Abstract
Probiotics are viable nonpathogenic microorganisms that are considered to confer health benefits to the host. Recent studies indicated that some Lactobacillus species function as probiotics and have been used as alternative treatments for diarrhea, which occurs due to increased secretion, decreased absorption, or both. However, the direct effects of probiotics on intestinal electrolyte absorption are not known. Therefore, we examined the effects of Lactobacillus on luminal chloride/hydroxyl (Cl−/OH−) exchange activity in human intestinal epithelial cells. Postconfluent Caco-2 cells were treated with the Lactobacillus species Lactobacillus acidophilus (LA), Lactobacillus casei, Lactobacillus plantarum, or Lactobacillus rhamnosus (LR) for 3 h at a multiplicity of infection of 50. Cl−/OH− exchange activity was measured as 4,4′-diisothiocyanostilbene-2, 2′-disulfonic acid-sensitive 36Cl uptake in base-loaded cells. Treatment with live, but not heat-killed, LA and LR significantly increased Cl−/OH− exchange activity (~50%), whereas other species were ineffective. Similarly, the conditioned medium (supernatant) of live LA increased Cl−/OH− exchange. The ability of LA or its conditioned culture medium to enhance Cl−/OH− exchange activity was blocked by PI-3 kinase inhibition but was unaffected by inhibition of mitogen-activated protein kinases. Corresponding to the increased Cl−/OH− exchange activity, LA treatment increased the surface expression of the apical anion exchanger, SLC26A3 [Down Regulated in Adenoma (DRA)]. The increased DRA membrane localization might contribute to the increased Cl− absorption by LA. Our results suggest that LA secretes soluble effector molecule(s) into the culture medium that stimulate apical Cl−/OH− exchange activity via phosphatidylinositol-3 kinase mediated mechanism.
Introduction
Probiotics are viable nonpathogenic microorganisms that, when supplied in adequate amounts, confer health benefits to hosts (1). The importance of a healthy gut microflora has long been recognized, but only recently has increased attention been focused on the potential of probiotics as preventive and therapeutic agents in gastrointestinal diseases (2,3). Several studies on probiotic bacterial treatments demonstrated promising results in ameliorating diseases, including inflammatory bowel disease(4), irritable bowel syndrome (5), pouchitis (6), and acute infantile or antibiotic-associated diarrhea (7,8). Although little is known about their mechanisms of action, probiotics have protective, trophic, and antiinflammatory effects on bowel mucosa (9). For example, pro- biotic bacteria enhance intestinal epithelial barrier function (10,11), mucin synthesis and secretion (12,13), cytoprotective heat-shock protein levels (14), and cell survival and growth (15,16), and inhibit enteropathogen binding and internalization (12,17,18). However, the mechanisms regulating epithelial cell responses to probiotics are complex and mostly undefined.
Lactobacilli are a main component of the commensal human bacteria in the gastrointestinal tract that have been shown to be protective against pathogen infection (17). Lactobacillus species have been successfully used in clinical trials to treat various forms of diarrhea (3). Diarrhea is considered to be a multifactorial event involving either increased secretion of fluid and electrolytes, decreased absorption, or both. Electroneutral NaCl absorption in the human ileum and colon involves coupling of luminal Na+/H+ exchange and chloride/hydroxyl (Cl−/OH−) (HCO3−) exchange activities (19,20). Our previous studies have shown regulation of luminal Cl−/OH− (HCO3−) exchange activity by phorbol ester (21), nitric oxide (22), serotonin (23), taurodeoxycholate (24), lysophosphatidic acid (25), and infection with enteropathogenic Escherichia coli (26). Modulation of Cl−/OH− (HCO3−) exchange activity by these agents involved various signaling pathways, including protein kinase C (21) and PI-3 kinases (21,24,25). Previous reports have shown that an enteropathogen-induced increase in Cl− secretion in human intestinal epithelial cells was counteracted by Lactobacillus treatment (10). Further, Lactobacillus acidophilus (LA)3 attenuated tumor-necrosis factor α-induced Cl− secretion in intestinal epithelial cells (11). However, to date, the effects of probiotics on intestinal electrolyte absorptive processes have not, to our knowledge, been examined. Therefore, current studies were designed to investigate the effects of Lactobacillus on apical Cl−/OH− exchange activity utilizing Caco-2 cells as an experimental model. The role of DRA (Down Regulated in Adenoma; SLC26A3), the potential apical anion exchanger (AE), in mediating the changes in luminal Cl−/OH− exchange in response to Lactobacillus treatment was also examined.
Materials and Methods
Materials
Caco-2 cells and probiotic Lactobacillus species were obtained from American Type Cell Collection. 36Cl was obtained from ARC, 4,4′-diisothiocyanostilbene-2, 2′ disulfonic acid (DIDS) was purchased from Sigma-Aldrich, pharmacological inhibitors U0126, SB203580, and LY294002 were procured from Biomol, and sulfo-NHS-SS-biotin was obtained from Pierce Biotechnology.
Cell culture
Caco-2 cells were grown at 37°C in an atmosphere of 5% CO2. Cells were maintained in DMEM with 4.5 g/L glucose, 50 kU/L penicillin, 5 mg/L streptomycin, 2 mg/L gentamycin, and 20% fetal bovine serum. Chloride uptake studies were performed using fully differentiated cells grown for 10–14 d postplating on 24-well plastic supports or on 0.4-μm polycarbonate membrane filters in 12-mm inserts.
Bacterial culture and preparation of conditioned medium
The following Lactobacilli species, with American Type Cell Collection strain numbers given in parentheses, were grown in Mann-Rogosa-Sharpe broth (Difco) for 24 h at 37°C without shaking: LA (4357), Lactobacillus rhamnosus (LR) (53103), Lactobacillus plantarum (14917), and Lactobacillus casei (393). The cultures were then centrifuged at 3000 × g; 10 min at 4°C. The supernatant, filtered through a 0.22-μm filter (Millex, Millipore) to sterilize and remove all bacterial cells, was designated as conditioned medium (CM). For treating the cell monolayers, the bacterial pellet was washed with DMEM/F-12 media (Invitrogen) containing 5 mg/L mannose and resuspended in the same media. Heat-killed bacteria were prepared by resuspending pellets and heating to 95°C for 20 min.
Treatment of cells and 36Cl− uptake
Bacterial suspensions in DMEM/F-12 media were diluted to OD600 nm = 0.2 in the same media and applied to the apical surface of cell monolayers at a multiplicity of infection (MOI) of 50 in 24-well plates for indicated time periods. For studies using pharmacological inhibitors of various kinases, Caco-2 monolayers were preincubated for 1 h with the inhibitors at the indicated concentrations [30 μmol/L U0126 (MEK1, MEK2 inhibitor); 10 μmol/L SB203580 (p38 inhibitor), or 10 μmol/L LY294002 (PI-3 kinase inhibitor]. Incubation with the inhibitor(s) was then further continued for the desired time periods in the presence or absence of the probiotic or the CM. Chloride uptake experiments were then performed as described previously (27) and modified by us (23). Briefly, Caco-2 cells were incubated with DMEM base medium containing 20 mmol/L HEPES/KOH, pH 8.5, for 30 min at room temperature. The medium was removed and the cells were rapidly washed with 1 mL tracer-free mannitol buffer containing 260 mmol/L mannitol, 20 mmol/LTris/2-(N-Morpholino) ethanesulfonic acid, pH 7.0. The cells were then incubated with the uptake buffer for 5 min in the absence or presence of 600 μmol/L DIDS, a specific inhibitor of anion exchange that inhibits the activity of AE by chemically modifying their functional groups through irreversible binding (28). The 5-min time period was selected because it was within the linear range of chloride uptake. The uptake buffer contained 1.4 μCi of 36Cl− (2.9 mmol/L) as hydrochloric acid (specific activity: 17.12 mCi/g) in the mannitol buffer. The uptake was terminated by removing the buffer and washing the cells rapidly 2 times with 1 mL of ice-cold PBS, pH 7.2. Finally, we solubilized the cells by incubation with 0.5 mol/L NaOH for 4 h. The protein concentration was measured by the method of Bradford (29) and the radioactivity was determined by a Packard Tri-Carb 1600TR Liquid Scintillation analyzer (Packard Instruments: Perkin Elmer). The Cl−/OH− exchange activity was assessed as DIDS-sensitive 36Cl− uptake and values were expressed as nmol/(mg protein−1·5 min).
Cell-surface biotinylation studies
Cell-surface biotinylation was performed using sulfo-NHS-SS-biotin (0.5 g/L) in borate buffer (in mmol/L: 154 NaCl, 7.2 KCl, 1.8 CaCl2, 10 H3BO3, pH 9.0) as previously described (26). Labeling was allowed to proceed for 60 min at 4°C to prevent endocytosis and internalization of antigens. After immunoprecipitation of biotinylated antigens with streptavidin agarose, biotinylated proteins were released by boiling in Laemmli buffer containing dithiothreitol, subjected to SDS-PAGE, and then labeled with anti-DRA antibody. The surface DRA was compared with total cellular DRA as determined by immunoblotting of the solubilized cell extract.
Statistical analyses
The data presented are means ± SEM of 3–4 independent experiments. Difference between control vs. various treatments was analyzed using 1-way ANOVA with Dunnett’s multiple comparison test. Differences were considered significant at P < 0.001.
Results
LA and LR increase Cl−/OH− exchange activity
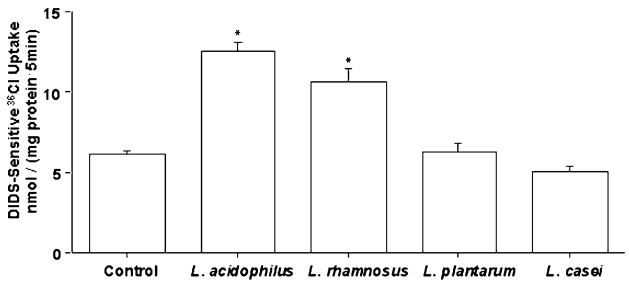
LA and LR caused a significant increase (~50%) in Cl−/OH− exchange activity (DIDS-sensitive 36Cl− uptake) compared with control, whereas L. plantarum and L. casei had no effect (Fig. 1). Subsequent experiments were performed with LA. A time course experiment showed that LA did not affect Cl−/OH− exchange activity at 1 h, but by 2 h, Cl−/OH− exchange activity increased essentially to the level at 3 h (data not shown). A 3-h time point was used to treat cells with probiotics in subsequent studies. We also compared the effects of the probiotics on Cl−/OH− exchange activity in Caco-2 cells grown on 0.4-μm polycarbonate membrane filters in 12-mm inserts and obtained similar results. LA and LR significantly enhanced Cl−/OH− exchange activity (36Cl− uptake values in nmol/(mg protein−1 ·5 min) were: LA, 8.75 ± 0.52; LR, 7.60 ± 0.32; control, 4.70 ± 0.22). On the other hand, L. plantarum and L. casei did not have any effect. We also performed time course of 36Cl− uptake for 5,10, 15, and 20 min on cells treated with various probiotics to ensure that the effects of other probiotics were not missed at the early 5-min time point. However, at all time points, L. plantarum and L. casei did not stimulate Cl−/OH− exchange activity (data not shown).
FIGURE 1.
Cl−/OH− exchange activity (DIDS-sensitive 36Cl− uptake) in Caco-2 cells following 3-h treatment with different Lactobacillus species. Values are means ± SEM, n = 4. *Different from control, P < 0.001.
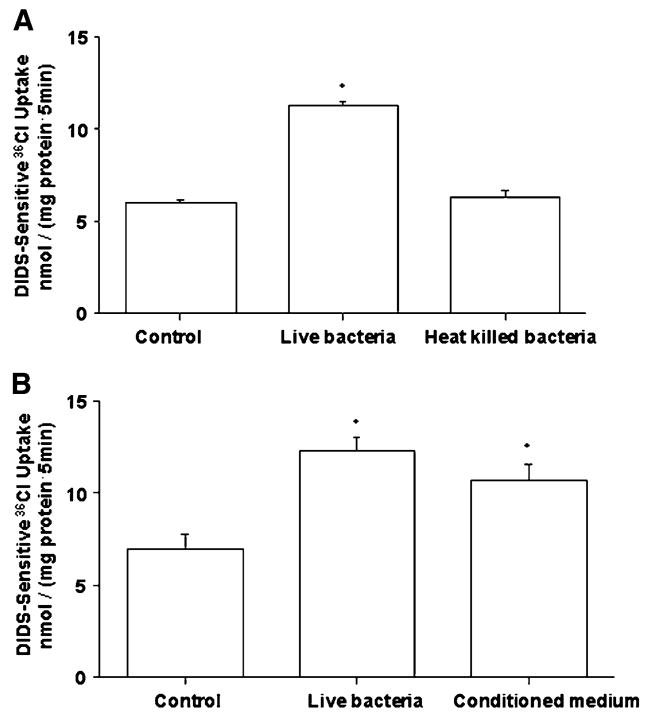
CM of LA, but not heat-killed bacteria, mimics the effects of live bacteria on Cl−/OH− exchange activity
We next examined whether the effects of LA on Cl−/OH− exchange activity required live organisms. Unlike live LA, the heat-killed bacteria did not stimulate apical Cl−/OH− exchange activity in Caco-2 cells (Fig. 2A). Previous studies have shown that CM of some Lactobacillus species had beneficial effects on host intestinal epithelial cells (14,16,30). We therefore tested the effects of CM of LA on Cl−/OH− exchange activity. CM of LA stimulated Cl−/OH− exchange activity to the same extent, compared with control, as that of live bacteria (Fig. 2B). CM was heat inactivated by boiling for 15 min in a water bath and effects on Cl−/OH− exchange activity were measured. Boiling did not alter the stimulatory effects of the CM on Cl−/OH− exchange activity [36Cl− uptake values in nmol/(mg protein·5 min) were: CM, 14.7 ± 0.37; heat-inactivated CM, 16.2 ± 0.50; control, 7.6 ± 0.22].
FIGURE 2.
Cl−/OH− exchange activity in Caco-2 cells following 3-h treatment with heat-killed LA (A) or CM of LA (B). Values are means ± SEM, n = 3. *Different from control, P < 0.001.
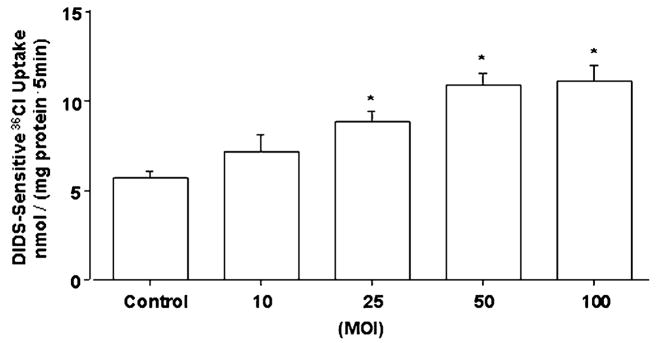
Simulation of Cl−/OH− exchange activity by LA or the CM is dose dependent
Dose response experiments of the effects of LA or CM on Cl−/OH− exchange activity showed a progressive increase in the stimulation of Cl−/OH− exchange activity with increasing MOI of LA from 10 to 100 (Fig. 3) or decreasing dilutions of CM (data not shown). For subsequent experiments, we used an MOI of 50 for LA and a 1:50 dilution of CM prepared from an overnight culture grown to a colony forming unit of 1.5 × 1010/L. At these dilutions, the pH of the media did not change over the course of incubation of Caco-2 cells with LA or CM (data not shown).
FIGURE 3.
Cl−/OH− exchange activity in Caco-2 cells following 3-h treatment with increasing doses (MOI) of LA. Values are means ± SEM, n = 4. *Different from control, P < 0.001.
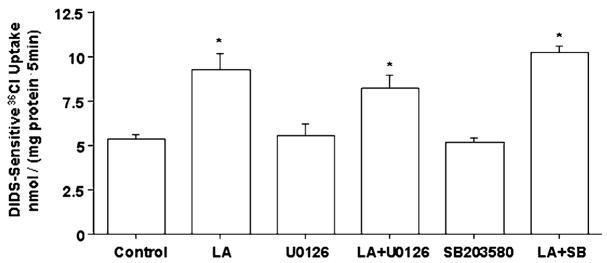
Inhibition of mitogen-activated protein kinase does not block the effects of LA on Cl−/OH− exchange activity
Previous studies have reported the involvement of various signaling pathways in Lactobacilli-mediated modulatory effects on intestinal epithelial cells (18). These pathways include mitogen-activated protein kinase (MAPK) (10,11,14), PI-3 kinase-Akt (10,11), and nuclear factor-κB-I-κB (9). Therefore, studies were designed to investigate the involvement of protein kinase signaling pathways in mediating the stimulation of Cl−/OH− exchange activity in response to LA treatment. Inhibition of MEK1 and MEK2 with U0126 or p38 MAPK with SB203580 did not alter the LA effects of Cl−/OH− exchange activity (Fig. 4), suggesting that these MAPK are not involved in stimulatory effects of LA on Cl−/OH− exchange activity. Similar results were obtained when Caco-2 cells were treated with the CM of LA (data not shown).
FIGURE 4.
Cl−/OH− exchange activity in Caco-2 cells following 3-h treatment with LA in the presence or absence of 30 μmol/L U0126 (MEK1 and MEK 2 inhibitors) or 10 μmol/L SB203580 (p38 inhibitor). Values are means ± SEM, n = 3. *Different from control, P < 0.001.
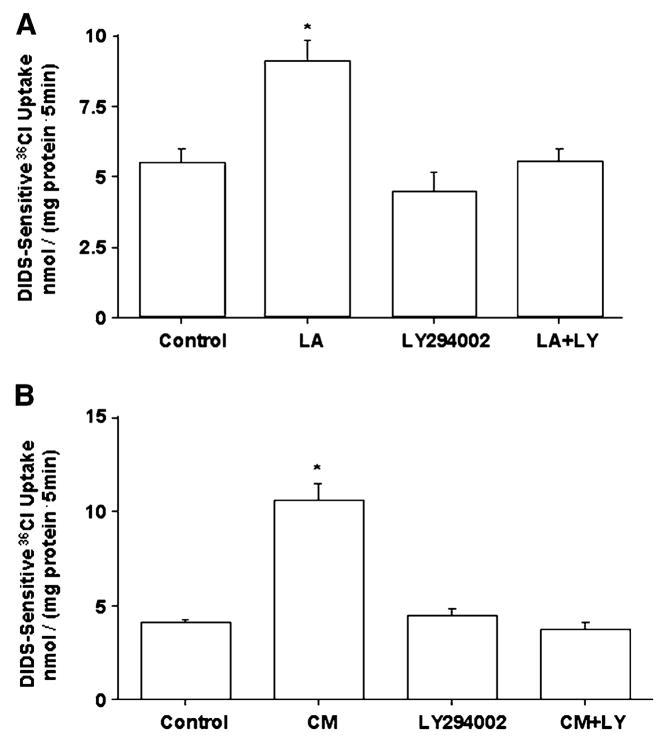
Involvement of PI3 kinase pathway
PI-3 kinase activity has previously been implicated in mediating the reversal of cytokine-induced dysfunctions (11) or the promotion of cell survival and growth (15,16) by Lactobacilli on intestinal epithelial cells. We therefore examined the effects of PI-3 kinase inhibition on LA-mediated stimulation of Cl−/OH− exchange activity. Inhibition of PI-3 kinase with 10 μmol/L LY294002 blocks the stimulatory effects of LA (Fig. 5A) or its CM (Fig. 5B) on Cl−/OH− exchange activity. This suggests that the PI-3 kinase signaling pathway is involved in mediating the effects of LA or its CM on Cl−/OH− exchange activity.
FIGURE 5.
Cl−/OH− exchange activity in Caco-2 cells following 3-h treatment with LA (A) or CM (B) in the presence or absence of 10 μmol/L Ly294002 (PI-3 kinase inhibitor). Values are means ± SEM, n = 3. *Different from control, P < 0.001.
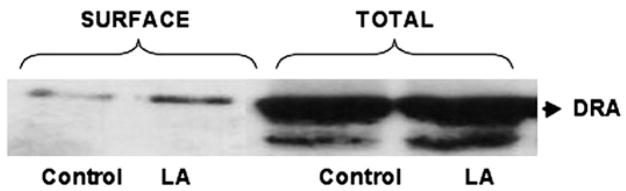
LA increases surface DRA expression
DRA, a member of the SLC26 gene family, has been implicated as one of the candidate genes for apical Cl−/OH− exchange in the human intestine (31,32). We have recently shown that infection of Caco-2 cells with enteropathogenic E. coli reduces surface DRA expression (26). Therefore, we utilized cell surface biotinylation studies to examine the effects of LA treatment for 3 h on cell surface DRA, defined as the biotin-accessible fraction of the total cellular DRA level. The surface level of DRA in LA-treated cells was considerably increased compared with the control, paralleling the increase in Cl−/OH− exchange activity; however, the total cellular DRA expression did not change (Fig. 6). Densitometric analysis of the protein bands (surface DRA:total DRA ratio: 1.5 ± 0.08 for LA-treated cells compared with 0.75 ± 0.03 for control) suggested that surface DRA expression increased by 50% in response to LA treatment for 3 h.
FIGURE 6.
Cell surface (biotin accessible) and total cellular DRA levels following 3-h treatment with LA. A representative blot of 3 independent experiments is shown.
Discussion
Cl−/OH− (HCO3−) exchangers or AE are responsible for the electroneutral exchange of Cl− for OH−/HCO3− across the plasma membranes of polarized epithelial cells and thus play an important role in the vectorial transport of Cl− and maintenance of intracellular Cl− concentration (19,20). We have previously demonstrated that apical Cl−/OH− exchange activity is inhibited by phorbol esters (21), nitric oxide (22), serotonin (23), and infection with enteropathogenic E. coli (26). The results of the present study demonstrate that LA, a probiotic bacterium, significantly stimulates Cl−/OH− exchange activity in Caco-2 cells after 3 h of incubation. Interestingly, the supernatants of LA also enhanced Cl−/OH− exchange, whereas heat-killed LA did not, suggesting that live LA secretes soluble factor(s) that mediate its stimulatory effects on Cl−/OH− exchange activity. Stimulation of Cl−/OH− exchange activity by either live LA, or its CM, was dose dependent. This stimulatory effect was not secondary to a change in external pH, because the dose of LA, or the dilutions of CM, used in the present study did not alter the pH of the incubation medium.
The molecular basis of the effects evoked by probiotics has not been well characterized and might reflect species-specific properties (13). Recent studies demonstrated the involvement of various intracellular signaling pathways in mediating probiotic effects, including MAPK (10,11,14), PI-3 kinase/Akt (10,11,16), and nuclear factor-κB/I-κB (9). A previous study reported the involvement of p38, ERK1, 2 MAPK, and PI3 kinase in mediating modulation of Cl− secretion by LA in Caco-2 and HT29/cl.19a cells (11). However, our studies utilizing pharmacological inhibitors showed that stimulation of Cl−/OH− exchange activity by LA or its CM was unaffected by inhibition of MEK1, MEK2, and p38 MAPK. On the other hand, inhibition of PI3-kinase activity by LY294002 abolished the stimulation of Cl−/OH− exchange activity by LA or its CM, suggesting the likely involvement of a PI3 kinase-mediated pathway. Our previous reports have demonstrated the involvement of PI-3 kinase-mediated pathway in the regulation of Cl−/OH− exchange activity in Caco-2 cells by phorbol ester phorbol myristate acetate (21), taurodeoxycholic acid (24), and lysophosphatidic acid (25).
PI-3 kinase and its downstream effector molecules have a well-established role in intracellular trafficking (33), which might be Akt-dependent or independent (34). It has been reported that interleukin-3–induced stimulation of glucose uptake was a PI-3 kinase-dependent process and was secondary to increased Glut-1 trafficking to membrane surface mediated by PI3-k/Akt pathway (35). On the other hand, insulin regulation of glucose uptake in adipocytes, which is secondary to increased trafficking of Glut-4, was dependent on PI-3 kinase activity but independent of Akt activity (33). Because our studies showed that LA-induced stimulation of Cl−/OH− exchange activity in Caco-2 cells is PI-3 kinase pathway dependent, it was of interest to examine if LA increases surface expression of DRA, the predominant transport protein shown to be responsible for intestinal apical Cl−/OH− (HCO3−) exchange activity. We found that LA significantly increased surface DRA although total cellular DRA did not change. This suggests that LA treatment increased trafficking of DRA to membrane surface but did not affect total cellular expression of DRA. Because intracellular trafficking events mediated by PI-3 kinase pathways are complex and require the involvement of several downstream effectors, including small GTPases (34), it will be of interest in the future to examine in detail if increased trafficking of DRA to membrane surface in response to LA treatment is mediated by a PI-3 kinase pathway. Previous studies have reported that LA reversed the cytokine-mediated downregulation of CFTR and NKCC1 (11). In future studies, it will be of interest to also examine the potential role of these transporters in probiotic-mediated modulation of apical Cl−/OH− exchange.
Our results also showed that the CM of LA was equally effective in stimulating Cl−/OH− exchange activity in a dose-dependent manner and involved PI-3 kinase pathway. These results indicate that soluble factors secreted by LA are responsible for stimulating Cl−/OH− exchange activity and attachment of live bacteria to the host cell surface is not necessary to produce this effect. Various other studies reported similar observations demonstrating that soluble factors secreted by probiotics are sufficient to beneficially modulate host cell functions and live bacteria are not necessary (14,16,30). Further characterization of these soluble factors is important to exploit their beneficial effects more efficiently. Interestingly, inactivation of the CM by heating at 95°C for 20 min did not alter the stimulatory effects of the CM on Cl−/OH− exchange activity. Although these results suggest that the soluble factors are unlikely to be proteins, this does not exclude the possibility that they might be heat-stable, small molecular weight peptides. A previous report (14) also demonstrated that induction of heat-shock protein expression in intestinal epithelial cells by Lactobacillus GG condition medium is mediated by a heat- and acid-stable low molecular weight peptide. Further characterization of the soluble factors present in LA culture supernatant that stimulate Cl−/OH− exchange activity will be of importance in future.
In summary, LA, a probiotic bacterium, stimulated apical Cl−/OH− exchange activity corresponding to increased surface expression of DRA in Caco-2 cells via the PI-3 kinase-mediated pathway. This effect of LA was mediated by soluble factors secreted by LA. The benefits of LA CM through stimulation of apical Cl−/OH− exchange may have broader therapeutic applicability in treating diarrhea and other intestinal inflammatory disorders involving impairment of electrolyte absorption. Future studies examining the characterization of the soluble factors and the effects of purified soluble factors on Cl−/OH− (HCO3−) exchange activity will be of therapeutic importance.
Footnotes
Supported by the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases grants DK 54016 (P.K.D.), DK 33349 (K.R.), DK 50694-10 (G.A.H.) and the Program Project grant DK 067887 (P.K.D., K.R., G.A.H.)
Author disclosures: A. Borthakur, R. K. Gill, S. Tyagi, A. Koutsouris, W. A. Alrefai, G. A. Hecht, K. Ramaswamy, and P. K. Dudeja, no conflicts of interest.
Abbreviations used: AE, anion exchanger; CM, conditioned medium; DIDS, 4,4′-diisothiocyanostilbene-2, 2′-disulfonic acid; DRA, Down Regulated in Adenoma; MAPK, mitogen-activated protein kinase; MOI, multiplicity of infection; LA, Lactobacillus acidophilus; LR, Lactobacillus rhamnosus.
Literature Cited
- 1.Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237–8. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 2.Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004;80:516–26. doi: 10.1136/pgmj.2003.008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mack DR, Lebel S. Role of probiotics in the modulation of intestinal infections and inflammation. Curr Opin Gastroenterol. 2004;20:22–6. doi: 10.1097/00001574-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Gionchetti P, Rizzello F, Venturi A, Campieri M. Probiotics in infective diarrhea and inflammatory bowel diseases. J Gastroenterol Hepatol. 2000;15:489–93. doi: 10.1046/j.1440-1746.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- 5.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–7. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 7.Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 8.Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, Hart CA. Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr. 1996;42:162–5. doi: 10.1093/tropej/42.3.162. [DOI] [PubMed] [Google Scholar]
- 9.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–87. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–97. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–46. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–33. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–26. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–30. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 15.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–65. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano J, Yoshida T, Sugiyama T, Koide N, Mori I, Yokochi T. The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Microbiol Immunol. 2003;47:405–9. doi: 10.1111/j.1348-0421.2003.tb03377.x. [DOI] [PubMed] [Google Scholar]
- 18.Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol. 2006;17:204–10. doi: 10.1016/j.copbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Dudeja PK, Gill RK, Ramaswamy K. Absorption-secretion and epithelial cell function. In: Koch TR, editor. Colonic diseases. Totowa (NJ): Humana Press; 2003. pp. 3–24. [Google Scholar]
- 20.Gill RK, Ramaswamy K, Dudeja PK. Research signpost. Recent research developments in physiology. Trivandrum (India): Research Signpost; 2003. Mechanisms and regulation of NaCl absorption in the human intestine; pp. 643–77. [Google Scholar]
- 21.Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Inhibition of apical Cl−/OH− exchange activity in Caco-2 cells by phorbol esters is mediated by PKCepsilon. Am J Physiol Cell Physiol. 2002;283:C1492–500. doi: 10.1152/ajpcell.00473.2001. [DOI] [PubMed] [Google Scholar]
- 22.Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Modulation of Cl-/OH- exchange activity in Caco-2 cells by nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2002;283:G626–33. doi: 10.1152/ajpgi.00395.2001. [DOI] [PubMed] [Google Scholar]
- 23.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl−/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem. 2005;280:11859–68. doi: 10.1074/jbc.M411553200. [DOI] [PubMed] [Google Scholar]
- 24.Alrefai WA, Saksena A, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholic acid modulates apical Cl−/OH− exchange activity in Caco-2 cells. Dig Dis Sci. 2007;52:1270–8. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 25.Dwivedi A, Gill RK, Pant N, Saksena S, Alrefai WA, Singla A, Ramaswamy K, Dudeja PK. Modulation of apical Cl−/OH− exchange activity by LPA in Caco-2 cells via LPA2 receptor and PI-3 kinase/Akt dependent pathways [abstract] Gastroenterology. 2007:A576. [Google Scholar]
- 26.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht GA, et al. Mechanism underlying inhibition of intestinal apical Cl−/OH− exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–37. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsnes S, Tonnessen TI, Ludt J, Sandvig K. Effect of intracellular pH on the rate of chloride uptake and efflux in different mammalian cell lines. Biochemistry. 1987;26:2778–85. doi: 10.1021/bi00384a019. [DOI] [PubMed] [Google Scholar]
- 28.Wieth JO, Andersen OS, Brahm J, Bjerrum PJ, Borders CL. Chloride-bicarbonate exchange in red blood cells: physiology of transport and chemical modification of binding sites. Philos Trans R Soc Lond B Biol Sci. 1982;B299:383–99. doi: 10.1098/rstb.1982.0139. [DOI] [PubMed] [Google Scholar]
- 29.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Frick JS, Schenk K, Quitadamo M, Kahl F, Koberle M, Bohn E, Aepfelbacher M, Autenrieth IB. Lactobacillus fermentum attenuates the proinflammatory effect of Yersinia enterocolitica on human epithelial cells. Inflamm Bowel Dis. 2007;13:83–90. doi: 10.1002/ibd.20009. [DOI] [PubMed] [Google Scholar]
- 31.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol. 1999;276:G185–92. doi: 10.1152/ajpgi.1999.276.1.G185. [DOI] [PubMed] [Google Scholar]
- 32.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–21. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–93. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vivanco I, Sawyers CL. The phosphatidyl inositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 35.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–46. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]