Abstract
Although the complement system has been implicated in atherosclerosis, the influence of membrane-bound complement regulators in this process has not been well understood. We studied the role of two membrane complement regulators, decay-accelerating factor (DAF) and CD59, in a murine model of atherosclerosis. DAF−/− and CD59−/− mice were crossed with apolipoprotein E (ApoE)-deficient mice to generate DAF−/−ApoE−/− and CD59−/−ApoE−/− mice. Mice were fed a high fat diet (HFD) for 8 or 16 weeks. En face analysis showed that CD59 deficiency led to more extensive lesions in female ApoE−/− mice both at 8 weeks (2.07±0.27 % vs.1.34±0.21%, P=0.06) and 16 weeks (17.13±1.14% vs. 9.72±1.14%, P<0.001). Similarly, lesions measured by aortic root sectioning were larger in female CD59−/−ApoE−/− mice than in controls at 8 weeks of HFD feeding (20.74±1.33% vs. 13.12±1.46%, P<0.005). On the other hand, DAF deficiency did not significantly influence atherosclerosis in ApoE−/− mice. Immunohistochemistry revealed more abundant membrane attack complex (MAC) deposition and more collagen staining in the aortic roots of CD59−/−ApoE−/− mice. Unexpectedly, total plasma cholesterol levels in female CD59−/−ApoE−/− mice were found to be elevated compared with CD59+/+ApoE−/− mice. We conclude that CD59 but not DAF offered protection in atherosclerosis in the context of ApoE deficiency. The protective role of CD59 was gender-biased and most likely involved prevention of MAC-mediated vascular injury, with possible contribution from an undefined effect on plasma cholesterol homeostasis.
1. Introduction
Recent studies have indicated that atherosclerosis is a chronic inflammatory condition in which many components of innate and adaptive immune systems are involved (Hansson, 2005). As an important part of innate immunity, complement proteins are present in the blood in precursor forms and can be activated during pathogen invasion or tissue injury (Mollnes et al., 2002; Song et al., 2000; Walport, 2001b; Walport, 2001c). Activation of complement can occur via three different pathways, the classical pathway, the lectin pathway and the alternative pathway (Mollnes et al., 2002; Song et al., 2000; Walport, 2001b; Walport, 2001c). Activated complement promotes inflammation through the generation of leukocyte chemoattractants C3a and C5a, and causes cellular injury by the formation of the membrane attack complex (MAC)(Walport, 2001a). To prevent complement-mediated autologous tissue injury, host cells, particularly those in the vascular space such as blood cells and endothelial cells, express several membrane-bound complement regulatory proteins. DAF and CD59 are two such proteins that link to the cell membrane via a glycosylphosphatidylinositol (GPI) anchor. DAF regulates complement activation by promoting the decay of C3 and C5 convertases, whereas CD59 inhibits the formation of the MAC (Lublin and Atkinson, 1989; Nicholson-Weller et al., 1982; Okada et al., 1989; Rollins and Sims, 1990).
A number of studies have implicated the complement system in the pathogenesis of atherosclerosis (Oksjoki et al., 2003). For example, various complement activation products, regulatory proteins, and complement receptors have been detected in human atherosclerotic lesions(Seifert and Hansson, 1989a; Vlaicu et al., 1985a), particularly in vulnerable and ruptured plaques(Oksjoki et al., 2003), and deposition of C5b-9 has been shown to correlate with the disease state (Vlaicu et al., 1985b). Experimental studies with complement deficient animals have also been performed to elucidate the role of the complement system in atherogenesis (Bhatia et al., 2007; Buono et al., 2002; Niculescu and Rus, 2004; Patel et al., 2001; Persson et al., 2004; Schmiedt et al., 1998). A cholesterol-rich diet was shown to induce less atherosclerosis in rabbits deficient in C6 than in controls with a fully functional complement system (Schmiedt et al., 1998). Contrasting results were obtained, however, in C5 deficient ApoE−/− mice, which showed a similar extent of atherosclerotic lesions compared to complement-competent control mice (Patel et al., 2001). In other studies, C3 deficiency was found to increase lipid-positive lesions in the mouse aorta (Buono et al., 2002; Persson et al., 2004), as well as altering the plasma lipid profile (Persson et al., 2004), whereas deficiency of factor B affected neither lipid levels nor lesion size (Bhatia et al., 2007). More recently, C1q deficiency was found to cause larger atherosclerotic lesions in low-density lipoprotein receptor knockout (LDLR−/−) mice, possibly reflecting a role of C1q in the disposal of dying cells (Bhatia et al., 2007). Together, these results suggested a variable and complex role of complement components in atherosclerosis.
In contrast to the above studies on individual complement components in atherogenesis, there have been few studies testing the role of complement regulatory proteins in the development of this disease. Previous studies have detected the presence of complement regulators in atherosclerotic lesions (Mackness et al., 1997; Niculescu et al., 1987; Niculescu et al., 1990; Seifert and Hansson, 1989a; Seifert and Hansson, 1989b) and recently, both statins and C-reactive protein (CRP), the latter a marker of chronic inflammation and strong predictor of vascular diseases, have been shown to induce the expression of endothelial complement regulators (Bhakdi et al., 1999; Li et al., 2004; Mason et al., 2002). In the present study, we have evaluated the role of DAF and CD59 in the ApoE−/− mouse model of atherosclerosis. We found that CD59 but not DAF deficiency exacerbated high fat diet-induced atherosclerosis in ApoE−/− mice in a gender-biased manner.
2. Materials and methods
2.1 Mice and diets
DAF knockout (DAF−/−) mice and CD59a knockout (CD59−/−) mice were generated as previously described (Miwa et al., 2002b; Sun et al., 1999). These mice were deficient in the widely distributed GPI-anchored Daf-1 gene and CD59a gene, respectively, and have been fully backcrossed (>9 generations) to C57BL/6J mice. ApoE−/− mice were purchased from the Jackson Laboratories (Bay Harbor, Maine, USA). To generate DAF−/−ApoE−/− or CD59−/−ApoE−/− mice, we first crossed DAF−/− or CD59−/− mice with ApoE−/− mice and produced DAF+/−ApoE−/− or CD59+/−ApoE−/− mice, respectively, as breeders. Brother/sister mating of the latter genotypes produced DAF−/−ApoE−/− and DAF+/+ApoE−/− mice as littermates, and CD59−/−ApoE−/− and CD59+/+ApoE−/− mice as littermates, respectively. The ApoE genotype was identified by polymerase chain reaction (PCR) of tail DNA. PCR conditions and primer information are available from the Jackson Laboratories. The DAF and CD59 genotype was screened by FACS analysis of erythrocytes as described previously (Miwa et al., 2002b).
Mice were genotyped and separated into male and female groups. Starting from 6 weeks of age, they were fed ad libitum a normal chow supplemented with 0.15% cholesterol and 20% buffer fat (Harlan Teklad, Madison, WI). At 14 or 22 weeks of age (i.e. 8 or 16 weeks after cholesterol diet feeding), they were fasted overnight and killed by CO2 asphyxiation. Blood was collected for plasma lipid analysis by vena cava nicking, and the aorta was perfused with cold PBS by inserting a cannula into the left ventricle. Perfused aortas were dissected from aortic arch to iliac bifurcation. All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania.
2.2 Processing and analysis of the aorta
The aortas were prepared and analyzed as described (Tangirala et al., 1995). Aortas were pinned on wax plates and stained with Sudan IV (Sigma-Aldrich, St. Louis, MO). The extent of atherosclerosis was expressed as the percentage of the aorta surface covered by positive staining. Atherosclerotic lesions were also quantified in the aortic root cross sections from frozen OCT-embedded hearts as described(Nicoletti et al., 1998). Briefly, serial 6-μm-thick cryostat sections were prepared from the origin of the aortic valve cusps and cross-sectional analysis for atherosclerotic lesion was performed every 60 μm over 360 μm by staining with oil-red O (Sigma-Aldrich, St. Louis, MO). The extent of atherosclerosis was expressed as the percentage of aortic root covered by lesions. Images were captured digitally with a video camera and analyzed by computerized image analysis (Image Pro Plus, Media Cybernetics). Samples were coded and acquisition of images and analysis of lesions were performed in a blinded fashion.
2.3 Histological analysis of aortic lesions
Sections were fixed in acetone, rehydrated in PBS, treated with 0.3% H2O2 in PBS to quench endogenous peroxides and then blocked with 5% goat serum. Subsequently, sections are stained with specific antibodies. To stain C9, a rabbit anti-rat C9 antibody (kindly provided by Dr. Paul Morgan, Cardiff University, Cardiff, UK) was used as primary antibody, and biotinylated goat anti-rabbit IgG antibody BA-1000 (Vector Laboratories, Burlingame, CA) as secondary antibody. For smooth muscle cells, FITC-conjugated mouse anti-α-smooth muscle actin clone 1A4 (Sigma-Aldrich, St Louis, Mo) was used as primary antibody, followed by a biotin anti-FITC antibody BA-0601(Vector Laboratories, Inc. Burlingame, CA). To stain macrophages, we used a rat anti-mouse CD68:FITC antibody (AbD serotec, Oxford, UK), followed by biotinylated anti-rat IgG BA-9400 (Vector Laboratories, Burlingame, CA) supplemented with 5% mouse serum. For C3 staining, biotinylated goat anti–mouse C3 antibodies (Kim et al., 2006) were used. A biotinylated goat IgG (Southern Biotech, Birmingham, AL) was used as a control. Antibody reactivity was detected with ABC Vectastain Elite kit (Vector Laboratories, Burlingame, CA), developed with diaminobenzidine tetrahydrochloride (DAB) as a substrate and then counterstained with hematoxylin. Collagen was stained with Picrosirius red (Sigma-Aldrich, St. Louis, MO), and sections were viewed under a polarized microscope. For T cell staining, FITC-conjugated anti-mouse CD4 (eBioscience Inc, San Diego, CA) or FITC-conjugated anti-mouse CD8a (BD Biosciences, San Jose, CA) was used as a primary antibody, followed by a biotin anti-FITC antibody BA-0601(Vector Laboratories, Inc. Burlingame, CA). For immunostaining experiments, protocols were first optimized to eliminate non-specific staining with no primary antibody or a control primary antibody added.
2.4 Serum and urine sample analysis
Plasma samples were collected at time of sacrifice (after 12 hours of fasting) and stored at −80°C. Total cholesterol was analyzed as described previously(Wang et al., 2008). Twenty-four hour urine samples were collected in metabolic cages. The nonenzymatic lipid peroxidation product, 8,12-iso-iPF2 -VI, was measured as previously described (Song et al., 2007). Metabolite levels were corrected for urinary creatinine (Oxford Biomedical Research, Oxford, Mich).
2.5 Statistical Analysis
Statistical analyses were performed with GraphPad software (Prism 3.0). Data were analyzed by two-tailed student’s t test (for data with normal distribution) or Mann-Whitney test (for data with non-parametric distribution). Results are considered to be significant at values of P<0.05 and are presented as mean ± SEM.
3. Results
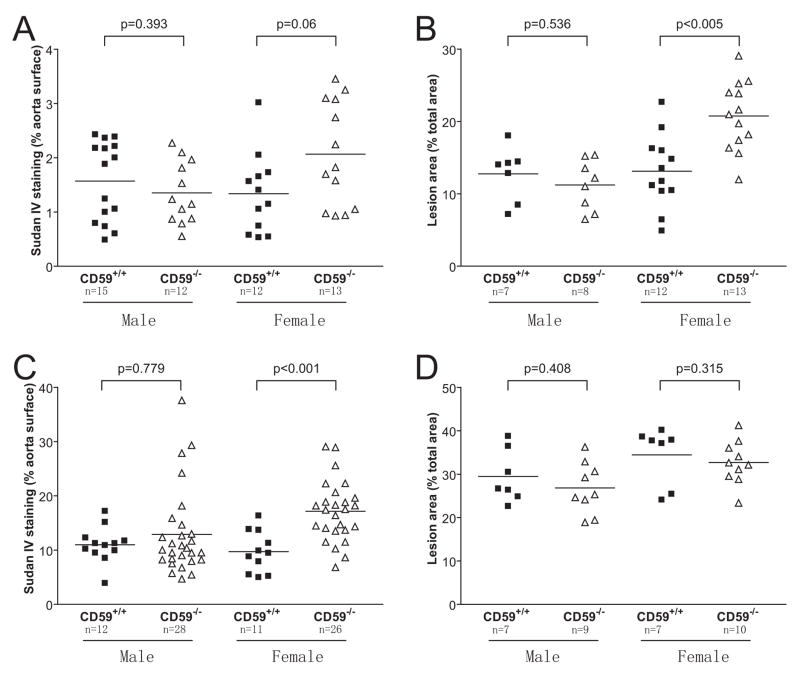
Aortic atherosclerotic lesions were observed in all groups of mice both at 8 weeks and 16 weeks of HFD feeding. As expected, the extent of atherosclerotic lesion development, evaluated by en face staining or by staining of aortic root sections, strongly correlated with the length of time of HFD feeding (Fig 1). After 8 weeks of HFD, we found that female CD59−/−ApoE−/− mice developed larger atherosclerotic lesions in their aortas, as shown by both en face (2.07±0.27% vs.1.34±0.21%, P=0.06; Figure 1A and 2A) and aortic root section analysis (20.74±1.33% vs. 13.12±1.46%, P<0.005; Figure 1A and 2B). Interestingly, the increased sensitivity to atherosclerosis development in CD59−/−ApoE−/− mice appeared to be gender-biased as we observed no significant difference between male CD59−/−ApoE−/− and CD59+/+ApoE−/− mice by either en face (1.35±0.16% vs. 1.57±0.19%, P=0.393; Figure 2A) or aortic root section analysis (11.23±1.23% vs. 12.75±1.41%, P=0.536; Figure 2B).
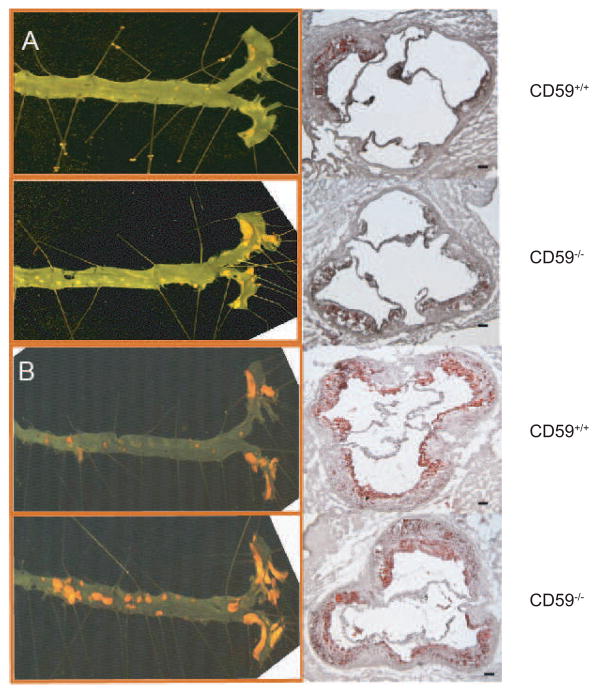
Figure 1.
Representative photomicrographs showing the influence of CD59 deficiency on atherosclerosis in ApoE−/− mice. A, aortic atherosclerotic lesions in female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice after 8 weeks of high fat diet. B, aortic atherosclerotic lesions in female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice after 16 weeks of high fat diet. Left panels show the en face method of Sudan IV staining of the whole aorta. Right panels show Oil red O staining of aortic root sections. Scale bar: 100 μm.
Figure 2.
Quantitative analysis of atherosclerotic lesions in CD59-deficient and -sufficient ApoE−/− mice. A, quantitation of lesion areas in the whole aorta (en face staining) after 8 weeks of high fat diet feeding. B. quantitation of lesion areas in the aortic root sections after 8 weeks of high fat diet feeding. C. quantitation of lesion areas in the whole aorta (en face staining) after 16 weeks of high fat diet feeding. D. quantitation of lesion areas in the aortic root sections after 16 weeks of high fat diet feeding. Samples in B and D were selected randomly from A and C, respectively. Data in B and D represent the mean±SEM lesion area in 6 sections for each mouse sample. P values are by Mann-Whitney test.
After 16 weeks of HFD, a more pronounced difference between female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice was observed by en face staining (17.13±1.14% vs. 9.72±1.14%, P<0.001; Figure 1B and 2C). However, a difference could not be detected between the two groups mice by aortic root section analysis at this advanced stage of lesion development (32.67±1.58%vs. 34.44±2.52%, P=0.315; Figure 1B and 2D). Similar to the finding at 8 weeks of HFD feeding, we detected no significant difference at 16 weeks between male CD59−/−ApoE−/− and CD59+/+ApoE−/− mice by either the en face (12.88±1.49%vs. 10.98±0.95%, P=0.779; Figure 2C) or aortic root section analysis (26.84±1.97% vs. 29.46±2.3%, P=0.408; Figure 2D). Nevertheless, 4 of the mice in the male CD59−/−ApoE−/− cohort were noted to have developed severe aortic lesions as assessed by the en face method (Fig 2C), suggesting that CD59 deficiency may have also exacerbate atherosclerosis in a subgroup of male CD59−/−ApoE−/− mice.
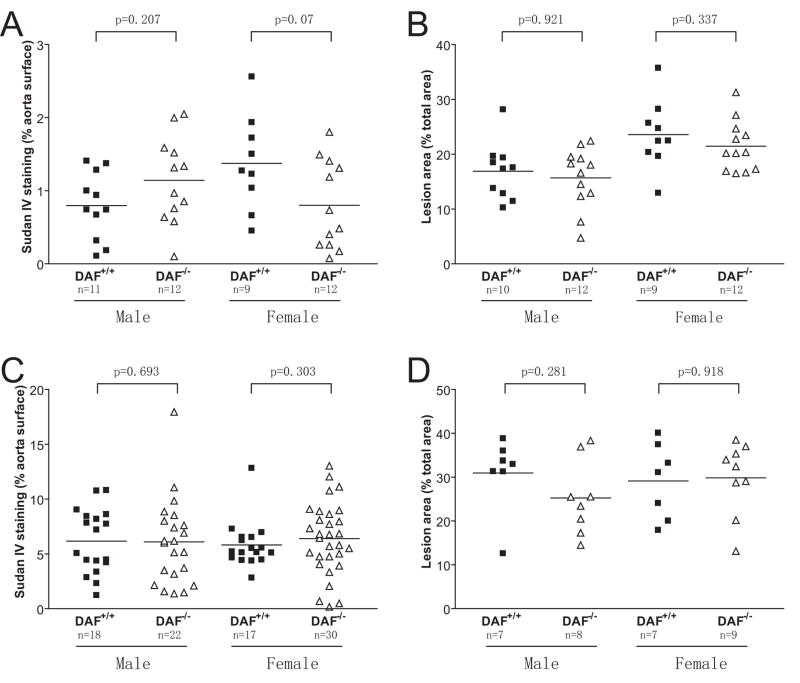
In contrast to the effect of CD59 deficiency on atherosclerosis in female ApoE−/− mice, we observed no significant difference in either gender between DAF−/−ApoE−/− and DAF+/+ApoE−/− mice, regardless of the length of HFD feeding (Fig 3). En face analysis showed that the average lesion areas in female DAF−/−ApoE−/− and DAF+/+ApoE−/− mice at 8 and 16 weeks of HFD feeding were 0.80±0.17% vs 1.37±0.22% (P=0.07; Figure 3A), and 6.39±0.59% vs 5.81±0.51%, (P =0.303; Figure 3C), and that for male mice were 1.14±0.17% vs 0.80±0.14% (P =0.207; Figure 3A), and 6.09±0.83% vs 6.16±0.69% (P =0.693; Figure 3C), respectively. Aortic root section analysis showed the average lesion areas in female DAF−/−ApoE−/− and DAF+/+ApoE−/− mice at 8 and 16 weeks of HFD to be 21.45±1.33% vs 23.58±2.10% (P =0.337; Figure 3B), and 29.81±2.78% vs 29.11±3.25% (P = 0.918; Figure 3D), and that for male mice were 15.68±1.58% vs 16.89±1.65% (P =0.921; Figure 3B), and 25.25±3.02% vs 30.94±3.22 % (P =0.281; Figure 3D), respectively.
Figure 3.
DAF deficiency did not impact atherogenesis in ApoE−/− mice. A and B, quantitative analysis of lesion areas in whole aortas (A) and in aortic root sections (B) in DAF−/−ApoE−/− and DAF55+/+ApoE−/− mice after 8 weeks of high fat diet feeding. C and D, quantitative analysis of lesion areas in whole aortas (C) and in aortic root sections (D) in DAF−/−ApoE−/− and DAF55+/+ApoE−/− mice after 16 weeks of high fat diet feeding. Samples in D were selected randomly from C, and data represent the mean±SEM lesion area in 6 sections for each mouse. P values are by Mann-Whitney test.
Because there was no significant influence on atherosclerosis by DAF deficiency, we focused our subsequent studies on CD59−/−ApoE−/− mice. Plasma lipid analysis showed a moderate increase in total plasma cholesterol levels in female CD59−/−ApoE−/− mice compared with their CD59+/+ApoE−/− littermates, but the difference reached statistical significance only at 8 weeks of HFD feeding (Table 1). To determine if exacerbated atherosclerosis in female CD59−/−ApoE−/− mice was correlated with increased oxidant stress, we analyzed and compared urinary 8,12-iso-iPF2 -VI levels in these mice and their CD59-sufficient littermates. We observed no significant difference between female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice either at 8 weeks or 16 weeks of HFD feeding (8 weeks: 2.98±1.01 vs. 3.12±0.96 ng/mg creatinine, P=0.92, Student t test, n=13 and n=12 for CD59-deficient and CD59-sufficient groups; 16 weeks: 1.59±0.38 vs. 1.65±0.56 ng/mg creatinine, P=0.92, Student t test, n=26 and n=11 for CD59-deficient and CD59-sufficient groups).
Table 1.
Plasma total cholesterol levels (mg/dL)
| CD59+/+ ApoE−/− | CD59−/− ApoE−/− | |
|---|---|---|
| 14-week-old mice | ||
| Male | 1337±71.32 (n=15) | 1518±101.1 (n=12) |
| Female | 1208±57.78 (n=12) | 1404±43.74 (n=13)* |
| 22-week-old mice | ||
| Male | 1078±102.7 (n=12) | 1047±80.86 (n=20) |
| Female | 988.2±38.33 (n=10) | 1112±65.28 (n=26) |
Mice consumed a high fat diet for 8 weeks or 16 weeks. Data are presented as mean±SEM.
P<0.05 by Student t test.
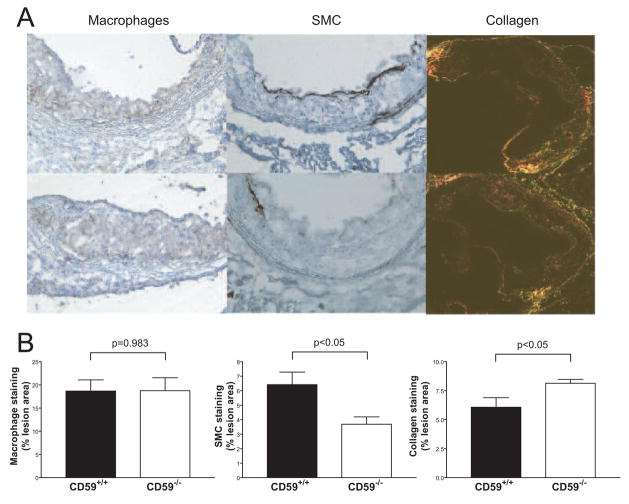
We next performed histological studies in female CD59-deficient and -sufficient mice to evaluate the complexity of the atherosclerotic lesions. We also carried out immunohistochemical analysis to determine the degrees of complement activation, macrophage infiltration, collagen deposition and smooth muscle cell proliferation. Morphological examination of aortic root sections after 8 weeks of HFD revealed lesions in all mice, ranging from fatty streaks composed of foam cells to advanced fibrofatty lesions covered by fibrous caps. After 16 weeks of HFD, all of the intima was covered by advanced fibrofatty lesions. Using CD68 as a marker, we measured the lesion areas that were positive for macrophage infiltration. No significant difference was observed between female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice in macrophage distribution and content (18.74±2.79% vs. 18.66±2.41%, P=0.983; Figure 4). On the other hand, at 8 weeks of HFD feeding, we detected more collagen (8.14±0. 33% vs. 6.07±0.82%, P<0.05; Figure 4) and less smooth muscle cell staining (3.69±0.51% vs. 6.42±0. 86%, P<0.05; Figure 4) in the lesions of CD59-deficient mice compared with their littermate controls. However, the difference in smooth muscle cell content was not observed at 16 weeks of HFD feeding (2.25±0.24% vs. 2.28±0.54% [n= 5 in each group], P=0.97, Student t test). We also examined CD4+ and CD8+ T cell infiltration into the lesions but observed no significant difference between female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice (for CD4+ T cells: 35.89 ±8.505 vs. 40.99±7.091/mm2 [n= 5 in each group], P=0.66, Student t test; for CD8+ T cells: 1.622±1.622 vs. 5.384±3.946/mm2 [n= 5 in each group], P=0.40, Student t test).
Figure 4.
Immunohistochemical analysis of macrophage infiltration, smooth muscle cell proliferation and collagen deposition in female DAF−/−ApoE−/− mouse atherosclerotic lesions. A, Representative photomicrographs of atherosclerotic plaques from the aortic root of CD59−/− ApoE−/− (bottom) and CD59+/+ ApoE−/− (top) mice stained for macrophages (left, CD68 staining), smooth muscle cells (middle, α-actin staining) or collagen (right, Sirius Red staining). B, Morphometric analysis of the stained areas for macrophages, smooth muscle cells and collagen. Mice were analyzed after 8 weeks of high fat diet feeding. Bars represent mean±SEM. N= 5 per group. Results are expressed as the percentage of positive area according to the lesion area in 6 sections for each mouse. P values are by Student t test.
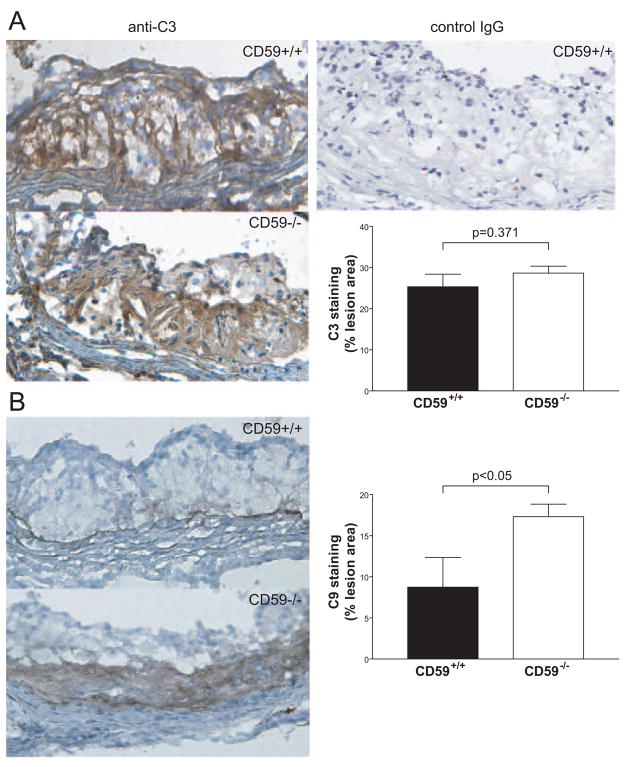
Staining of aortic root sections with anti-C3 and anti-C9 antibodies showed a similar C3 deposition (28.65±1.69% vs. 25.33±3.07%, P=0.371) in female CD59−/−ApoE−/− mice and controls (Figure 5A), but significantly increased C9 deposition was found in the sections of female CD59−/−ApoE−/− mice than those of the controls (17.32±1.52% vs. 8.74±3.63%, P<0.05) after 8 weeks of HFD feeding (Figure 5B). This result suggested that CD59 deficiency led to more deposition of the membrane attack complex within the atherosclerotic lesions.
Figure 5.
CD59 deficiency increased the deposition of membrane attack complex within the atherosclerotic lesions. A, C3 staining of aortic root sections from female CD59−/− ApoE−/− and CD59+/+ ApoE−/− mice after 8 weeks of high fat diet (n=5 in each group). Left, representative photographs of sections stained for C3. Upper right, background staining with a goat IgG isotype control antibody. Lower right, morphometric analysis of C3-stained area in the lesions. B, C9 staining of aortic root sections from female CD59−/− ApoE−/− and control mice after 8 weeks of high fat diet (n=7 in each group). Left, representative photographs of sections stained for C9. Right, quantitative analysis of the C9 stained area of lesions. Results are expressed as the percentage of positive area. Lesion areas in 6 sections for each mouse were measured. Bars represent mean±SEM. P values are by Student t test.
4. Discussion
Complement activation has been suggested to contribute to the progression of atherosclerotic lesions and its regulatory components are also found in human atherosclerotic lesions (Niculescu and Rus, 1999; Niculescu and Rus, 2004). To examine the role of membrane-bound complement regulatory proteins DAF and CD59 in this process, we crossed DAF−/− and CD59−/− mice with ApoE−/− mice and evaluated the impact of DAF or CD59 deficiency on the progression of diet-induced atherosclerosis in ApoE−/− mice. While we observed no impact of DAF deficiency, we found CD59 deficiency significantly exacerbated atherosclerosis development in female ApoE−/− mice. Our result is consistent with the finding of a recent study showing CD59 deficiency accelerated atherosclerosis development in Ldlr−/− mice (Yun et al., 2008), and supports the conclusion that CD59 is protective on a pro-atherogenesis genetic background. Interestingly, the protective activity of CD59 in this disease setting was gender-biased, with the effect being detected only in female mice. Of note, in the study involving CD59−/− and Ldlr−/− crosses (Yun et al., 2008), only female mice were studied.
The lack of effect by DAF deficiency on atherosclerosis development in ApoE−/− mice contrasted with other inflammatory disease models wherein DAF was shown to be relevant (Liu et al., 2005; Miwa et al., 2002a; Sogabe et al., 2001). It also contrasted with the result of an Ldlr−/− mouse study showing that DAF deficiency exacerbated atherosclerosis (Leung et al, unpublished data). The reason for these discrepancies is unknown, but may involve disease context-specific up-regulation of redundant C3 convertase inhibitors such as factor H or complement receptor 1-related gene/protein y (Crry) (Kim et al., 2008; Molina et al., 2002), which could have compensated for the lack of DAF in the ApoE−/− atherosclerosis model but not in other settings. Arguing against such an explanation, however, was the finding of abundant C3 deposition on the atherosclerotic lesions of both CD59+/+ApoE−/− and CD59−/−ApoE−/− mice (Fig 5A), suggesting that even in the presence of DAF and other C3 regulators, there was active C3 activation in the ApoE−/− mouse lesions. Thus, at least in the ApoE−/− model, deleting DAF may be inconsequential. An alternative interpretation is that C3 activation may play a paradoxical role in suppressing lesion development. Indeed, both in the ApoE−/− and the Ldlr−/− mouse model, deletion of C3 was shown to increase the lesion size (Buono et al., 2002; Persson et al., 2004).
It is interesting that although we found a good correlation between the en face and the aortic root section methods at 8 weeks, such a correlation was not seen at 16 weeks of HFD feeding. Thus, we observed a clear difference in lesion size between female CD59−/−ApoE−/− and CD59+/+ApoE−/− mice at 16 weeks by the en face but not the aortic root section analysis. The lack of a correlation between the two methods was also reported in other studies using different strains of atherosclerosis-prone mice (Babaev et al., 2000; Persson et al., 2004; Yun et al., 2008). The aortic roots may be particularly sensitive to lesion development caused by ApoE- or LDLR-deficiency, and any additive or synergistic effect brought upon by other pro-atherogenic genetic alterations could be difficult to detect, especially after prolonged HFD feeding. A second notable observation in our study was the lack of correlation between atherosclerosis and isoprostane production. Isoprostanes are free radical-catalyzed products of arachidonic acid which reflect oxidative stress and lipid peroxidation in vivo(Patrono and FitzGerald, 1997). Urinary isoprostanes are regarded as a good marker of oxidative stress-related vascular wall injury in human patients of atherosclerosis and in several mouse models of atherosclerosis (Pratico et al., 1997; Rokach et al., 1997; Rudic et al., 2005). We measured urinary 8, 12 -iso iPF(2alpha)-VI levels in our study but found no difference among CD59-deficient and sufficient ApoE−/− mice. This suggested that the anti-atherogenic role of CD59 in our model is independent of oxidant stress.
CD59 prevents the assembly of the MAC on host cells (Okada et al., 1989; Rollins and Sims, 1990). Increased MAC deposition on endothelial cells as a result of CD59 deficiency could have a multitude of consequences. Previous studies have shown that sublytic deposition of MAC on endothelial cells could cause their activation and stimulate the release of bFGF and PDGF (Benzaquen et al., 1994; Halperin et al., 1993), as well as the expression of IL-8, MCP-1, P-selectin, E-selectin and other vascular cell adhesion molecules(Oksjoki et al., 2003). These factors may together increase the infiltration and retention of monocytes and the formation of foam cells. Our immunostaining analysis revealed no significant difference in the macrophage content between CD59−/−ApoE−/− and CD59+/+ApoE−/− mouse lesions, which would argue against sublytic MAC-induced monocyte trafficking and retention as a responsible mechanism. More likely, a higher level of MAC deposition, as supported by significantly increased C9 staining, could have caused lyses of endothelial cells and the resulting disruption in proper endothelial barrier function could have contributed to the accelerated atherogenesis in female CD59−/−ApoE−/− mice. A similar MAC-mediated lysis of vascular smooth muscle cells could have occurred and accounted for a reduction in smooth muscle cell staining in the lesions of CD59−/−ApoE−/− mice.
The finding of increased total plasma cholesterol levels in CD59−/−ApoE−/− mice was unexpected. Although the increase was modest and reached statistical significance only at 8 weeks of HFD feeding, we can not exclude the possibility that this alteration in plasma lipid profile contributed to the exacerbated atherosclerosis in female CD59−/−ApoE−/− mice. Apart from being an inhibitor of MAC formation, recent studies have revealed a novel function of CD59 in T cell biology (Longhi et al., 2006; Longhi et al., 2005; Morgan et al., 1993; Murray and Robbins, 1998). Whether CD59 has an uncharacterized role in lipid metabolism and/or acted through modulating T cell immunity in the ApoE−/− model remain to be clarified in further studies. Regardless, it is reasonable to conclude that inhibition by CD59 of MAC-mediated endothelial injury contributed to its protective effect in atherosclerosis and therapies aimed at inhibiting terminal complement activity may benefit patients clinically.
Acknowledgments
This study was supported by National Institutes of Health grants AI-49344, AI-44970 and AI-62388. We thank Dr Paul Morgan for rabbit anti-rat C9 antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babaev VR, Patel MB, Semenkovich CF, Fazio S, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J Biol Chem. 2000;275:26293–9. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994;179:985–92. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19:2348–54. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–26. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, Lichtman AH. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–31. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- Halperin JA, Taratuska A, Nicholson-Weller A. Terminal complement complex C5b-9 stimulates mitogenesis in 3T3 cells. J Clin Invest. 1993;91:1974–8. doi: 10.1172/JCI116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Kim DD, Miwa T, Kimura Y, Schwendener RA, van Lookeren Campagne M, Song WC. Deficiency of decay-accelerating factor and complement receptor 1-related gene/protein y on murine platelets leads to complement-dependent clearance by the macrophage phagocytic receptor CRIg. Blood. 2008;112:1109–19. doi: 10.1182/blood-2008-01-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DD, Miwa T, Song WC. Retrovirus-mediated over-expression of decay-accelerating factor rescues Crry-deficient erythrocytes from acute alternative pathway complement attack. J Immunol. 2006;177:5558–66. doi: 10.4049/jimmunol.177.8.5558. [DOI] [PubMed] [Google Scholar]
- Li SH, Szmitko PE, Weisel RD, Wang CH, Fedak PW, Li RK, Mickle DA, Verma S. C-reactive protein upregulates complement-inhibitory factors in endothelial cells. Circulation. 2004;109:833–6. doi: 10.1161/01.CIR.0000117087.27524.0E. [DOI] [PubMed] [Google Scholar]
- Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–77. doi: 10.1084/jem.20040863. Epub 2005 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27:102–8. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD4+ T cell activity in a complement-independent manner. J Immunol. 2005;175:7098–102. doi: 10.4049/jimmunol.175.11.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Mackness B, Hunt R, Durrington PN, Mackness MI. Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:1233–8. doi: 10.1161/01.atv.17.7.1233. [DOI] [PubMed] [Google Scholar]
- Mason JC, Ahmed Z, Mankoff R, Lidington EA, Ahmad S, Bhatia V, Kinderlerer A, Randi AM, Haskard DO. Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury. Circ Res. 2002;91:696–703. doi: 10.1161/01.res.0000038151.57577.19. [DOI] [PubMed] [Google Scholar]
- Miwa T, Maldonado MA, Zhou L, Sun X, Luo HY, Cai D, Werth VP, Madaio MP, Eisenberg RA, Song WC. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol. 2002a;161:1077–86. doi: 10.1016/S0002-9440(10)64268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Zhou L, Hilliard B, Molina H, Song WC. Crry, but not CD59 and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement attack. Blood. 2002b;99:3707–16. doi: 10.1182/blood.v99.10.3707. [DOI] [PubMed] [Google Scholar]
- Molina H, Miwa T, Zhou L, Hilliard B, Mastellos D, Maldonado MA, Lambris JD, Song WC. Complement-mediated clearance of erythrocytes: mechanism and delineation of the regulatory roles of Crry and DAF. Decay-accelerating factor. Blood. 2002;100:4544–9. doi: 10.1182/blood-2002-06-1875. Epub 2002 Aug 1. [DOI] [PubMed] [Google Scholar]
- Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends Immunol. 2002;23:61–4. doi: 10.1016/s1471-4906(01)02129-9. [DOI] [PubMed] [Google Scholar]
- Morgan BP, van den Berg CW, Davies EV, Hallett MB, Horejsi V. Cross-linking of CD59 and of other glycosyl phosphatidylinositol-anchored molecules on neutrophils triggers cell activation via tyrosine kinase. Eur J Immunol. 1993;23:2841–50. doi: 10.1002/eji.1830231118. [DOI] [PubMed] [Google Scholar]
- Murray EW, Robbins SM. Antibody cross-linking of the glycosylphosphatidylinositol-linked protein CD59 on hematopoietic cells induces signaling pathways resembling activation by complement. J Biol Chem. 1998;273:25279–84. doi: 10.1074/jbc.273.39.25279. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A, Burge J, Fearon DT, Weller PF, Austen KF. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982;129:184–9. [PubMed] [Google Scholar]
- Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest. 1998;102:910–8. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu F, Rus H. Complement activation and atherosclerosis. Mol Immunol. 1999;36:949–55. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30:73–80. doi: 10.1385/IR:30:1:073. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus HG, Vlaicu R. Immunohistochemical localization of C5b-9, S-protein, C3d and apolipoprotein B in human arterial tissues with atherosclerosis. Atherosclerosis. 1987;65:1–11. doi: 10.1016/0021-9150(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus HG, Vlaicu R. Decay-accelerating factor regulates complement-mediated damage in the human atherosclerotic wall. Immunol Lett. 1990;26:17–23. doi: 10.1016/0165-2478(90)90170-u. [DOI] [PubMed] [Google Scholar]
- Okada N, Harada R, Fujita T, Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1:205–8. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Oksjoki R, Kovanen PT, Pentikainen MO. Role of complement activation in atherosclerosis. Curr Opin Lipidol. 2003;14:477–82. doi: 10.1097/00041433-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Patel S, Thelander EM, Hernandez M, Montenegro J, Hassing H, Burton C, Mundt S, Hermanowski-Vosatka A, Wright SD, Chao YS, Detmers PA. ApoE(−/−) mice develop atherosclerosis in the absence of complement component C5. Biochem Biophys Res Commun. 2001;286:164–70. doi: 10.1006/bbrc.2001.5276. [DOI] [PubMed] [Google Scholar]
- Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol. 1997;17:2309–15. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- Persson L, Boren J, Robertson AK, Wallenius V, Hansson GK, Pekna M. Lack of complement factor C3, but not factor B, increases hyperlipidemia and atherosclerosis in apolipoprotein E−/− low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2004;24:1062–7. doi: 10.1161/01.ATV.0000127302.24266.40. [DOI] [PubMed] [Google Scholar]
- Pratico D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Rokach J, Maclouf J, Violi F, FitzGerald GA. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–34. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokach J, Khanapure SP, Hwang SW, Adiyaman M, Lawson JA, FitzGerald GA. Nomenclature of isoprostanes: a proposal. Prostaglandins. 1997;54:853–73. doi: 10.1016/s0090-6980(97)00184-6. [DOI] [PubMed] [Google Scholar]
- Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–83. [PubMed] [Google Scholar]
- Rudic RD, Brinster D, Cheng Y, Fries S, Song WL, Austin S, Coffman TM, FitzGerald GA. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res. 2005;96:1240–7. doi: 10.1161/01.RES.0000170888.11669.28. [DOI] [PubMed] [Google Scholar]
- Schmiedt W, Kinscherf R, Deigner HP, Kamencic H, Nauen O, Kilo J, Oelert H, Metz J, Bhakdi S. Complement C6 deficiency protects against diet-induced atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998;18:1790–5. doi: 10.1161/01.atv.18.11.1790. [DOI] [PubMed] [Google Scholar]
- Seifert PS, Hansson GK. Complement receptors and regulatory proteins in human atherosclerotic lesions. Arteriosclerosis. 1989a;9:802–11. doi: 10.1161/01.atv.9.6.802. [DOI] [PubMed] [Google Scholar]
- Seifert PS, Hansson GK. Decay-accelerating factor is expressed on vascular smooth muscle cells in human atherosclerotic lesions. J Clin Invest. 1989b;84:597–604. doi: 10.1172/JCI114204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogabe H, Nangaku M, Ishibashi Y, Wada T, Fujita T, Sun X, Miwa T, Madaio MP, Song WC. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001;167:2791–7. doi: 10.4049/jimmunol.167.5.2791. [DOI] [PubMed] [Google Scholar]
- Song WC, Sarrias MR, Lambris JD. Complement and innate immunity. Immunopharmacology. 2000;49:187–98. doi: 10.1016/s0162-3109(00)80303-3. [DOI] [PubMed] [Google Scholar]
- Song WL, Lawson JA, Wang M, Zou H, FitzGerald GA. Noninvasive assessment of the role of cyclooxygenases in cardiovascular health: a detailed HPLC/MS/MS method. Methods Enzymol. 2007;433:51–72. doi: 10.1016/S0076-6879(07)33003-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci U S A. 1999;96:628–33. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–8. [PubMed] [Google Scholar]
- Vlaicu R, Rus HG, Niculescu F, Cristea A. Immunoglobulins and complement components in human aortic atherosclerotic intima. Atherosclerosis. 1985a;55:35–50. doi: 10.1016/0021-9150(85)90164-9. [DOI] [PubMed] [Google Scholar]
- Vlaicu R, Rus HG, Niculescu F, Cristea A. Quantitative determinations of immunoglobulins and complement components in human aortic atherosclerotic wall. Med Interne. 1985b;23:29–35. [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001a;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001b;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001c;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–9. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- Yun S, Leung VW, Botto M, Boyle JJ, Haskard DO. Accelerated Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice Lacking the Membrane-Bound Complement Regulator CD59. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.169912. [DOI] [PubMed] [Google Scholar]