 4.65 vs 10.65 ± 5.54 vs 21.88 ± 13.81, P < 0.001) and a longer hospital stay (9.17 ± 5.34 vs 14.63 ± 8.65 vs 24.47 ± 15.52, P < 0.001) than other groups. Also, the complication rate of pancreatitis was higher in-group 3 than in the other two groups (P < 0.005).
Table 3.
Univariate analysis of predictive factors
| G-1 | G-2 | G-3 | P-value | |
| BMI | 2.5 ± 0.7 | 2.3 ± 0.7 | 2.2 ± 0.6 | 0.16 |
| Leukocytosis (/mm3) | 11 249.0 ± 6964.3 | 10 555.0 ± 4212.5 | 17 700.6 ± 23 913.3 | 0.048a |
| Hemoglobin (g/dL) | 13.7 ± 1.9 | 13.4 ± 2.1 | 14.8 ± 2.3 | 0.016a |
| Platelet (103/mm2) | 223.3 ± 92.1 | 220.5 ± 61.2 | 232.0 ± 95.8 | 0.829 |
| AST (IU/L) | 233.2 ± 253.9 | 337.9 ± 645.8 | 176.3 ± 393.7 | 0.31 |
| ALT (IU/L) | 212.0 ± 221.8 | 201.5 ± 235.2 | 139.2 ± 237.9 | 0.368 |
| Total bilirubin (mg/dL) | 2.8 ± 2.2 | 2.2 ± 1.9 | 1.5 ± 1.1 | 0.023a |
| Albumin (g/dL) | 3.9 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.6 | 0.51 |
| S-amylase (IU/L) | 768.3 ± 926.4 | 762.7 ± 517.3 | 1243.1 ± 1531.7 | 0.079 |
| S-lipase (IU/L) | 1944.2 ± 1560.4 | 2820.3 ± 2521.4 | 3862.8 ± 3586.6 | 0.009a |
| Ranson (initial) | 1.7 ± 1.3 | 2.1 ± 1.2 | 1.8 ± 1.1 | 0.272 |
| Ranson | 0.7 ± 0.7 | 1.2 ± 1.0 | 0.9 ± 0.9 | 0.052 |
| (24 h after) | ||||
| A PACH-II (initial) | 4.8 ± 4.3 | 7.5 ± 4.3 | 6.0 ± 4.5 | 0.017a |
| APACH-II (24 h after) | 3.5 ± 3.0 | 5.5 ± 3.8 | 4.7 ± 3.8 | 0.031a |
| CRP (initial) (mg/dL) | 4.5 ± 5.2 | 5.5 ± 4.5 | 11.9 ± 7.1 | 0.000a |
| CRP (f/u) | 2.4 ± 3.7 | 2.4 ± 2.5 | 6.6 ± 5.5 | 0.003a |
P < 0.05.
Table 4.
Multivariate analysis of predictive factors
| Factors | P value |
| Leukocytosis | 0.29 |
| Hemoglobin | 0.07 |
| Total bilirubin | 0.31 |
| S-lipase | 0.44 |
| APACH-II (initial) | 0.65 |
| APACH-II (24 h after) | 0.47 |
| CRP (initial) | 0.001a |
| CRP (f/u) | 0.004a |
P < 0.05.
Table 5.
Likelihood ratio for prognostic markers at two time intervals
| Marker | Initial | 24 h after |
| CRP | 2.22/0.39 | 2.09/0.46 |
| Ranson | 1.22/0.71 | 1.09/0.87 |
| APACH-II | 1.23/0.70 | 1.01/1.00 |
Positive likelihood ratio/negative likelihood ratio; Positive likelihood ratio = sensitivity/(1-specificity); Negative likelihood ratio = (1-sensitivity)/specificity.
According to the ROC curve (Figure 1), when we set the CRP cut off value at 83 mg/L, the likelihood ratio for a positive CPR test was 3.84 and the likelihood ratio for a negative CPR test was 0.26 in group 3. This result showed good correlation with the radiological grade in the course of acute pancreatitis (Table 6).
Figure 1.
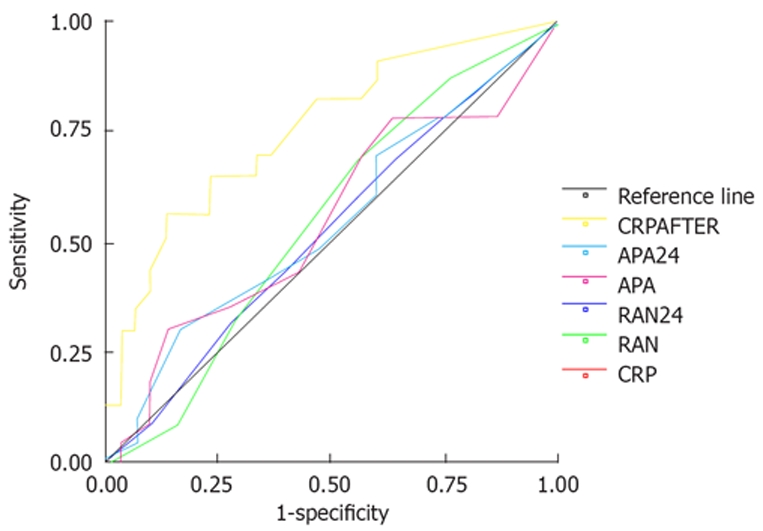
ROC curve predictive severe radiologic severity.
Table 6.
CRP test
| Cut-off value | 8.3 | 5.8 | 2.7 |
| Sensitivity | 70.6% | 73.5% | 69.6% |
| Specificity | 81.6% | 68.4% | 68.7% |
| Likelihood for positive test | 3.84 | 2.33 | 2.22 |
| Likelihood for negative test | 0.26 | 0.44 | 0.45 |
CRP: C-reactive protein (reference value: 0-5 mg/L).
DISCUSSION
Acute pancreatitis arises from a variety of causes. The process of this disease varies from mild to severe necrotic pancreatitis. Most of these patients recover through the conservative management[11,12]. But, a few patients progress to pancreatic necrosis and multi-organ dysfunction. In this study, the mortality was very high and near 30%[13].
There was no gold standard therapy for acute pancreatitis except for conservative management such as fasting and injection of analgesics. Therefore, prediction of severity has not yet been important for the treatment of acute pancreatitis. However, this will be an important task in the future for the preventive and treatment of complications because various drugs and therapy strategies are now being tried[6].
We know that various methods have been used to predict the progress of acute pancreatitis, such as clinical evaluation (include multivariate analysis of the prognostic factors), radiological evaluation via abdominal CT, and testing of various serological markers[14–18]. The development of abdominal CT allows precise estimation of the severity of pancreas and retroperitoneal lesion. Furthermore, changes of parenchymal necrosis are now being evaluated using a dye that shows the changes of the microcirculation in the pancreas. Abdominal CT was recently used for determining the presence and diagnosis of complications in acute pancreatitis[8,9,19]. The CT grade has shown good correlations with the clinical course and the prediction of mortality, so this is good method as compared to multivariate estimation of different factors[17,20]. In our study, the CT grade was a more predictive method for the clinical course, that is, the higher the radiological grade, the longer the pain duration, the fasting duration and the hospital stay. But, those factors that represent the progress of acute pancreatitis have limitations.
We investigated the correlation between the changes of the pancreatic enzyme, the biochemical markers and the clinical results according to the Balthazar CT grade. We knew that acute pancreatitis was more frequent in six-decade old males. The biliary pancreatitis patients were older than the alcoholic pancreatitis patients, but any radiological differences were not present. Most patients were of an alcoholic and biliary origin. We reported the difference of the pancreatic enzyme concentrations according to the etiology of pancreatitis. Our study showed that the serum amylase and lipase concentrations are higher in the biliary pancreatitis patients than the pancreatitis patients of an alcoholic origin. In another study, patients with severe biliary pancreatitis tended to have higher serum amylase levels at admission than the other groups[21].
On the univariate analysis, the factors that affected the radiological grade were the leukocyte count at admission, the hemoglobin level, the total bilirubin concentration, serum lipase, CRP and the follow up CRP. The serum lipase concentration was used to diagnose acute pancreatitis due to its high specificity and sensitivity, but it cannot predict disease prognosis and severity. Also, in our study, the serum lipase concentration at admission was elevated in proportion to radiological severity, but it is not correctly correlated with the radiological severity on multivariate analysis. There were significant differences in each group for the APACH-II score at admission and also at 24 h. The estimation of severity through the Ranson criteria is not precise and is not an appropriate method because this method needs 48 h to complete and it has low specificity and sensitivity (77% and 75%, respectively). There was no significant correlation between the Ranson criteria and the radiological grade in our study.
The APACH-II score is the sum of the various physiologic parameters. This score has been used for evaluation of severe patients[22–24]. It has high specificity and sensitivity for acute pancreatitis. In our study, groups 2 and 3 showed high sensitivity and specificity compared to group 1. But, the APACHE II score allows monitoring both the disease progression and the response to therapy, but the system is complex, difficult to perform and less accurate for identification of local complications[25]. On our multivariate analysis, the complex APACHE II system was not correlated with the radiological grade when compared to the easily detectable CRP concentration, and the APACHE II system had a lower positive likelihood ratio and accuracy rate.
CRP is an acute stage protein that’s synthesized in the liver. This parameter is usually used because it is simple and cheap[26,27]. CRP is known to be a significant factor in the differential prognosis of acute pancreatitis[5,28–30]. In this study, the CRP titer was only a predicative factor with good correlation to the radiological grade on multivariate analysis. Our results show statically significant differences at admission for prediction of severity. Also, changes of the CRP level during treatment reflect the prognosis of disease. When the cut-off value of CRP was 8.3 g/L, the positive predictive value was 38.4 mg/L in-group 3, and the negative predictive value was 0.26 in group 3. This single factor was the most precise and accurate for determining the radiological severity.
In conclusion, our study suggests that the CRP with the radiological severity may be used to estimate the severity of acute pancreatitis.
COMMENTS
Background
The diagnostic markers (pancreatic enzymes such as amylase and lipase) and the risk factors that affect the clinical outcome are not well established in acute pancreatitis. Abdominal computer tomography (CT) has recently been used for determining the presence and diagnosis of complications in acute pancreatitis patients.
Research frontiers
Previous studies haven’t showed that clinical evaluation and biochemical marker predict severity in acute pancreatitis. The CT grade has shown good correlations with clinical course and the prediction of mortality.
Innovations and breakthroughs
C-reactive protein (CRP) and radiological severity have good correlation and may be used to estimate the severity of acute pancreatitis. CRP is easily measurable and is a simple method. CT is a good method which allows the prediction of clinical course and mortality, but it is expensive. Therefore, CRP is alternative method and clinically useful for prediction severity.
Peer review
This interesting study suggests that the CRP with the radiological severity may be used to estimate the severity of acute pancreatitis. The contents of the manuscript are reasonable, and this may be a useful method for prediction of clinical course and mortality, as the author’s state.
Peer reviewer: CS Pitchumoni, Professor, Robert Wood Johnson School of Medicine, Robert Wood Johnson School of Medicine, New Brunswick, NJ 08903, United States
S- Editor Yang RH L- Editor Rippe RA E- Editor Lu W
References
- 1.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 2.Gullo L, Migliori M, Olah A, Farkas G, Levy P, Arvanitakis C, Lankisch P, Beger H. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223–227. doi: 10.1097/00006676-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Lee HS. Diagnosis and predicting severity in acute pancreatitis. Korean J Gastroenterol. 2005;46:333–338. [PubMed] [Google Scholar]
- 4.Buchler MW, Gloor B, Muller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner J, Hartwig W, Uhl W, Muller C, Buchler MW. Useful markers for predicting severity and monitoring progression of acute pancreatitis. Pancreatology. 2003;3:115–127. doi: 10.1159/000070079. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg AA, Borgstrom A. Early prediction of severity in acute pancreatitis. Is this possible? JOP. 2002;3:116–125. [PubMed] [Google Scholar]
- 7.Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002;34:167–176. doi: 10.1097/00004836-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 9.Simchuk EJ, Traverso LW, Nukui Y, Kozarek RA. Computed tomography severity index is a predictor of outcomes for severe pancreatitis. Am J Surg. 2000;179:352–355. doi: 10.1016/s0002-9610(00)00375-5. [DOI] [PubMed] [Google Scholar]
- 10.Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767–772. doi: 10.1148/radiology.156.3.4023241. [DOI] [PubMed] [Google Scholar]
- 11.Werner J, Waldernar UHL, Buchler MW. Acute pancreatitis. In: Cameron JL, editor. Current Surgical Therapy, 8th ed. Philadelphia: Elsevier Mosby; 2004. pp. 459–464. [Google Scholar]
- 12.Eachempati SR, Hydo LJ, Barie PS. Severity scoring for prognostication in patients with severe acute pancreatitis: comparative analysis of the Ranson score and the APACHE III score. Arch Surg. 2002;137:730–736. doi: 10.1001/archsurg.137.6.730. [DOI] [PubMed] [Google Scholar]
- 13.Robert JH, Frossard JL, Mermillod B, Soravia C, Mensi N, Roth M, Rohner A, Hadengue A, Morel P. Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers. World J Surg. 2002;26:612–619. doi: 10.1007/s00268-001-0278-y. [DOI] [PubMed] [Google Scholar]
- 14.Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901–907. doi: 10.1097/00003246-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Windsor JA. Search for prognostic markers for acute pancreatitis. Lancet. 2000;355:1924–1925. doi: 10.1016/S0140-6736(00)02317-5. [DOI] [PubMed] [Google Scholar]
- 16.Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309–1318. doi: 10.1111/j.1572-0241.2002.05766.x. [DOI] [PubMed] [Google Scholar]
- 17.Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36:253–260. doi: 10.1097/00004836-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004;183:1261–1265. doi: 10.2214/ajr.183.5.1831261. [DOI] [PubMed] [Google Scholar]
- 19.Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Deviere J, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–723. doi: 10.1053/j.gastro.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Leung TK, Lee CM, Lin SY, Chen HC, Wang HJ, Shen LK, Chen YY. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol. 2005;11:6049–6052. doi: 10.3748/wjg.v11.i38.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiatt JR, Calabria RP, Passaro E Jr, Wilson SE. The amylase profile: a discriminant in biliary and pancreatic disease. Am J Surg. 1987;154:490–492. doi: 10.1016/0002-9610(87)90261-3. [DOI] [PubMed] [Google Scholar]
- 22.Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:294–299. [PubMed] [Google Scholar]
- 23.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–205. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 24.Osvaldt AB, Viero P, Borges da Costa MS, Wendt LR, Bersch VP, Rohde L. Evaluation of Ranson, Glasgow, APACHE-II, and APACHE-O criteria to predict severity in acute biliary pancreatitis. Int Surg. 2001;86:158–161. [PubMed] [Google Scholar]
- 25.Gurleyik G, Emir S, Kilicoglu G, Arman A, Saglam A. Computed tomography severity index, APACHE II score, and serum CRP concentration for predicting the severity of acute pancreatitis. JOP. 2005;6:562–567. [PubMed] [Google Scholar]
- 26.Viedma JA, Perez-Mateo M, Dominguez JE, Carballo F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut. 1992;33:1264–1267. doi: 10.1136/gut.33.9.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Formela LJ, Galloway SW, Kingsnorth AN. Inflammatory mediators in acute pancreatitis. Br J Surg. 1995;82:6–13. doi: 10.1002/bjs.1800820105. [DOI] [PubMed] [Google Scholar]
- 28.Riche FC, Cholley BP, Laisne MJ, Vicaut E, Panis YH, Lajeunie EJ, Boudiaf M, Valleur PD. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133:257–262. doi: 10.1067/msy.2003.70. [DOI] [PubMed] [Google Scholar]
- 29.Pezzilli R, Melzi d'Eril GV, Morselli-Labate AM, Merlini G, Barakat B, Bosoni T. Serum amyloid A, procalcitonin, and C-reactive protein in early assessment of severity of acute pancreatitis. Dig Dis Sci. 2000;45:1072–1078. doi: 10.1023/a:1005525329939. [DOI] [PubMed] [Google Scholar]
- 30.Wilson C, Heads A, Shenkin A, Imrie CW. C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg. 1989;76:177–181. doi: 10.1002/bjs.1800760224. [DOI] [PubMed] [Google Scholar]


