Abstract
The substantia nigra pars compacta (SNpc) is a compact brain structure that contains a variable distribution of cells in both medial to lateral and rostral to caudal dimensions. The SNpc is the primary brain structure affected in Parkinson’s disease, where loss of dopaminergic neurons is one of the major hallmarks of the disorder. Neurotoxic and genetic models of Parkinson’s disease, as well as mechanisms to treat this disorder, are modeled in the mouse. To accurately assess the validity of a model, one needs to be assured that the method(s) of analysis is accurate. Here, we determine the total number of dopaminergic neurons in the SNpc of the C57BL/6J mouse by serial reconstruction then compared that value to estimates derived using model-based stereology and design-based stereology. Serial reconstruction of the SNpc revealed the total number of SNpc dopaminergic neurons to be 8305±540 (SEM). We compared this empirically derived neuron number to model based and design-based stereological estimates. We found that model based estimates gave a value of 8002±91 (SEM) while design-based estimates were 8716±338 (SEM). Statistical analysis showed no significant difference between estimates generated using model- or design-based stereological methods compared to empirically-derived counts using serial reconstruction.
Keywords: Dopaminergic Neuron, Parkinson’s disease, stereology, cell counts
Introduction
The need for reliable methods for counting neurons and other cells within the nervous system is clearly understood. However, there is considerable controversy as to what is the best method for achieving this end. Many papers have examined both the theoretical and practical strengths and weaknesses of model based (2D) versus design-based (3D) stereological methods, and it is clear that each have their proponents (Gundersen, 1978, West et al., 1991, Guillery and Herrup, 1997, Benes and Lange, 2001, von Bartheld, 2001, Schmitz and Hof, 2005). However, beyond theoretical considerations, it is critical to identify method(s) that yield true unbiased estimates of cell number in each particular system, then standardize this method(s), so that different groups of researchers can compare and contrast each other’s data. This is particularly important in studies that examine models of neurodegeneration or those testing and validating methods for neuroprotection.
Cell counts have been used to validate animal models of human neurological disorders (Hirsch et al., 2003, Smeyne et al., 2007). In the field of neuroprotection, cell counts have been used to demonstrate efficacy of treatments (Tomac et al., 1995) that impact the basis for starting or stopping human clinical trials. One neurological disease where cell counts play a significant role is in the development of models and treatments of Parkinson’s disease.
Parkinson’s disease is a progressive neurological disorder that most conspicuously affects the dopaminergic (DA) neurons located in the substantia nigra pars compacta (SNpc). In humans, the disease generally strikes in the 6th decade, with a clinical finding of progressive loss of DA neurons over a 5–15 year period. It has been suggested that an individual must lose approximately 70% of their SNpc dopaminergic neurons in order to develop the cardinal motor symptoms associated with this disorder; thus most animal models rely on generating cell loss in this structure (German et al., 1989, Tomac et al., 1995, Przedborski and Vila, 2001, Mentis and Delalot, 2005, Richard, 2005). Free radical generating agents such as 6-OHDA, MPTP, rotenone, or paraquat are the most common chemical methods for generating lesions of the SNpc. Administration of any of these compounds results in a fairly specific loss of the SNpc DA neurons, although the amount of cell loss depends on the route of administration, the concentration of the chemical, and the schedule in which they are administered (Sotelo et al., 1973, Betarbet et al., 2000, Jackson-Lewis and Smeyne, 2004, Shepherd et al., 2006). Other models have used transgenic technology to recapitulate genetic lesions known to be associated with familial models of Parkinson’s disease (Fleming et al., 2005, Terzioglu and Galter, 2008). However, regardless of the neurological insult, determination of the extent of the lesion can only be accomplished by comparing it to the baseline number of SNpc DA neurons. In a survey of the literature, we found that the reported estimates of bilateral numbers of TH-positive neurons in the SNpc number of C57BL/6 mouse ranged from approximately 5200 (Coban and Filipov, 2007) to 23,670 (Jakowec et al., 2004) although most studies ranged from 8000–12,000 (Liberatore et al., 1999, Walters et al., 1999, Zou et al., 2000, Sugama et al., 2003, Thiruchelvam et al., 2003, Baquet et al., 2005, McCormack et al., 2005, Smeyne et al., 2005, Cao et al., 2006, McCormack et al., 2006, Vijitruth et al., 2006). Even at this more limited range, this variance makes it imperative to empirically determine the actual number of SNpc dopamine neurons to facilitate comparisons between studies using stereological estimates.
In this study, we used serial sections to reconstruct the SNpc from C57BL/6J mouse brains and directly count the number of dopaminergic neurons present. We then compared this number to estimates determined using model-based and design-based stereology and found no significant statistical differences in cell number using all three methods.
Experimental Procedures
Animals
Adult 3–4 month old male and female C57BL/6J mice (Jackson Labs, Bar Harbor, ME) were used in this study. All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and were approved by the SJCRH Animal Care and Use Committee. The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
Serial reconstruction of the SNpc
Eight 3–4 month old C57BL/6J mice were anesthetized with an overdose of Avertin. Following induction of deep anesthesia, animals were intracardially perfused with physiologic saline followed by 4% paraformaldehyde in 1× phosphate-buffered saline (PBS), pH 7.4. Each brain was dissected out of the skull following perfusion and post-fixed overnight in fresh fixative. Brains were then dehydrated through a graded series of ethanols, defatted in xylene and embedded in paraffin (Paraplast-X-tratm, Fisher Scientific). Brains were blocked and cut in the coronal plane and serially-sectioned at 10 μm. Every section from the rostral hippocampus to the anterior aspects of the cerebellar-midbrain junction was saved and mounted, 5 sections per slide, onto Superfrost-Plus slides (Fisher Scientific), immunolabeled for TH (TH polyclonal P40101-0, PelFreeze, Rogers, AK: 1:250), and visualized using the ABC method (Vector labs, Burlingame, CA). All sections were subsequently counterstained with the Nissl stain, Neutral Red. To ensure that this antibody had complete penetration, we immunostained alternate sections from these brains with a fluorescent secondary antibody (AlexaFluor 488, 1:250, Invitrogen, Carlsbad, CA). Sections were examined using a Scanning Laser Confocal Microscope (Carl Zeiss LSM 510 Meta) at 0.5 micron z-planes. We found TH-immunolabling at all levels of the z-stack ensuring that the antibody completely penetrated the section.
Of these 8 brains, we examined the region of the SN for the following characteristics: 1) completeness, i.e. were any sections missing, 2) integrity, i.e. were any sections folded, 3) immunolabeling, i.e. were any sections poorly stained. If any of these factors were less than optimal, the brain was deemed not useful for the reconstruction. Of the eight brains processed, we identified 4 that met this strict criteria and these brains were used in the study.
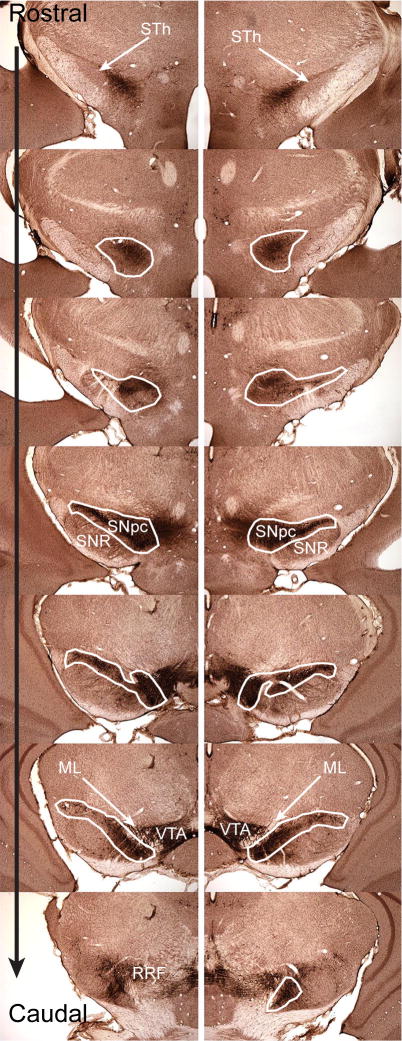
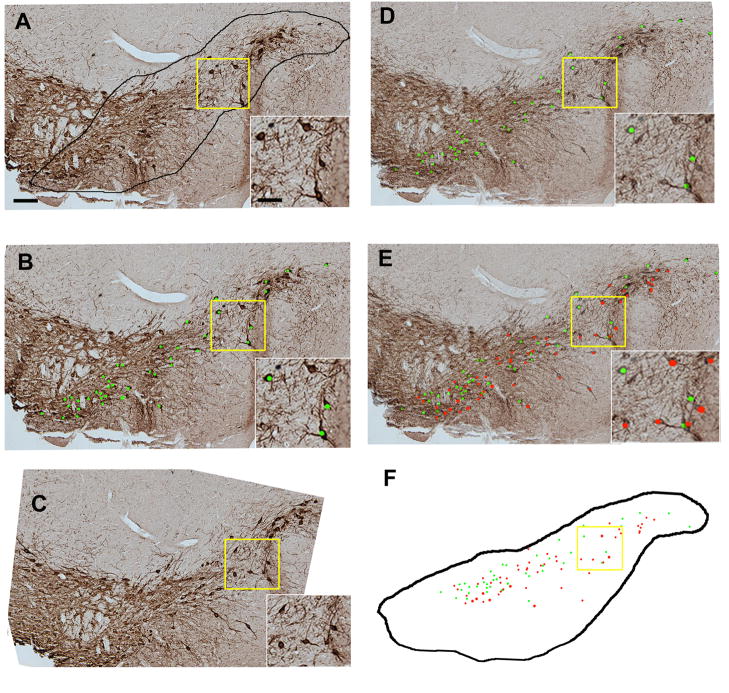
The anterior to posterior extent of the SN (Figure 1) was identified based on a standard mouse brain atlas (Paxinos and Franklin, 2001) using landmarks shown in Nelson et al (Nelson et al., 1996). The rostral portion of the SNpc starts with the first TH positive cells near the end of the subthalamic nucleus and is lateral to the TH-positive fibers in the medial forebrain bundle. The caudal SNpc ends where the retrorubral field becomes visible. The outlines used include the substantia nigra lateralis. The dorsal portion of the SN pars reticulata defines the ventrolateral boundary. The anterior medial boundary is defined by the ventral tegmental area and by size and orientation of stained cells. DA neurons of the SNpc are larger than ventral tegmental area DA neurons (Nelson et al., 1996), and SNpc DA neurons orient along the long axis of the SNpc. The posterior medial portion of the SNpc is defined by the medial lemniscus. The SN on each side of the brain was photographed at 10× using the Microbrightfield system Virtual Slice Module that rasterized areas seamlessly. Using Adobe Photoshop CS3, each image was imported into a master file, and magnified 500× on screen. At this magnification, at least 4 identified cells in each section, along with other landmarks such as blood vessels, outlines of the brain and ventricles, were used to align each section. On the section to be counted, the SN was outlined (Figure 2A). Within this outline, all neurons including both TH-positive and a few TH−, Nissl+ cells with the characteristics of dopaminergic neurons (Hamre et al., 1999)were labeled using a green marker (Figure 2B). Once all cells were marked, the adjacent section was overlaid on the previous section and aligned (Figure 2C). Once aligned, the opacity of the overlying section was reduced so that the cells marked from the underlying section were visible (Figure 2D). Cells in the new section that were not marked in the previous section were labeled with red marks (Figure 2E). This was repeated, alternating green and red markings, until all cells were counted (Figure 2F). This was done on each side of the brain for each animal and then left and right values were added to generate a total dopaminergic SNpc neuron count.
Figure 1.
Outline of Substantia Nigra pars compacta used to determine cell number. Forty micron free-floating cryostat sections were stained for tyrosine hydroxylase expression. The sections shown are spaced every 240 microns and thus represent every 6th section from a single brain. White outlines indicate areas used to define the SNpc for the purposes of stereology. The rostral portion of the SNpc starts with the first TH positive cells near the end of the subthalamic nucleus (STh) and the caudal SNpc is where the retrorubral field (RRF). The outlines used include the substantia nigra lateralis. The mediodorsal boundary of the SNpc is defined by TH expression. The dorsal portion of the SN pars reticulata (SNR) defines the ventrolateral boundary. The anterior medial boundary is defined by the ventral tegmental area (VTA) and by size and orientation of stained cells. DA neurons of the SNpc are larger than ventral tegmental area DA neurons, and SNpc DA neurons orient along the long axis of the SNpc. The posterior medial portion of the SNpc is defined by the medial lemniscus (ML).
Figure 2.
A representative overlay with individual dots representing single dopaminergic neurons used for serial reconstuction of the SNpc. (A) The SNpc in a 10 micron paraffin section stained for tyrosine hydroxylase was outlined. Inset: Higher power of box outlined in yellow. (B) Individual dopaminergic neurons within an outline of the SNpc on each section were labeled with a green dot. Inset: Higher power of box outlined in yellow.(C) Once all cells were marked in a single section, the adjacent section was overlaid on the previous section and aligned (D). Once aligned, the opacity of the overlying section was reduced so that the cells marked from the underlying section were visible. Inset: Higher power of box outlined in yellow. (E) Dopaminergic neurons in the adjacent section that contained cells not marked as present in the previous section were labeled with red dots. Inset: Higher power of box outlined in yellow. (F) A representative overlay with the dots the represent total dopaminergic neurons in 2 sections. Scale bar = 60 microns, Scale bar, inset= 30 microns
Model-based (2D) cell counting
Ten 3–4 month old C57BL/6J mice were anesthetized with an overdose of Avertin. Following induction of deep anesthesia, animals were intracardially perfused with physiologic saline followed by 4% paraformaldehyde in 1× phosphate-buffered saline (PBS), pH 7.4, and processed, sectioned, mounted, immunostained and counterstained in an identical manner as described for the serial reconstructions.
Following processing, and using 100× magnification to focus from the top to bottom of at least 10 representative sections/brain, we measured the final section thickness and found that they had shrunk less than 1 micron (the average section thickness ± SEM was measured at 9.5 ± .1 microns). This value was subsequently used to generate the correction factor. Both TH+, Nissl-positive cells (which accounted for approximately 97% of the counted cells) and TH−, Nissl+ cells with the characteristics of dopaminergic neurons (similar size and shape as the TH+, Nissl+ neurons, accounting for approximately 3% of the counted cells) within the SNpc (both left and right sides) in one section per slide (chosen randomly and then maintained throughout all sections, ( i.e. the 3rd section on each slide) were counted using a 40× objective (total magnification 400×). Once a section on each slide was counted, we multiplied by 5 to correct for uncounted sections and corrected for split nuclei using the Abercrombie correction factor (Abercrombie, 1946). The formula for the correction was:
where N equals the estimated cell number, n= raw cells counts, t= section thickness measured for each brain, and × equals the empirically determined counting particle size).
To generate the correction factor, we measured the nuclear size of dopaminergic neurons in the x,y and z axes in each of three sections at the rostral, intermediate and caudal boundaries of the SNpc (total number of cells was ≥ 200 cells/animal/axes). This allowed us to derive an average counting particle size across the entire structure. The cell diameter measurements were summed and a mean was generated. This direct measurement was found to be comparable to measurements using disector models (Pover et al., 1993). Our nuclear size measurement used for the correction factor was made using the longest visible nuclear length with the realization that this would give us a lower “corrected value”. However, in a pilot study where we measured the same profiles in the x, y and z planes, we did not find a statistical difference in nuclear size. This supports previous studies that showed that nuclei are generally spherical (Hatton and von Bartheld, 1999) in histological sections.
Design-based (3D) cell counting
Ten 3–4 month old C57BL/6J mice were anesthetized with an overdose of Avertin. Following induction of deep anesthesia, animals were intracardially perfused with physiologic saline followed by 4% paraformaldehyde in 1× phosphate-buffered saline (PBS), pH 7.4. Each brain was dissected out of the skull following perfusion and postfixed overnight in fresh fixative. Brains were then placed in 30% sucrose/PBS until cryoprotected and frozen sectioned on a calibrated cryostat at 40 microns. All sections were saved and immunolabeled for TH and visualized using the ABC method (Vector labs, Burlingame, CA) as free floating sections. All sections were subsequently counterstained with the Nissl stain, Neutral Red. Following staining, the sections were mounted on Superfrost-Plus slides in 6 series. To ensure that this antibody had complete penetration, we immunostained alternate sections from these brains with a fluorescent secondary antibody (AlexaFluor 488, 1:250, Invitrogen, Carlsbad, CA). Sections were examined using a Scanning Laser Confocal Microscope at 1.0 micron z-planes. We found TH-immunolabling at all levels of the z-stack ensuring that the antibody completely penetrated the section.
Using the morphometry and design-based stereology software package, StereoInvestigator, (version 7.0; MicroBrightField, Colchester, VT), estimates of dopaminergic (DA) cell number in the SNpc of 3 to 4 months old C57BL/6J male mice were obtained. The total number of tyrosine hydroxylase (TH) positive neurons + TH-negative, Nissl-positive neurons with the characteristics of dopaminergic neurons in both hemispheres of the SNpc was estimated using a fractionator-sampling design (Gundersen et al., 1988, West et al., 1991). Using the StereoInvestigator’s optical fractionator probe, cell counts were made at regular predetermined intervals, (x 200 μm; y 200 μm; 40,000 μm2) within an unbiased counting frame of known area (60 × 60 μm; 3600 μm2) superimposed on a video image of the SNpc (area fraction of 0.09). Particular attention was paid to following the criterion described in the User’s Guide for StereoInvestigator v7, including that sample counts for each total brain were between 100 and 600. A grid size was chosen so that an average of 10 or more sampling sites per section was achieved. Gundersen Coefficients of Error for m=1 were all less than or equal to 0.10. Sections were viewed under a 100×, 1.3 numerical aperture objective (Olympus) on a BX51 microscope (Olympus) with a MAC5000 motorized XYZ axis computer-controlled stage and a CX9000 CCD video camera (Microbrightfield) giving at least 4 distinct focal planes through the section. The unbiased counting frame was positioned randomly on the outline of the SNpc by the software, thereby creating a systematic random sample of the area. Within each section counted, the actual mounted thickness was determined at every sixth counting site and averaged 22 microns (45% shrinkage from the unprocessed section thickness). 20μm was defined as the z-dimension of the disector thus providing a 1 μm guard on either side. TH staining delineated the broad outline of the SNpc (Figure 1) as these areas encompassed all of the dopaminergic neurons that were counted as well as the immediately surrounding TH-positive fiber (for position of individual subnuclei of the SNpc of rodents see (German and Manaye, 1993) but also the fibers surrounding them since we have observed that dopamine neuron position was not immutably fixed and we wanted to ensure that all SNpc dopamine neurons had a chance of being counted. StereoInvestigator was used to make outlines of both hemispheres of the SNpc at low power (20×) as defined by this staining using landmarks described for serial reconstruction. The counting unit was the same as described for the Model-based stereology and was counted only if the nucleus came into focus within the counting frame (West et al., 1991). Analysis was performed starting with the first anterior appearance of TH-positive neurons and extending to the most caudal parts of the SNpc.
Results
Serial reconstruction of the dopaminergic neurons in the SNpc of the C57BL/6J mouse
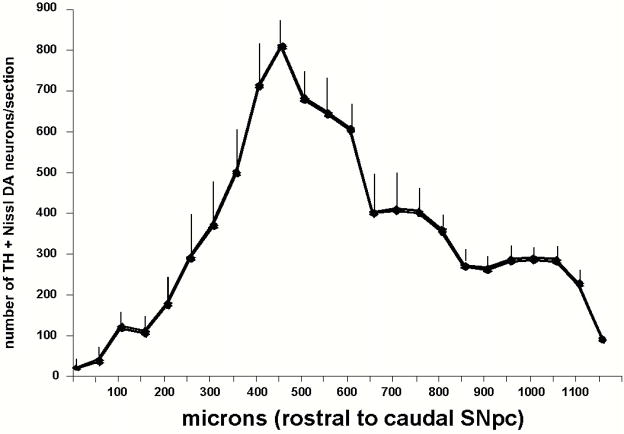
The total bilateral number of DA neurons (± SEM) in the SNpc from the 4 serially reconstructed brains was 8305 ± 539 (n=4, range 7464–9763). On average, the rostral to caudal length of the paraffin embedded SNpc was 1150 microns. The distribution of the cells demonstrates that the majority of cells are present 400–700 microns into the SNpc (rostral to caudal) (Figure 3). This is similar to the distribution of C57BL/6J SNpc dopaminergic neurons previously reported (Nelson et al., 1996, Hamre et al., 1999).
Figure 3.
Bilateral distribution of dopaminergic neurons (number ± SEM) in the SNpc based on serial reconstruction of four individual brains. The majority of the dopaminergic neurons are located 400–700 microns (rostral to caudal) within the SNpc.
Model-based (2D) Stereological estimates of dopaminergic neurons in the C57BL/6J SNpc
Model-based stereological estimates were performed by sampling sections equally spaced every 50 microns. On average, we counted 26 sections per animal. The average raw count (± SEM) we computed was 14943 ± 160 neurons. This raw count was then corrected for split nuclei in each individual brain using the empirically determined section thickness and average counting particle size. On average, the counting particle size in the horizontal (x,y) axis was 8.23 ± .03 microns with a range of 5.5 to 11.6 microns. Measurement of the nuclear height in the z-axis showed an average height of 8.32 ± 0.29 microns, which was not significantly different that measurement in the x,y axis and for this reason we used measurement in the x,y axis to generate the correction factor.
We also empirically measured section thickness and determined an average thickness after processing averaged 9.5 ± 0.1 microns. Thus, the correction factor (as described in the Experimental Procedures) for split nuclei averaged 0.5352 and for a corrected value of 8002 ± 91 (range=7563–8415, n=10), slightly lower (96% of the reconstructed number of DA neurons in the SNpc) but not statistically different from the number of SNpc DA neurons determined by serial reconstruction.
Design-based stereological estimates
Our design-based stereological estimates were determined with the optical fractionator probe in the StereoInvestigator software package. Using an oversampling technique described by Slomianka and West (Slomianka and West, 2005) we estimated cell number by examining every section of the brain that included the SNpc and each grid site established by the Microbrightfield StereoInvestigator program. We then systematically removed data from consecutive sections and sampling sites to determine the number of sections and sampling sites that count be excluded and still retain precision. We established empirically that counting every 6th section could provide the necessary precision (less than 10% variability around the mean) (Figure 4A). Next we used the same oversampling procedure to determine the number of sampling sites necessary for precision (Figure 4B). In this analysis, we allowed a 10% variance from the mean then concluded from empirical data that we could sample every 3rd counting site to maintain precision. Using these parameters, we estimated the number of SNpc DA neurons in the C57BL/6J mouse to be 8716±338 (range=7546–9869, −n=10), a 5% increase compared to the reconstructed number of DA neurons in the SNpc.
Figure 4.
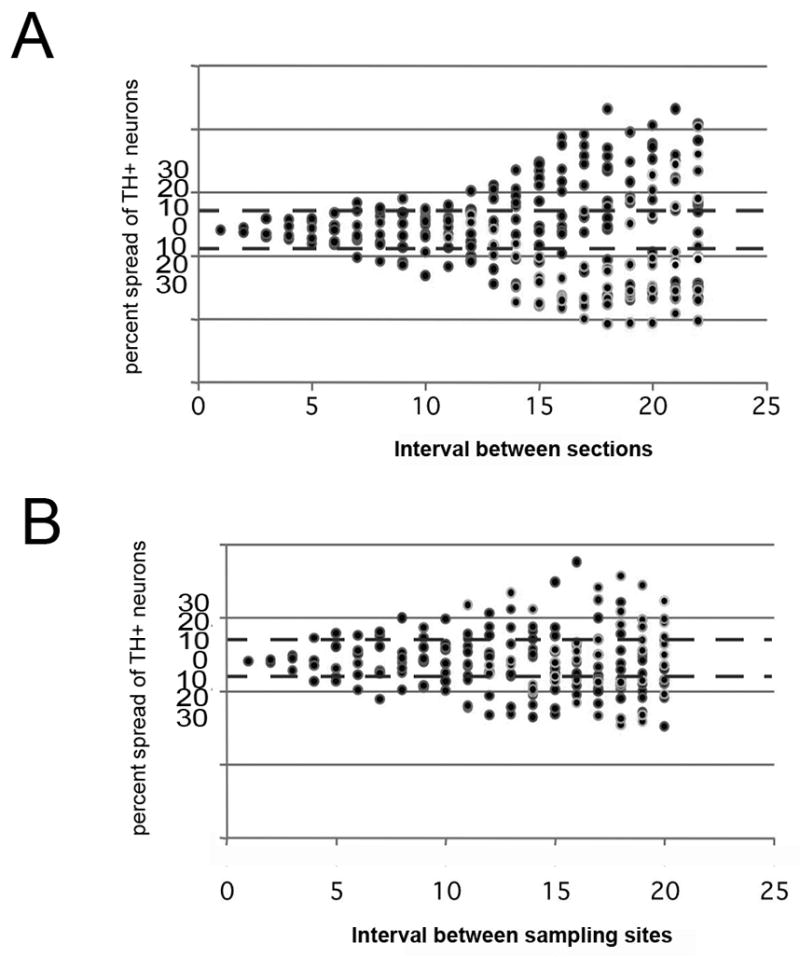
Effect of section interval and number of sampling sites on neuron number estimation in SNpc. (A) Using an oversampling technique described by Slomianka and West (Slomianka and West, 2005) we determined the estimated deviation of dopaminergic neurons within the SNpc using different sampling intervals in a single C57BL/6J mouse. Counting every section through every 6th section gives numerical estimates within 10% (B) Using the same oversampling technique we estimated that an investigator could sample up to every third site and generate estimates within 10%.
Comparison of each counting method
The estimates from each of the methods were compared by one way ANOVA. This analysis demonstrated that there was no significant difference between each of the three methods (F=1.844)(p≤0.183).
Discussion
Establishment of a gold standard for the number of neurons within the SNpc of mice is necessary in order for investigators to both assess the accuracy of the estimated values of the given experiments as well as to allow comparisons between experiments. This is especially important with regards to determining the efficacy of particular treatments that purport to cause or prevent neuronal loss. Here we used serial reconstruction of the entire adult SNpc of the C57BL/6J mouse to reveal that it contains 8305±540 dopaminergic neurons. We included in these counts, both the TH+ dopaminergic neurons as well as the few dopaminergic neurons, based on neuron shape, size and location, that were TH−, Nissl+. While these cells, at most, accounted for less than 3% of the total dopaminergic neuron number, to generate an accurate total dopaminergic neuron count it is critical to include them. In addition, although not addressed in this paper, many treatments that induce dopaminergic cell death via oxidative stress, such as MPTP or 6-OHDA have been shown to inhibit tyrosine hydroxylase (Ozaki et al., 1988, Zhu et al., 1993)without causing neuronal death (Boyd et al., 2007). One must also note that we found some variability in the reconstructed number of neurons in the C57BL/6J SNpc, although the SEM did not differ more than 10% from the mean of the 4 animals counted. This was of interest since these counts were for an inbred strain of mice and thus in theory all animals should have the same number of neurons. The variability of our neuronal counts, including those from serial reconstruction, suggests that there are environmental factors and/or random somatic mutations/polymorphisms that can contribute to the final number of SNpc neurons in adult C57BL/6 animals.
The goal of this paper was to compare the empirically determined number of SNpc dopaminergic neurons to estimates obtained by model-based (2D) and design-based (3D) stereology estimation methods. In addition to defining the actual number of dopaminergic neurons in the SNpc of the C57BL/6 mouse, this type of study is also useful as a necessary mechanism for calibrating methods of estimation in either method- or design-based stereology (Coggeshall, 1992, Popken and Farel, 1996, 1997, Hatton and von Bartheld, 1999, Saper, 1999, Farel, 2002, von Bartheld, 2002) (for opposing views see (Mayhew and Gundersen, 1996, Howard and Reed, 2005). We discovered that both methods can yield estimates (8002 ± 91 for model-based and 8716±338 for design-based) that were not significantly different from the empirically-derived reconstructed value. Although the potential for type II errors has been stated when comparing stereological estimates (especially when using density to determine cell number) (Schmitz et al., 2005), our comparisons of design and methods-based protocols show that when the rules are observed, one can generate estimates that are statistically the same as those empirically determined by serial reconstruction. Although there was no observed statistical difference between the empirically defined number of dopaminergic neurons and either the method- or design-based stereology, we found that the model-based method generated slightly low estimates while the design-based method rendered slightly high estimates. There are several reasons why we may have seen the non-significant variations from the empirically calculated SNpc dopaminergic neuron number. In this study we find that the design-based methods give a low estimate. Correct estimates in this procedure requires a robust correction factor, and this issue has been specifically addressed in numerous papers (Coggeshall, 1992, Benes and Lange, 2001, von Bartheld, 2002). We have chosen to use the standard Abercrombie correction, and based on our measurements of the counting particle in the x,y, and z axes, these can be considered spherical. We also choose to use the largest value obtained from x,y, axes measurements, which lowered the corrected numbers by approximately 4%. It is also possible that although the measurement of this counting particle in all places was equal, it has a differential shrinkage during processing from the rest of the cell. If this is the case, the counting particle (in this case the nucleus) might be overrepresented, and therefore lowering the numerical value of the corrected number. In addition, Hatton and Von Bartheld (Hatton and von Bartheld, 1999) have suggested that lost caps in the sectioning process can also account for low estimates.
We also found a systemic overestimation of dopaminergic counts using the optical fractionator, although there was no statistical difference from the empirically determined number derived from serial reconstruction. The literature suggests several possible reasons for this finding. First, we only counted these cells in the coronal plane, not sagittal or horizontal, due to the less defined boundaries of the SNpc in these planes. Slomianka and West (Slomianka and West, 2005) have shown that this can have a systemic impact on cell counts. Additionally, the substantia nigra is composed of several tiers of clustered cells, with small areas of cell free zones; thus there is not a consistent density. Although the placement of the counting frame in the number of animals we examined (n=10) should have accounted for this, it is possible that we oversampled the cell-rich regions would lead to a slight overestimation of the cell number.
Thus, there appear to be strengths, biases and caveats for each method depending on the structure being analyzed.
Model-based estimation has been an established method for estimating cell populations since the publication by Abercrombie in 1946 (Abercrombie, 1946) and has been further reappraised (Clarke, 1992, Coggeshall, 1992, Clarke, 1993). Since this method makes investigator based choices it has been termed “biased”. West et al (West, 1999) suggested that a less pejorative term of “assumption-based” or “indirect counting techniques” be used so long as potential sources of error other than the average height of an object (the optimal measure of counting particle size) is taken into account. In this paper, we demonstrate that if one chooses an appropriate counting particle (such as nuclei or nucleolus) and adequately samples the complete structure, one can achieve surprisingly accurate (minimally biased) estimates. The caveat is that these choices are not to be reached without forethought. In terms of sampling, we empirically determined that for counts of the SNpc, a sampling interval of 100 microns is appropriate. When counts were taken every 50 microns (data not shown), we observed no change in cell number; however, when we counted every 150 microns, we lost adequate precision (based on a comparison with the number of neurons in the SNpc empirically determined by serial reconstruction). In terms of counting particle, one must be cognizant of sampling bias based on the size, shape, and orientation of the feature relative to the direction of sampling. In this study, we used the nucleus of the DA neurons. We measured this counting particle to average 8.23 microns, which is less than the measured section thickness, but as we note, the measurement was made in the in the x-y axes rather than the z-axis, which is the optimal measurement for this analysis but is difficult if not impossible to measure using sectioned material. The relative impact of this geometrical bias (West, 1999) depends on the degree of sphericity of the feature in question, with less bias for more spherical features. While we acknowledge that the nucleus of dopaminergic neurons are not perfectly spherical, we did find that there were no significant differences in nuclear size whether they were measured through the x,y, or z axis of the visible nucleus. This confirms the finding that neuronal nuclei in paraffin are essentially spherical (Hatton and von Bartheld, 1999), thus measurement in any of the x,y, or z planes should yield similar correction factors.
In terms of counting particle size, our present results suggest that the counting particle used in an analysis should only be at most in 2 sections to get an accurate measure. We meet this by examining 10 micron sections. On average, our nuclear size measures meet this criteria. In a previous study from our lab (Hamre et al., 1999), we used the same counting particle and interval counts, but used 5 micron sections. Here, we got extremely consistent and precise counts, although they were significantly lower than the true value for DA neurons in the SNpc defined by our serial reconstruction.
Estimates from design-based stereology are performed so that they are independent of the size, shape, spatial orientation, and spatial distribution of the objects to be counted. By using these methods, one can lessen the inherent sources of error that must be carefully considered using model-based estimates.
The general estimation equation for design-based counts is:
Where N is the estimate of total cell number; Q is the number of units counted; t is the section thickness, h is the height of the disector; asf is the area sampling fraction; and ssf is the section sampling fraction. Discussion of this equation can be found elsewhere (Petersen et al., 2006).
One of the key determinants for achieving both minimal bias and precision is to establish how much sampling is necessary. This is the average sampling fraction and is defined as:
The choice of an appropriate asf is dependent on a number of factors, including the size of the sample to be counted and the density of the particles contained within the structure. In our study, the asf was calculated to be approximately 0.09. Thus, we sampled approximately 10% of each section. Since the SNpc of the mouse is relatively small and compact, the amount of work to achieve this 10% sample is not unreasonable (Long et al., 1998, Smith et al., 1999). In other larger structures, significantly lower asf’s are generally used (Schumann and Amaral, 2006)
With design-based stereology, the parameters for setting up each experiment are defined empirically and can be arrived at by a variety of means and logic. Perusal of the literature yields a wide range of predicted values from stereological techniques for the total bilateral number of SNpc DA neurons in C57BL/6 mice from as low as ~5200 (Coban and Filipov, 2007) to as high as 23,670 (Jakowec et al., 2004) with the average around 12,000 neurons (Walters et al., 1999, Zou et al., 2000, Bezard et al., 2003, Sugama et al., 2003, Thiruchelvam et al., 2003, Robertson et al., 2004, Smeyne et al., 2005, Cao et al., 2006, McCormack et al., 2006, Vijitruth et al., 2006). This clearly demonstrates that different parameters are capable of generating equivalent estimates although not necessarily with the desired accuracy or consistency. Therefore, when utilizing design-based estimation methods, experimenters need to be able to compare their estimates back to a true empirically-determined value to know if their parameters are workable. It is imperative to report the parameters used in each study so that labs can reproduce the published data and/or compare it to results from others. Schmitz and Hof (Schmitz and Hof, 2005) have suggested that when using an optical fractionator method investigators report, at a minimum, 1) the number of investigated sections, 2) the mean number of investigated microscopic fields, 3) the mean actual section thickness after processing, 4) the mean number of counted cells, and 5) and the coefficient of error (CE).
For our analysis we used SNpc from the C57BL/6J mouse strain. This strain is the one primarily used in experimental Parkinson’s disease research, mostly due to its sensitivity to oxidative stress inducing agents such as MPTP (Hamre et al., 1999, Jackson-Lewis and Przedborski, 2007). The importance of strain cannot be emphasized enough. Although we have previously reported that the total number of SNpc neurons is similar in 7 different inbred strains of mice as well as two different F1 crosses were not different (Hamre et al., 1999), Nelson et al (Nelson et al., 1996) have reported a significantly larger number of SNpc neurons in the FVB/J strain that is commonly used to generate transgenic animals (Taketo et al., 1991). This suggests that baseline SNpc numbers need to be empirically determined prior to any analysis of experimental effects and is especially necessary when using examining animals that are composed of one or more genetic backgrounds as is often the case following generation of null mutations in mice (Ross et al., 1976, Sundstrom et al., 1987, Muthane et al., 1994, Zaborszky and Vadasz, 2001, Vadasz et al., 2007).
In conclusion, we believe that the empirically-determined number of dopaminergic neurons of the SNpc reported here, along with the multiple methods for estimating this value will be beneficial to many using this system to assess disease models and potential treatments. Surpisingly, we found that the accuracy and precision of the design-based stereological methods were equal to that of the method-based analysis. Thus, since both methods can be used in obtaining accurate neuronal estimates, one needs to consider the advantages and disadvantages of each. Model-based estimation methods are quicker and less expensive but require more tissue for sampling. This increased sampling can result in higher costs associated with sample collection, antibody usage and histological supplies. Design-based estimation, once parameters are determined, allows for reduced section sampling thus making more tissue available for other studies; however, it can be more time consuming and costly (in terms of the initial purchase of equipment and software). The broader impact of our analysis is that by defining the true number of SNpc neurons in the mouse SNpc, studies from different labs can be compared and contrasted and the efficacy of potential treatments for Parkinson’s disease related disorders can be more accurately judged.
Acknowledgments
The authors acknowledge support from the National Parkinson’s Foundation, NIH (NS39006 to RJS) and P30 CA 21765 and the American Lebanese Syrian Associated Charities (ALSAC). The authors thank Haeman Jang for assistance with photomicroscopy and Yun Jiao for assistance with histology. We thank the staff at MicroBrightField Inc., especially Dan Peruzzi, for comments on this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ. Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantia nigra pars compacta. Brain Res. 2007;1175:107–116. doi: 10.1016/j.brainres.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XQ, Arai H, Ren YR, Oizumi H, Zhang N, Seike S, Furuya T, Yasuda T, Mizuno Y, Mochizuki H. Recombinant human granulocyte colony-stimulating factor protects against MPTP-induced dopaminergic cell death in mice by altering Bcl-2/Bax expression levels. J Neurochem. 2006;99:861–867. doi: 10.1111/j.1471-4159.2006.04125.x. [DOI] [PubMed] [Google Scholar]
- Clarke PG. How inaccurate is the Abercrombie correction factor for cell counts? Trends Neurosci. 1992;15:211–212. doi: 10.1016/0166-2236(92)90036-8. [DOI] [PubMed] [Google Scholar]
- Clarke PG. An unbiased correction factor for cell counts in histological sections. J Neurosci Methods. 1993;49:133–140. doi: 10.1016/0165-0270(93)90117-a. [DOI] [PubMed] [Google Scholar]
- Coban A, Filipov NM. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem. 2007;100:1177–1187. doi: 10.1111/j.1471-4159.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Farel PB. Trust, but verify: the necessity of empirical verification in quantitative neurobiology. Anat Rec. 2002;269:157–161. doi: 10.1002/ar.10111. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Estimators of the number of objects per area unbiased by edge effects. Microsc Acta. 1978;81:107–117. [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. J Comp Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Hirsch EC, Hoglinger G, Rousselet E, Breidert T, Parain K, Feger J, Ruberg M, Prigent A, Cohen-Salmon C, Launay JM. Animal models of Parkinson’s disease in rodents induced by toxins: an update. J Neural Transm Suppl. 2003:89–100. doi: 10.1007/978-3-7091-0643-3_6. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology: Three-dimensional Measurement in Microscopy (Advanced Methods) New York: Taylor & Francis; 2005. [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Smeyne RJ. From Man to Mouse: The MPTP Model of Parkinson’s Disease. In: LeDoux M, editor. Animal Models of Movement Disorders. Elsevier; 2004. pp. 151–162. [Google Scholar]
- Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res. 2004;76:539–550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Hengemihle JM, Jucker M, Calhoun ME, Ingram DK, Mouton PR. Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Methods. 1998;84:101–108. doi: 10.1016/s0165-0270(98)00100-9. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me’: a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188 ( Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Langston JW, Di Monte DA. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience. 2006;141:929–937. doi: 10.1016/j.neuroscience.2006.03.069. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Delalot D. Depression in Parkinson’s disease. Adv Neurol. 2005;96:26–41. [PubMed] [Google Scholar]
- Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994;126:195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- Nelson EL, Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: computer-assisted mapping. J Comp Neurol. 1996;369:361–371. doi: 10.1002/(SICI)1096-9861(19960603)369:3<361::AID-CNE3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Nakahara D, Mogi M, Harada M, Kiuchi K, Kaneda N, Miura Y, Kasahara Y, Nagatsu T. Inactivation of tyrosine hydroxylase in rat striatum by 1-methyl-4-phenylpyridinium ion (MPP+) Neurosci Lett. 1988;85:228–232. doi: 10.1016/0304-3940(88)90356-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Petersen MS, Petersen CC, Agger R, Hokland M, Gundersen HJ. A simple method for unbiased quantitation of adoptively transferred cells in solid tissues. J Immunol Methods. 2006;309:173–181. doi: 10.1016/j.jim.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Farel PB. Reliability and validity of the physical disector method for estimating neuron number. J Neurobiol. 1996;31:166–174. doi: 10.1002/(SICI)1097-4695(199610)31:2<166::AID-NEU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Farel PB. Sensory neuron number in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol. 1997;386:8–15. [PubMed] [Google Scholar]
- Pover CM, Orr MH, Jr, Coggeshall RE. A method for producing unbiased histograms of neuronal profile sizes. J Neurosci Methods. 1993;49:123–131. doi: 10.1016/0165-0270(93)90116-9. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M. The last decade in Parkinson’s disease research. Basic sciences. Adv Neurol. 2001;86:177–186. [PubMed] [Google Scholar]
- Richard IH. Anxiety disorders in Parkinson’s disease. Adv Neurol. 2005;96:42–55. [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Ross RA, Judd AB, Pickel VM, Joh TH, Reis DJ. Strain-dependent variations in number of midbrain dopaminergic neurones. Nature. 1976;264:654–656. doi: 10.1038/264654a0. [DOI] [PubMed] [Google Scholar]
- Saper CB. Unbiased Stereology: Three-Dimensional Measurement in Microscopy by C.V. Howard and M.G. Reed Trends Neurosci. 1999;22:94– 95. [Google Scholar]
- Schmitz C, Born M, Dolezel P, Rutten BP, de Saint-Georges L, Hof PR, Korr H. Prenatal protracted irradiation at very low dose rate induces severe neuronal loss in rat hippocampus and cerebellum. Neuroscience. 2005;130:935–948. doi: 10.1016/j.neuroscience.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd KR, Lee ES, Schmued L, Jiao Y, Ali SF, Oriaku ET, Lamango NS, Soliman KF, Charlton CG. The potentiating effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on paraquat-induced neurochemical and behavioral changes in mice. Pharmacol Biochem Behav. 2006;83:349–359. doi: 10.1016/j.pbb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, Hatler M, Wolf R, Henderson C, Smeyne RJ. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci U S A. 2007;104:1977–1982. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Jiao Y, Shepherd KR, Smeyne RJ. Glia cell number modulates sensitivity to MPTP in mice. Glia. 2005;52:144–152. doi: 10.1002/glia.20233. [DOI] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci U S A. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Javoy F, Agid Y, Glowinski J. Injection of 6-hydroxydopamine in the substantia nigra of the rat. I. Morphological study. Brain Res. 1973;58:269–290. doi: 10.1016/0006-8993(73)90001-2. [DOI] [PubMed] [Google Scholar]
- Sugama S, Yang L, Cho BP, DeGiorgio LA, Lorenzl S, Albers DS, Beal MF, Volpe BT, Joh TH. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987;405:26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzioglu M, Galter D. Parkinson’s disease: genetic versus toxin-induced rodent models. Febs J. 2008;275:1384–1391. doi: 10.1111/j.1742-4658.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Vadasz C, Smiley JF, Figarsky K, Saito M, Toth R, Gyetvai BM, Oros M, Kovacs KK, Mohan P, Wang R. Mesencephalic dopamine neuron number and tyrosine hydroxylase content: Genetic control and candidate genes. Neuroscience. 2007;149:561–572. doi: 10.1016/j.neuroscience.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. Journal of neuroinflammation. 2006;3:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld C. Counting particles in tissue sections: choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–648. doi: 10.14670/HH-17.639. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Comparison of 2-D and 3-D counting: the need for calibration and common sense. Trends Neurosci. 2001;24:504–506. doi: 10.1016/s0166-2236(00)01960-3. [DOI] [PubMed] [Google Scholar]
- Walters TL, Irwin I, Delfani K, Langston JW, Janson AM. Diethyldithiocarbamate causes nigral cell loss and dopamine depletion with nontoxic doses of MPTP. Exp Neurol. 1999;156:62–70. doi: 10.1006/exnr.1998.6997. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Vadasz C. The midbrain dopaminergic system: anatomy and genetic variation in dopamine neuron number of inbred mouse strains. Behav Genet. 2001;31:47–59. doi: 10.1023/a:1010257808945. [DOI] [PubMed] [Google Scholar]
- Zhu YS, Jones SB, Burke RE, Franklin SO, Inturrisi CE. Quantitation of the levels of tyrosine hydroxylase and preproenkephalin mRNAs in nigrostriatal sites after 6-hydroxydopamine lesions. Life Sci. 1993;52:1577–1584. doi: 10.1016/0024-3205(93)90058-b. [DOI] [PubMed] [Google Scholar]
- Zou L, Xu J, Jankovic J, He Y, Appel SH, Le W. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci Lett. 2000;281:167–170. doi: 10.1016/s0304-3940(00)00853-3. [DOI] [PubMed] [Google Scholar]