Abstract
Phenylketonuria (PKU) is an inborn metabolic error in which metabolism of phenylalanine into tyrosine is disrupted. If the diet of an infant with PKU is not restricted, blood phenylalanine levels are elevated, leading to irremediable brain damage and severe mental retardation. Children with PKU who are placed early and continuously on a low-phenylalanine diet develop normal levels of intelligence, and brain damage is largely prevented. However, if the diet of a mother with PKU is unrestricted during her pregnancy, high phenylalanine levels in her blood can cross the placental barrier and damage the developing fetus in multiple ways. These results demonstrate how genes and environmental factors combine to create prenatal environments that can have profound effects on the growth and development of offspring during infancy and childhood.
Keywords: phenylketonuria (PKU), intelligence, behavior genetics, prenatal influences
The history of research on phenylketonuria (PKU) is a fascinating story of scientific endeavors to understand the nature of a genetic defect, develop tests to diagnose it, and apply appropriate environmental manipulations to prevent its effects. The success of this program of research demonstrates that just because an inherited defect is present does not mean the behavioral outcomes typically associated with it are immutable or inevitable. Even more intriguing are studies of offspring of mothers with PKU, which reveal teratogenic effects (a teratogen is a factor that causes malformation of a developing embryo or fetus) of prenatal exposure to high levels of the amino acid phenylalanine. These findings illustrate how genes and environments combine to create prenatal environments that can have profound influences on behavioral development. These findings are consistent with Barker’s (1998) hypothesis that prenatal environments can influence patterns of development in infancy, childhood, and beyond.
PKU
Historical Milestones
As recounted by Koch and de la Cruz (1999a), PKU was identified in 1934 by Følling, who noted the presence of phenylketone (a byproduct of phenylalanine breakdown or metabolism) in the urine of several patients who had severe mental retardation. Later work established that PKU is caused by identifiable genetic defects and is associated with high levels of phenylalanine in the blood. Phenylalanine is an essential amino acid—essential because it is necessary for protein synthesis and must be derived from the diet. Normally, phenylalanine is metabolized into tyrosine, a nonessential amino acid that, in turn, is a precursor of several key neurotransmitters and hormones, including epinephrine, norepinephrine, and dopamine. In PKU, metabolism of phenylalanine into tyrosine is disrupted, leading to high levels of phenylalanine and low levels of tyrosine in the blood. Research showed that high levels of blood phenylalanine during infancy and childhood lead to severe, permanent brain damage, but the exact nature of the biological impact on neuronal function is not yet understood. Two pathophysiological mechanisms of PKU have been proposed—one emphasizing the deficiency of dopamine and the other emphasizing a generalized slowing of the sheathing (myelination) of neuronal fibers (Dyer, 1999). Current data support both hypotheses, and researchers have not yet identified either as the more crucial underlying mechanism.
In 1953, Bickel and colleagues demonstrated beneficial effects of a low-phenylalanine diet in treating a young child with PKU. A low-phenylalanine diet typically consists of a phenylalanine-free medical formula drink; carefully selected amounts of fruits, vegetables, and low-protein breads and pastas; and avoidance of high-protein foods. In 1961, Guthrie devised a simple test to identify PKU within the first week after birth. The ease, accuracy, and inexpensiveness of the Guthrie test led to its rapid adoption across the United States, and screening tests are now routinely used on births throughout the world.
Genetic Defect and Mutation Severity
PKU is one of the most common inborn errors of metabolism, occurring in 1 in 10,000 to 15,000 live births, and is caused by mutations on the gene coding for the enzyme phenylalanine hydroxylase (PAH). PKU is a recessive trait, so a person will exhibit symptoms of PKU only if the person receives a defective PAH gene from each parent. If a person receives a defective PAH gene from only one parent, the person shows no symptoms of PKU but is a carrier of the defect. The PAH Locus Knowledge-base Web site (http://www.pahdb.mcgill.ca/) lists state-of-the-art information about PKU, including the over 500 different mutations on the PAH gene identified to date.
Classifications of PKU severity are often used in treatment. One common classification is shown in Table 1, based on levels of blood phenylalanine when the person is on an unrestricted diet. In treatment, most attention is paid to individuals in the first three categories of Table 1: classic, moderate, or mild PKU, all of whom have phenylalanine levels above 10 milligrams per deciliter (mg/dL) when on an unrestricted diet. By placing such infants on a low-phenylalanine diet by the third week after birth, physicians hope to keep phenylalanine levels between 3 and 10 mg/dL, because it is assumed that levels below 10 mg/dL do not lead to brain damage. Infants with phenylalanine levels between 3 and 10 mg/dL when on an unrestricted diet are identified as having mild hyperphenylalaninemia (MHP) and are usually not placed on a restricted diet.
TABLE 1.
Severity of PAH Gene Defect, Indexed by Phenylalanine Level in Blood When on Unrestricted Diet
| Level of phenylalanine in blood |
||
|---|---|---|
| Severity category | mg/dL | μmol/L |
| Classic PKU | > 20 | > 1200 |
| Moderate PKU | 15–20 | 900–1200 |
| Mild PKU | 10–15 | 600–900 |
| MHP | 3–10 | 180–600 |
| Normal | < 3 | < 180 |
Note: PAH = phenylalanine hydroxylase; PKU = phenylketonuria; MHP = mild hyperphenylalaninemia; mg/dL = milligrams per deciliter; μmol/L = micromols per liter. Mg/dL and μmol/L are alternative ways of indexing phenylalanine level in blood.
Debate on the level of phenylalanine required to ensure normal development continues, with some arguing that levels should be kept below 6 mg/dL and others arguing that levels of 10 mg/dL or below are good enough. Indeed, professional recommendations regarding the level of blood phenylalanine to maintain and the age at which it is safe to discontinue the restricted diet vary widely across European countries and the United States (Burgard, Link, & Schweitzer-Krantz, 2000; Schweitzer-Krantz & Burgard, 2000). Moreover, effects of phenylalanine exposure are likely to be threshold effects, with no effect up to the threshold and a teratogenic effect of exposure beyond the threshold. Thus, more informative results are likely to come from treating phenylalanine level as a continuous variable and searching for the crucial threshold of exposure than from continuing to coarsely categorize persons (as in Table 1). Useful information on many aspects of diagnosing and managing PKU is contained in the statement by a National Institutes of Health Consensus Panel, Phenylketonuria: Screening and Management (National Institutes of Health, 2000).
Behavioral Effects
The potentially devastating effects of PKU on development are well known. If the diet of an infant with PKU is unrestricted, its intellectual functioning declines precipitously. Apparently normal at birth, such an infant will fall to the level of severe mental retardation (mean IQ of 50) by age 2—a decline that cannot be reversed. But if an infant with PKU is placed on a low-phenylalanine diet early in life and continues strictly on such a diet until age 20 years or later, he or she will exhibit normal or near-normal development.
Two recent studies that included cognitive measures deserve special mention. In the first, Koch et al. (2002) retested adults who had participated in a large study of diet adherence and discontinuance during the developmental period. Results revealed that the mean IQ of a group of persons who had never discontinued their low-phenylalanine diets was 17 points higher than that of a group of persons who had discontinued their diets at mean age 8 years. Further, of 16 individuals who later resumed the low-phenylalanine diet, 9 who maintained the diet into adulthood showed a significant rise in mean IQ from childhood to adulthood, whereas 7 who later discontinued the diet had a significant drop in mean IQ from childhood to adulthood. In the second study, Diamond, Prevor, Callender, and Druin (1997) tested infants and children with PKU or MHP and compared the performance of a low-phenylalanine group (levels between 2 and 6 mg/dL) and a high-phenylalanine group (levels between 6 and 10 mg/dL) against the performance of multiple comparison groups on cognitive tasks relying on prefrontal cortex functions. The low-phenylalanine group performed similarly to comparison groups, whereas the high-phenylalanine group exhibited significant deficits in performance. Thus, the Koch et al. study supports recommendations that a low-phenylalanine diet should be continued at least through the developmental period, and the Diamond et al. study suggests that phenylalanine levels be maintained below 6 mg/dL.
In many ways, research on PKU represents a scientific success story (see Koch & de la Cruz, 1999b, for a review of PKU research). Until the early 1960s, biology was destiny for persons with PKU. Diagnosis of PKU usually happened so late during development—even if during infancy or early childhood—that severe brain damage had already occurred and could not be remedied. If behavior-genetic studies had been conducted, the heritability (i.e., the estimated proportion of variance in a phenotypic trait that is attributable to genetic variation among individuals) for the PKU syndrome would have been very high. However, after development of the Guthrie screening test, infants could be placed early and continuously on low-phenylalanine diets, and brain damage could be largely or completely prevented. Thus, heritability of the PKU syndrome would have fallen to low levels in a single generation because of a key manipulation of the environment—the use of a low-phenylalanine diet.
MATERNAL PKU
Initial Findings
The success of medical science in treating PKU is well known, but less widely known are the potentially devastating effects of maternal PKU. Lenke and Levy (1980) reported results of over 500 pregnancies of women with PKU who were not on low-phenylalanine diets during pregnancy: Infants born to mothers with PKU had a high rate of birth defects and developmental disabilities. These infants never would have exhibited symptoms of PKU, because they received a PAH gene defect only from their mothers, but phenylalanine in the mother’s blood passed the placental barrier and exposed the fetus to high levels of phenylalanine prenatally. These prenatal-exposure effects showed a dose-response relation, with higher levels of exposure leading to higher levels of disability.
The Maternal PKU Collaborative Study
The Maternal PKU Collaborative (MPKUC) Study (Koch, de la Cruz, & Azen, 2003) was initiated in 1984 to monitor pregnancies of women with PKU, to maintain mothers on a low-phenylalanine diet throughout their pregnancies, and to study relations between levels of phenylalanine in the mothers’ blood during pregnancy and birth and developmental outcomes of their offspring. All 413 children in the MPKUC Study received a gene defect only from their mothers. Consequently, they never would have exhibited symptoms of PKU as they would have metabolized phenylalanine normally. However, these children were exposed prenatally to differing levels of phenylalanine, with varying teratogenic effects.
Due to less-than-complete adherence to low-phenylalanine diets during their own development, mothers in the MPKUC Study tended to have lower-than-average IQ scores (mean = 86). Because pregnancy alters food preferences and makes it more difficult to adhere to a diet, the phenylalanine level in a mother’s blood was monitored at each prenatal visit (if possible) throughout her pregnancy. Prenatal visits occurred weekly for some mothers, biweekly for others, and less regularly for the rest. Despite attempts by medical personnel to maintain MPKUC Study participants on the diet, mothers exhibited wide individual differences in mean blood phenylalanine level across the pregnancy (ranging from 1.3 to 28.3 mg/dL).
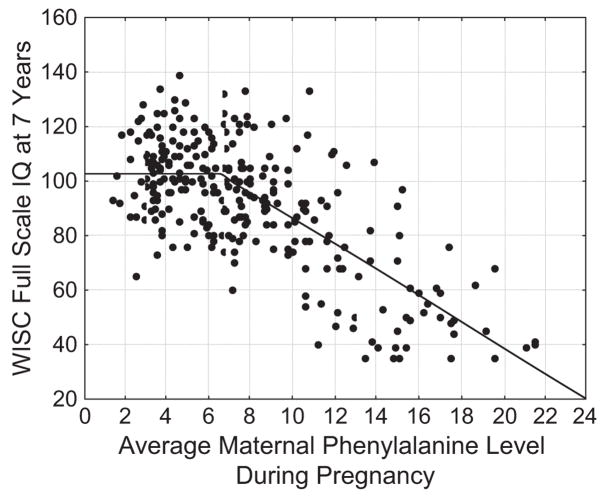
Offspring in the MPKUC Study were assessed using the Bayley Scales of Infant Development at 1 year and 2 years, the McCarthy Scales of Children’s Abilities at 4 years, and the Wechsler Intelligence Scale for Children–Revised (WISC-R) at 7 years. For brevity, I will concentrate on WISC-R scores, although similar results were found for outcomes at earlier ages. To represent most accurately the relation between average phenylalanine exposure during pregnancy and WISC-R scores, Widaman and Azen (2003) contrasted the fit of a standard linear regression model with that of a two-piece linear spline model. In a linear regression model, a single straight line is used to represent the relation between phenylalanine level and WISC-R scores. In a two-piece linear spline, two straight lines that represent predicted WISC-R can have different slopes and intersect at what is called a knot point. A scatterplot of scores on the two variables is shown in Figure 1, along with the predicted outcome from the two-piece linear spline model, which fit the data better than did the linear regression model. Inspection of individual data points in Figure 1 appears to support those who argue that phenylalanine levels up to 10 mg/dL are nonteratogenic, as IQ scores below 70 occurred, with few exceptions, only for children exposed to prenatal phenylalanine levels above this threshold. However, rather than relying on visual inspection of the scatterplot to identify the point where damage occurs, the spline regression model yielded a statistical estimate of the threshold for teratogenic effects. As shown in Figure 1, predicted IQ scores under the spline model have a value of about 103 between 0 mg/dL and the knot point, which was estimated at 6.6 mg/dL of phenylalanine exposure; after the knot point, the model predicts a drop in IQ of 4.7 points for every 1 mg/dL increase in exposure. These results are consistent with those by Diamond et al. (1997), suggesting that exposure to phenylalanine levels above 6 or 6.5 mg/dL may be teratogenic, whether elevated levels are experienced during the developmental period for children with PKU or during gestation for offspring of mothers with PKU.
Fig. 1.
Relation of average phenylalanine in maternal blood during pregnancy and child Wechsler Intelligence Scale for Children (WISC)-Revised IQ scores at age 7 years. (Note: solid line represents predicted IQ scores under the two-piece linear spline model.)
One additional set of findings for intellectual measures deserves mention. Average phenylalanine exposure during gestation was the strongest single correlate of offspring intellectual functioning; it correlated strongly and negatively with Bayley Mental Development Index scores at 1 year of age but exhibited an even stronger negative correlation with WISC-R scores at 7 years of age. This surprising pattern—higher correlations of prenatal phenylalanine exposure during gestation with later IQ scores than with earlier ones—could be due to many different factors. For example, problems with slowed myelination of neuronal fibers might continue during development, so brain development of highly exposed children could fall steadily farther behind normal patterns of brain development, resulting in ever higher correlations. Or, the damage from prenatal exposure to high phenylalanine levels may be to brain areas supporting higher intellectual functions that are assessed more adequately with intelligence tests during childhood and adolescence than with tests in infancy. Regardless of the basis, these correlational findings are intriguing.
CONCLUSIONS AND FUTURE DIRECTIONS
Behavior–Gene Relations
Findings from the MPKUC Study support an alternative to typical behavior-genetic approaches to understanding gene–behavior relations. Behavior–genetic analyses yield heritability coefficients that are sometimes misinterpreted as indices of the immutability of behavioral traits or as stable estimates of how much phenotypic variation is determined by genes. Neither interpretation is warranted. Despite clear genetic bases, the PKU phenotype is mutable and can be prevented by an appropriate environmental manipulation (a low-phenylalanine diet), which leads to drastically reduced gene–phenotype relations due to reduced phenotypic variation. But, even if heritability of the PKU phenotype falls to zero, the gene defect can still exert its teratogenic effect. Specifically, a gene defect in one organism (the mother) can, through interaction with the environment (an unrestricted diet), result in a toxic environment (the intrauterine environment) that can have devastating teratogenic effects on another organism (the developing fetus), effects that mimic features of phenotypes of infants with PKU who are on an unrestricted diet. Standard behavior-genetic statistical models are unable to capture such phenomena. The findings of the MPKUC Study illustrate one way to move beyond behavior-genetic statistical analyses to investigate the place of genes—in maternal PKU as codeterminers of crucial environments—in processes that influence bodily structures and behaviors in fundamental ways.
The findings are also consistent with Barker’s (1998) hypothesis that prenatal environmental effects may underlie many behavioral outcomes that are often presumed to have genetic origins. With daily advances in our ability to identify genetic markers, future research should establish the ways in which genes and environmental factors interact and co-act to produce variance in phenotypic traits. Moreover, we may find that effects of prenatal gene–environment interactions, as in the MPKUC Study, are at least as powerful and long lasting as those that occur postnatally (e.g., Caspi et al., 2002).
Medical Therapy
Gene therapy and other forms of medical therapy (e.g., medication) have potential for correcting disordered phenylalanine metabolism by persons with PKU. If such therapies were to become reality, they might solve problems of both PKU and maternal PKU by restoring normal phenylalanine metabolism. Early screening would still be needed to identify infants needing gene therapy, and phenylalanine levels in persons with PKU would still need to be monitored to verify success of therapy. Unfortunately, medical therapies are probably many years away, and the presence of multiple PAH gene mutations suggests that a “one size fits all” form of therapy is unlikely. Regardless, the possibility of future medical therapies is a cause for optimism.
In most prior research, average phenylalanine level across the entire pregnancy was used as the primary index of prenatal exposure. But researchers continue to search for different ways of representing phenylalanine exposure that better capture the relation of exposure to outcomes. Approaches most likely to generate research advances are the continued exploration of effects of elevated phenylalanine exposure at different stages of prenatal development and the identification of child characteristics that are protective against prenatal exposure to high levels of phenylalanine.
Acknowledgments
This research was supported in part by Grants NOI-HD-2-3148, NOI-HD-2-3149, NOI-HD-2-3155, and NOI-HD-2-3156 from the National Institute of Child Health and Human Development (Richard Koch, Principal Investigator) and Grants DA017902 from the National Institute on Drug Abuse and MH051361 from the National Institute of Mental Health (Rand Conger, Principal Investigator).
References
- Barker DJP. Mothers, babies, and health in later life. 2. New York: Churchill Livingstone; 1998. [Google Scholar]
- Burgard P, Link R, Schweitzer-Krantz S. Phenylketonuria: Evidence-based clinical practice. European Journal of Pediatrics. 2000;159(Suppl 2):S69–S168. doi: 10.1007/pl00014387. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development (No. 4, Serial No. 252) 1997;62 [PubMed] [Google Scholar]
- Dyer CA. Pathophysiology of phenylketonuria. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:104–112. [Google Scholar]
- Koch R, Burton B, Hoganson G, Peterson R, Rhead W, Rouse B, et al. Phenylketonuria in adulthood: A collaborative study. Journal of Inherited Metabolic Diseases. 2002;25:333–346. doi: 10.1023/a:1020158631102. [DOI] [PubMed] [Google Scholar]
- Koch R, de la Cruz F. Historical aspects and overview of research on phenylketonuria. Mental Retardation and Developmental Disabilities Research Reviews. 1999a;5:101–103. [Google Scholar]
- Koch R, de la Cruz F. Phenylketonuria. Mental Retardation and Developmental Disabilities Research Reviews. 1999b;5:101–161. [Google Scholar]
- Koch R, de la Cruz F, Azen CG. The Maternal Phenylketonuria Collaborative Study: New developments and the need for new strategies. Pediatrics. 2003;112:1513–1587. [Google Scholar]
- Lenke RR, Levy HL. Maternal phenylketonuria and hyperphenylalaninemia. New England Journal of Medicine. 1980;202:1202–1208. doi: 10.1056/NEJM198011203032104. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Phenylketonuria: Screening and management. [Retrieved January 6, 2009];NIH Consensus Statement. 2000 October 16–18;17(3):1–27. from http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat4.chapter.20932. [PubMed]
- Schweitzer-Krantz S, Burgard P. Survey of national guidelines for the treatment of phenylketonuria. European Journal of Pediatrics. 2000;159(Suppl 2):S70–S73. doi: 10.1007/pl00014385. [DOI] [PubMed] [Google Scholar]
- Widaman KF, Azen C. Relation of prenatal phenylalanine exposure to infant and childhood cognitive outcomes: Results from the International Maternal PKU Collaborative Study. Pediatrics. 2003;112:1537–1543. [PubMed] [Google Scholar]
Recommended Reading
- Burgard P, Link R, Schweitzer-Krantz S. A clearly written set of papers from contributors around the world that portray recommendations for clinical practice, many varying from country to country, based on current research findings 2000 (See References) [Google Scholar]
- Koch R, de la Cruz F. A thorough review of theories and findings on phenylketonuria in general and on maternal phenylketonuria in particular 1999b (See References) [Google Scholar]
- Koch R, de la Cruz F, Azen CG. A comprehensive, accessible set of papers on various outcomes from the MPKUC Study, documenting effects of prenatal exposure to PKU 2003 (See References) [Google Scholar]
- Lenke RR, Levy HL. A historical classic on the teratogenic effects of prenatal exposure to phenylketonuria, the paper that raised attention to the unexpected effects of maternal PKU 1980 (See References) [Google Scholar]
- National Institutes of Health. The results of a National Institutes of Health consensus conference that reviewed findings and offered general recommendations for clinical practice in phenylketonuria and maternal phenylketonuria 2000 October 16–18; (See References) [Google Scholar]