Abstract
Objective
To determine the association between 25-hydroxyvitamin D (25(OH)D) levels and the prevalence of peripheral arterial disease (PAD) in the general United States population.
Methods and Results
We analyzed data from 4,839 participants of the National Health and Nutrition Examination Survey 2001–2004 to evaluate the relationship between 25(OH)D and PAD (defined as an ankle-brachial index <0.9). Across quartiles of 25(OH)D, from lowest to highest, the prevalence of PAD was 8.1%, 5.4%, 4.9%, and 3.7% (p-trend<0.001). After multivariable adjustment for demographics, co-morbidities, physical activity level and laboratory measures, the prevalence ratio of PAD for the lowest, compared to the highest, 25(OH)D quartile (<17.8 and ≥29.2 ng/mL, respectively) was 1.80 (95% confidence interval: 1.19, 2.74). For each 10 ng/mL lower 25(OH)D level, the multivariable-adjusted prevalence ratio of PAD was 1.35 (95% confidence interval: 1.15, 1.59).
Conclusions
Low serum 25(OH)D levels are associated with a higher prevalence of PAD. Several mechanisms have been invoked in the literature to support a potential anti-atherosclerotic activity of vitamin D. Prospective cohort and mechanistic studies should be designed to confirm this association.
Keywords: 25-hydroxyvitamin D, peripheral arterial disease, phosphate, PTH, cardiovascular disease
Introduction
Peripheral arterial disease (PAD) affects approximately 5 million US adults and it is associated with a high risk of morbidity and mortality from cardiovascular disease1, 2. Established risk factors for PAD include typical atherosclerosis risk factors such as dyslipidemia, diabetes mellitus, hypertension, smoking, and reduced kidney function 2, 3. In addition, non-traditional cardiovascular risk factors such as elevated C-reactive protein (CRP) and fibrinogen have been associated with PAD 4.
Basic science research indicates that low vitamin D levels may be linked with cardiovascular risk. Vascular smooth muscle cells possess the 1-α hydroxylase enzyme that locally activates circulating vitamin D5. In animal models, active vitamin D is an inhibitor of the renin-angiotensin system6 and myocardial cell hypertrophy7. Cellular experiments show that active vitamin D and its analogs exhibit anti-coagulant activity 8. Conflicting data are available on the association between low 25(OH)D levels and cardiovascular disease in the general population 9–11.
Among adults, 25-hydroxyvitamin D (25(OH)D) levels ≥30 ng/ml are considered optimal 12. These levels are associated with reduced fracture rates and have been postulated to be associated with better health outcomes 12. The goal of the current study was to evaluate the association between serum 25(OH)D levels and the prevalence of PAD in the general population. To accomplish this goal, we analyzed data from a nationally representative sample of the United States adult population aged ≥40 years in NHANES 2001–2004. In addition, elevated serum calcium, phosphate and intact parathyroid hormone (iPTH) levels have been associated with cardiovascular disease in the general population13, 14. Because these serum markers are associated with vitamin D levels, we assessed the association between these serum markers and PAD in a secondary analysis.
Methods
Study Population
NHANES 2001–2004 was a nationally representative cross-sectional survey of the civilian, non-institutionalized United States population performed by the National Center for Health Statistics. All participants underwent standardized interviews, physical examinations and laboratory testing. Ankle-brachial index (ABI) was measured in NHANES 2001–2004 participants ≥40 years of age. Serum 25(OH)D levels were measured for 4,839 participants, ≥40 years of age, who had complete data on all other study variables.
Study Variables
Study procedures in NHANES 2001–2004 consisted of an in-home interview followed by a medical evaluation and blood sample collection at a mobile examination center. Of relevance to the current analysis, variables collected during the in-home interview included age, gender, race-ethnicity, cigarette smoking, education, leisure-time physical activity, and a history of diabetes mellitus and myocardial infarction. For the current analysis, self-reported race-ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican-American, and other. Participants who reported having smoked ≥100 cigarettes during their lifetime were classified as current or former smokers if they answered affirmatively or negatively, respectively, to the question “Do you smoke cigarettes now?” Diabetes mellitus was defined as a self-report of a physician diagnosis, not during pregnancy, with concurrent use of oral hypoglycemic or insulin medication. A history of myocardial infarction was based on participants’ self-report of a previous physician diagnosis on the medical history questionnaire. Being physically active was defined as participating in 30 or more minutes of moderate or vigorous activity or strength training in the 30 days preceding the NHANES visit.
The NHANES 2001–2004 examination procedures included measurement of height, weight and blood pressure. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Normal weight, overweight and obesity were defined as a BMI of <25 kg/m2, 25 to <30 kg/m2 and ≥30 kg/m2, respectively. Three blood pressure measurements were taken during a single examination visit by a physician using a protocol adapted from the American Heart Association. Based on the average of all three blood pressure measurements, hypertension was defined as systolic or diastolic blood pressure ≥ 140 mmHg or 90 mmHg, respectively, and/or self-reported current use of blood pressure lowering medication. Medication and supplement use data was obtained for use of statins, anti-hypertensives, anti-diabetes medications, and vitamin D supplementation through questionnaires and pill bottle reviews.
Detailed descriptions of blood collection and processing procedures are provided in the NHANES Laboratory/Medical Technologists Procedures Manual15. Serum creatinine concentrations was measured using the modified kinetic method of Jaffe, calibrated to the assays used for the development of the Modification of Diet in Renal Disease (MDRD) Study equation, and glomerular filtration rate was estimated with the simplified MDRD equation16. Participants with an estimated glomerular filtration rate of <60 ml/min/1.73 m2 were considered to have chronic kidney disease (CKD). High-sensitivity CRP was measured by latex-enhanced nephelometry. Elevated CRP was defined as ≥ 1.0 mg/L. Total plasma homocysteine levels were measured by the Abbott Homocysteine assay. Total and HDL-cholesterol, serum calcium and phosphate levels were measured on the Beckman Synchron LX20. Serum calcium was corrected for serum albumin using the formula: adjusted calcium = measured calcium - [(4.0- serum albumin in g/dl) × 0.8]. Serum calcium and phosphate were measured from 1999–2004 and were available for analysis in 6,984 participants. Serum intact parathyroid hormone (iPTH) was measured on an Elecsys 1010 autoanalyzer (Roche Diagnostics, Mannheim, Germany), using a electrochemiluminescent process. Serum iPTH was measured only in 2003–2004 (n= 2,391). Serum vitamin D was measured using the Diasorin (formerly Incstar) 25(OH)D assay. Three levels of control specimens were used to assess the quality of each 25(OH)D run. Coefficients of variation for serum 25(OH)D remained less than 10% throughout the study period.
Outcome
The primary outcome for the current study was PAD defined using ABI. Details of the methods used to measure ABI in NHANES have been described previously17. In brief, for participants with at least one arm and weighing ≤400 pounds, systolic blood pressure for the ABI was measured via blood pressure cuffs on the right brachial artery and both posterior tibial arteries. For participants aged 40–59 years, two measures were taken and averaged at each site, while for participants aged ≥60 years, one measure was taken. For individuals with conditions precluding measurement of the right arm, left brachial artery systolic blood pressure was taken. ABI was calculated as the ratio of the average ankle systolic blood pressure to arm systolic blood pressure. The smaller of the two measurements was considered the ABI for this study. Participants with ABI ≥ 1.5 may have severe arterial rigidity and were therefore excluded from all analyses (n=10). PAD was defined as ABI <0.9.
Statistical Analysis
Participant characteristics were calculated by PAD status. The statistical significance of differences for these characteristics was determined using least squares and maximum likelihood for continuous and dichotomous variables, respectively. Next, the prevalence of PAD was calculated by quartile of 25(OH)D, with the statistical significance of trends across quartile assessed using maximum likelihood. Prevalence ratios of PAD associated with quartile of serum 25(OH)D were calculated using log binomial regression models. An initial model included adjustment for age, gender, and race-ethnicity with a subsequent model including additional adjustment for education, current and former cigarette smoking, leisure-time physical activity, diabetes mellitus, total to HDL cholesterol ratio, body mass index, systolic blood pressure, log homocysteine, glycated hemoglobin, statin use, anti-hypertensive medication use, vitamin D supplement use, history of myocardial infarction, elevated CRP and chronic kidney disease. To further explore the dose–response relationship of 25(OH)D with PAD, we used restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of the 25(OH)D distribution (12.4, 23.4, 34.3 ng/mL, respectively). The multivariable-adjusted association between 25(OH)D, as continuous variable, and PAD was determined overall and for sub-groups defined by age, gender, race-ethnicity, BMI, physical activity, diabetes mellitus, a history of myocardial infarction, chronic kidney disease, and vitamin D supplement use. For analyses as a continuous variable, the prevalence ratio of PAD was calculated for one standard deviation lower serum 25(OH)D (10 ng/mL). Differences in the association of 25(OH)D and PAD across sub-group were tested by using a multiplicative term in the regression models. Additionally, the associations between serum calcium, phosphate and iPTH, each modeled by quartile and as continuous variables, and PAD were determined. Data were analyzed using SUDAAN (version 9.0; Research Triangle Institute, Research Triangle Park, NC) to account for the complex NHANES sampling design including unequal probabilities of selection, over-sampling, and non-response.
Results
Participant Characteristics
Participants with PAD were older, more likely to be non-Hispanic black, have less than a high school education, be a former smoker, and have diabetes mellitus, higher glycated hemoglobin levels, hypertension, a history of myocardial infarction, chronic kidney disease, use statins and have elevated homocysteine and CRP (Table 1). Additionally, those with PAD were less likely to be physically active. There was no difference in serum calcium, phosphate or iPTH levels between participants with and without PAD. In contrast, mean 25(OH)D levels were significantly lower among participants with PAD (p<0.001).
Table 1.
Participant characteristics by peripheral arterial disease (PAD)
| Variable | PAD (n=406) |
No PAD (n=4433) |
p-Value | |
|---|---|---|---|---|
| Age, years | 67.1 (0.8) | 55.2 (0.3) | <0.001 | |
| Male, % | 42.8 | 48.7 | 0.117 | |
| Ethnicity, % | ||||
| Non-Hispanic White | 81.1 | 85.1 | ||
| Non-Hispanic Black | 15.1 | 10.1 | <0.001 | |
| Mexican American | 3.8 | 4.9 | ||
| Less than a high school education, % | 28.9 | 17.0 | <0.001 | |
| Physically active, % | 49.4 | 66.6 | <0.001 | |
| Smoking, % | ||||
| Never | 32.8 | 47.2 | ||
| Former | 42.6 | 32.0 | <0.001 | |
| Current | 24.6 | 20.9 | ||
| BMI, kg/m2 | 29.0 (0.4) | 28.3 (0.1) | 0.096 | |
| Diabetes mellitus, % | 17.5 | 7.5 | <0.001 | |
| Anti-diabetes medication use, % | 17.7 | 7.2 | <0.001 | |
| Glycated hemoglobin, % | 5.90 (0.07) | 5.61 (0.02) | <0.001 | |
| Systolic BP, mmHg | 137.4 (1.4) | 128.1 (0.8) | <0.001 | |
| Diastolic BP, mmHg | 66.8 (0.9) | 73.8 (0.3) | <0.001 | |
| Anti-hypertensive medication use, % | 50.8 | 26.4 | <0.001 | |
| Hypertension, % | 71.7 | 41.1 | <0.001 | |
| History of myocardial infarction, % | 13.9 | 4.6 | <0.001 | |
| Chronic kidney disease, % | 30.2 | 10.6 | <0.001 | |
| Total cholesterol, mg/dL | 205.6 (3.0) | 209.9 (1.0) | 0.185 | |
| HDL cholesterol, mg/dL | 51.6 (1.2) | 53.8 (0.4) | 0.089 | |
| Statin use, % | 30.6 | 14.2 | <0.001 | |
| Homocysteine†, µmol/L | 10.3 (8.1 – 12.8) | 8.6 (7.3 – 10.6) | <0.001 | |
| Elevated C-reactive protein, % | 17.2 | 9.2 | <0.001 | |
| Bone alkaline phosphatase, µg/L | 14.7 (0.6) | 13.6 (0.2) | 0.017 | |
| Serum calcium, mg/dL | 9.66 (0.05) | 9.69 (0.02) | 0.590 | |
| Serum phosphate, mg/dL | 3.73 (0.03) | 3.72 (0.01) | 0.863 | |
| Parathyroid hormone†, pg/mL | 44.6 (31.1 – 61.4) | 40.2 (30.5 – 52.7) | 0.070 | |
| Vitamin D supplement use, % | 18.7 | 16.2 | 0.261 | |
| Serum vitamin D, ng/mL | 21.5 (0.6) | 24.6 (0.5) | <0.001 | |
PAD is defined as an ankle brachial index < 0.9
Values are median (25th – 75th percentiles).
Associations between Serum 25 (OH) Vitamin D Levels and PAD
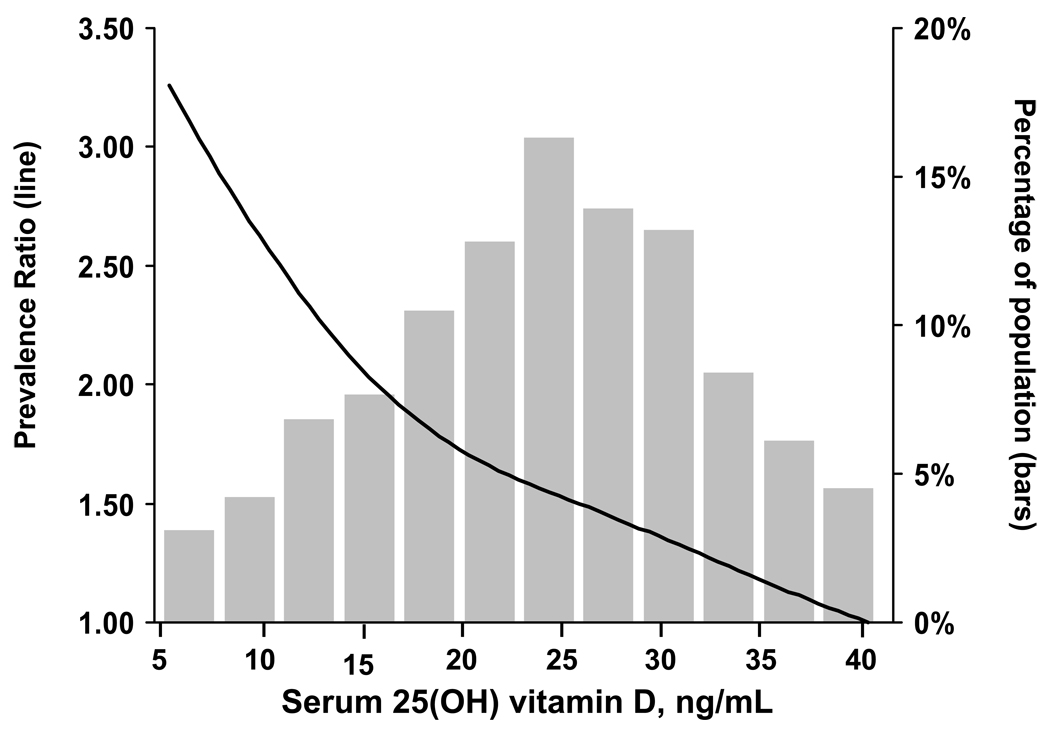
A graded association was present between lower 25(OH)D levels and a higher prevalence of PAD (Table 2). This association was present after age, gender, race-ethnicity and multivariable adjustment. Compared to their counterparts in the highest 25(OH)D quartile (≥29.2 ng/ml), participants in the lowest 25(OH)D quartile (<17.8 ng/ml) were 2.18 (95% confidence interval [CI]: 1.50, 3.16) and 1.80 (1.19, 2.74) times more likely to have PAD, after age, gender, race-ethnicity and multivariable adjustment, respectively. In a multivariable adjusted spline regression model, a progressive increase in PAD was evident at lower levels of 25(OH)D levels (Figure 1).
Table 2.
Age, gender, race-ethnicity and multivariable adjusted† prevalence ratios of peripheral arterial disease (ankle/brachial index<0.9) by quartiles of serum 25(OH) vitamin D.
| Quartile of Serum 25(OH) vitamin D (range) | |||||
|---|---|---|---|---|---|
| 1 – Lowest (<17.8) |
2 (17.8 –23.4) |
3 (23.5 – 29.1) |
4 - Highest (≥ 29.2) |
P-trend | |
| N | 1445 | 1292 | 1153 | 949 | |
| Median 25(OH)D | 12.9 | 20.4 | 26.0 | 33.8 | |
| Prevalence, % (95% CI) | 8.1 (6.4 – 9.9) | 5.4 (4.2 – 6.6) | 4.9 (3.8 – 5.9) | 3.7 (2.5 – 4.8) | <0.001 |
| Prevalence ratio | |||||
| Age, gender, race-ethnicity Adjusted | 2.18 (1.50 – 3.16)*** | 1.42 (0.92 – 2.20) | 1.28 (0.92 –1.76) | 1.00 (ref) | <0.001 |
| Multivariable† adjusted | 1.80 (1.19 – 2.74)*** | 1.49 (0.95 –2.34) | 1.28 (0.91 – 1.81) | 1.00 (ref) | <0.001 |
Cut-points for the 25(OH)D quartiles were based on the weighted distribution. Therefore, the un-weighted sample size (N) is not equal within each quartile.
CI – confidence interval
p-value<0.001 compared to the reference group of 25(OH) vitamin D ≥ 29.2 ng/mL
Multivariable model adjusted for: age, gender, race-ethnicity, education, current and former cigarette smoking, leisure-time physical activity, diabetes mellitus, total to HDL cholesterol ratio, body mass index, systolic blood pressure, glycated hemoglobin, statin use, anti-hypertensive medication use, vitamin D supplement use, history of myocardial infarction, log homocysteine, elevated CRP and chronic kidney disease.
Figure 1. Prevalence ratio of peripheral artery disease associated with serum 25(OH) vitamin D levels between 5 and 40 ng/mL.
† Adjusted for age, gender, race-ethnicity, education, current and former cigarette smoking, leisure-time physical activity, diabetes mellitus, total to HDL-cholesterol ratio, body mass index, systolic blood pressure, glycated hemoglobin, statin use, anti-hypertensive medication use, vitamin D supplement use, history of myocardial infarction, log homocysteine, elevated CRP and chronic kidney disease.
4.4% of the population had 25(OH)D levels >40 ng/mL and are not included in the graph
Subgroup Analysis
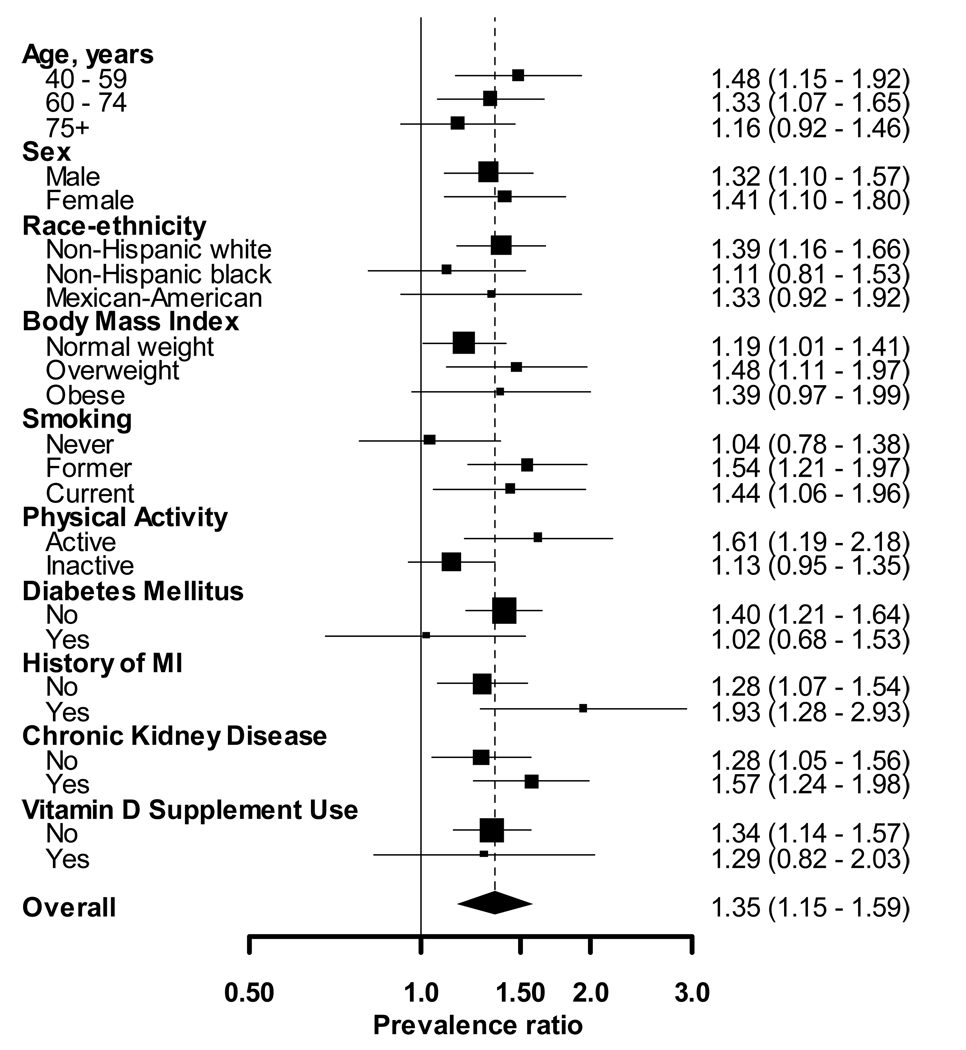
After multivariable adjustment, the prevalence ratio of PAD associated with each 10 ng/ml lower 25(OH)D was 1.35 (95% CI: 1.15, 1.59) (Figure 2). The association between 25(OH)D and PAD was similar (p-value for interaction>0.10) in all sub-groups except physical activity. Specifically, the prevalence ratio of PAD per each 10 ng/ml lower 25(OH)D was 1.61 (95% CI: 1.19, 2.18) and 1.13 (95% CI: 0.95, 1.35) for those who were and were not physically active, respectively (p-interaction=0.06).
Figure 2. Multivariable adjusted† prevalence ratios of PAD (ABI<0.9) associated with one standard deviation lower serum 25(OH)D (10 ng/mL) by sub-group.
† Adjusted for age, gender, race-ethnicity, education, current and former cigarette smoking, leisure-time physical activity, diabetes mellitus, total to HDL-cholesterol ratio, body mass index, systolic blood pressure, glycated hemoglobin, statin use, anti-hypertensive medication use, vitamin D supplement use, history of myocardial infarction, log homocysteine, elevated CRP and chronic kidney disease.
Associations of Serum Calcium, Phosphate and iPTH Levels and PAD
Higher quartiles of serum calcium, phosphate and iPTH levels were not associated with PAD (Table 3). Additionally, modeled as a continuous variable, there was no association between serum calcium, phosphate, or iPTH and PAD, overall, or in sub-groups (data not shown).
Table 3.
Prevalence and age, gender, race-ethnicity and multivariable adjusted† prevalence ratios of peripheral arterial disease (ankle/brachial index<0.9) by quartiles of serum phosphate, calcium, and parathyroid hormone.
| Quartile of Phosphate, mg/dL (range) | |||||
|---|---|---|---|---|---|
| 1 – Lowest (<3.22) |
2 (3.22 –3.55) |
3 (3.56 – 3.91) |
4 - Highest (≥3.92) |
P-trend | |
| N | 1740 | 1522 | 1969 | 1753 | |
| Median | 2.99 | 3.37 | 3.70 | 4.19 | |
| Prevalence, % (95% CI) | 5.9 (4.4 – 7.4) | 5.4 (3.9 – 7.0) | 4.8 (3.7 – 5.9) | 5.6 (4.2 – 7.0) | 0.478 |
| Prevalence ratio | |||||
| Age, gender, race-ethnicity adjusted | 1.00 (ref) | 0.96 (0.73, 1.28) | 0.93 (0.69, 1.26) | 1.15 (0.89, 1.49) | 0.313 |
| Multivariable† adjusted | 1.00 (ref) | 0.98 (0.76, 1.27) | 0.96 (0.71, 1.29) | 1.00 (0.75, 1.33) | 0.962 |
| Quartile of Calcium, mg/dL (range) | |||||
| 1 – Lowest (<9.42) |
2 (9.42 – 9.79) |
3 (9.80 – 10.17) |
4 - Highest (≥ 10.18) |
||
| N | 2153 | 2012 | 1660 | 1159 | |
| Median | 9.18 | 9.58 | 9.96 | 10.38 | |
| Prevalence, % (95% CI) | 6.1 (5.0 – 7.2) | 4.9 (3.9 – 5.8) | 4.9 (4.1 – 5.8) | 5.4 (3.9 – 7.0) | 0.508 |
| Prevalence ratio | |||||
| Age, gender, race-ethnicity adjusted | 1.00 (ref) | 0.84 (0.67, 1.05) | 0.91 (0.69, 1.20) | 1.00 (0.72, 1.38) | 0.986 |
| Multivariable† adjusted | 1.00 (ref) | 0.87 (0.66, 1.15) | 1.02 (0.74, 1.40) | 1.01 (0.71, 1.42) | 0.807 |
| Quartile of Parathyroid hormone, pg/mL (range) | |||||
| 1 – Lowest <30 |
2 30 –39 |
3 40 – 51.7 |
4 - Highest ≥ 51.8 |
||
| N | 505 | 612 | 467 | 807 | |
| Median | 24.7 | 35.4 | 45.1 | 62.5 | |
| Prevalence, % (95% CI) | 5.6 (3.8 – 7.4) | 3.1 (1.7 – 4.5) | 6.8 (4.6 – 9.0) | 6.6 (4.6 –8.6) | 0.128 |
| Prevalence ratio | |||||
| Age, gender, race-ethnicity adjusted | 1.00 (ref) | 0.47 (0.32, 0.69)*** | 1.01 (0.63, 1.64) | 0.74 (0.47, 1.17) | 0.803 |
| Multivariable† adjusted | 1.00 (ref) | 0.42 (0.27, 0.65)*** | 0.97 (0.68, 1.39) | 0.69 (0.41, 1.15) | 0.714 |
Cut-points for the phosphate, calcium, and parathyroid hormone quartiles were based on the weighted distributions. Therefore, the un-weighted sample sizes (N) are not equal within each quartile.
CI – confidence interval
Multivariable model adjusted for: age, gender, race-ethnicity, education, current and former cigarette smoking, leisure-time physical activity, diabetes mellitus, total to HDL cholesterol ratio, body mass index, systolic blood pressure, glycated hemoglobin, statin use, anti-hypertensive medication use, vitamin D supplement use, history of myocardial infarction, log homocysteine, elevated CRP and chronic kidney disease.
p<0.001 for comparison with reference group.
Discussion
In this nationally representative sample of US adults, there was a strong, graded association between lower levels of 25(OH)D levels and PAD. This association was present after adjustment for several cardiovascular risk factors and potential confounders. In contrast, no association was present between elevated serum calcium, phosphate or iPTH levels and PAD in this population.
Epidemiologic studies have shown an inverse association between blood pressure and vitamin D levels18 and a direct association between increasing latitude, as a surrogate of lower vitamin D levels, and blood pressure19. One small clinical trial suggested that oral vitamin D supplementation20 reduces systolic blood pressure. Additionally, in another trial, patients randomized to ultraviolet light exposure three times a week experienced a 180% increase in 25(OH)D levels and improved blood pressure control21. However, another small study did not show this effect on blood pressure22.
The association of 25(OH)D deficiency with glucose intolerance23, and the metabolic syndrome24 is another potential mechanism for increased cardiovascular disease risk. In one study of older adults with impaired fasting glucose, supplementation with vitamin D and calcium attenuated the progression of insulin resistance and hyperglycemia25. However, in the current study, a graded association between 25(OH)D and PAD remained present after adjustment for diabetes mellitus and glycated hemoglobin.
The association of both 25(OH)D and 1,25(OH)2 vitamin D levels with cardiovascular events remains controversial. In some studies, low 25(OH)D levels have been associated with increased prevalence of coronary heart disease (CHD)9, stroke,26 and congestive heart failure27. However, low 25(OH)D has been associated with a protective association in other studies. For example, in a case-control study of 143 patients with CHD by Rajasree, 25(OH)D levels >89 ng/ml were associated with a multivariable-adjusted odds ratio for CHD of 3.18 ( 95% CI :1.31, 7.73) 10. In contrast, in a case-control study of acute myocardial infarction by Scragg, 25(OH)D levels above the median (≥12.8 ng/mL) were protective against CHD (multivariable adjusted odds ratio=0.43, 95% CI: 0.27, 0.69)9. In the Scragg study, 25(OH)D levels were notably lower than in the study by Rajasree10.
Among 1739 Framingham Offspring Study participants free of cardiovascular disease at baseline, a 25(OH)D level <15 ng/ml was associated with a multivariable-adjusted 62% higher hazard of developing a first cardiovascular event11. Interestingly, PAD, defined as the onset of new claudication, was included in the outcome definition. However, PAD only comprised 8 out of the 120 events in the study.
There are several lines of evidence suggesting that vitamin D may play a role in the pathogenesis of cardiovascular disease. Cardiac myocytes possess vitamin D receptors28. Several in vitro studies have shown that active vitamin D inhibits cardiac myocyte hypertrophy 7, 29. Paricalcitol, an active vitamin D compound, attenuated the development of left ventricular hypertrophy in Dahl salt-sensitive rats30. Vitamin D has been shown to be an inhibitor of the renin-angiotensin system6. In addition, several small studies have shown that supplementation with various forms of vitamin D may improve the cytokine profile (CRP, TNFα levels) of patients with vitamin D deficiency31 and congestive heart failure32. Cellular experiments showed that active vitamin D and its analogs exhibit anti-coagulant activity8. Finally, the vitamin D-24-hydroxylase transgenic rat, a model of vitamin D deficiency due to continuous degradation of active vitamin D, develops aortic atherosclerosis33. Interestingly, an early experimental model of atherosclerosis was the cholesterol and vitamin D fed rat. These rats were given an extremely high dose of vitamin D2 (1.8 million units/kg), and developed aortic atherosclerosis34. It is interesting that animal models of both excessive and insufficient vitamin D develop atherosclerosis and that there is conflicting human data on very high levels and very low levels of 25(OH)D being associated with cardiovascular disease9–11 suggesting that there may be an optimal level that is neither too high nor too low.
The results of the current study show that the association between low 25(OH)D levels and the prevalence of PAD was consistent across multiple sub-groups. In the sub-group analyses, the association was stronger for non-Hispanic whites and for participants without diabetes mellitus. However, the interaction terms were not significant for non-Hispanic blacks versus whites (p-interaction=0.31) or diabetes status (p-interaction=0.19). Although one should be cautious interpreting the results from sub-group analyses because of the reduced sample size and statistical power, these results are similar to those reported by Scragg et al35. This line of investigation deserves further research.
The association we report is cross-sectional, similar to two previous smaller studies of 25(OH)D and PAD36, 37. In a study of 161 patients with angiographically proven PAD, those with Stage II PAD, defined as the presence of claudication symptoms, had higher mean 25(OH)D levels (23.4 ng/mL) compared to patients with Stage IV PAD, defined as the presence of ulcers, (9.4 ng/mL)37. Similarly, in a separate study of 95 patients with angiographically proven PAD, patients with Stage II PAD had higher 25(OH)D levels compared to Stage IV PAD36. The results of the current study extend these findings to a large nationally representative population with clinical and subclinical PAD.
High iPTH levels have been associated with cardiovascular disease in the general population13. Elevated serum phosphate levels have also been associated with cardiovascular events, a composite outcome which included peripheral vascular disease, in a community-based study14. Despite these suggestive findings, we did not find an association between serum calcium, phosphate and iPTH levels and the prevalence of PAD.
There are potential limitations to the current study. Most notably, the study was cross-sectional. As in any cross-sectional study, one must be cautious in interpreting the direction of the association. It has been hypothesized that patients with PAD may be less mobile, and therefore, receive less sun exposure and have lower 25(OH)D levels. While this may be the case for severe PAD, all participants in the current study were non-institutionalized and mobile enough to attend the NHANES visit. An additional limitation is the lack of data in NHANES on sun exposure, geographic location, and the season during which participants attended their study visits. Another potential limitation is that angiography was not used to detect PAD, which was impractical in a large, population-based survey. Despite these limitations, the current study maintains several strengths. Using the ABI allowed us to identify the presence of sub-clinical PAD. NHANES included a broad range of data collection. This allowed us to study 25(OH)D, calcium, phosphate, and iPTH and perform statistical analyses after adjustment for several potential confounding factors. Additional strengths include standardized protocols with rigorous quality control for data collection and inclusion of a large, nationally representative study sample. The large sample size allowed the investigation of the association between 25(OH)D and PAD in several important sub-groups. The consistency of the results in these sub-groups is noteworthy.
In summary, in this nationally representative study, US adults with low 25(OH)D levels had a higher prevalence of PAD. This association was strong, graded and present after adjustment for multiple cardiovascular risk factors. Prospective and mechanistic studies are needed to confirm these associations.
ACKNOWLEDGEMENTS
Acknowledgements: The National Center for Health Statistics (NCHS) is the source of the data used in this analysis. All analyses, interpretations and conclusions are made by the authors and do not represent views of the NCHS.
Sources of funding: MLM was supported by grant K23-DK078774 from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health and by an American Heart Association Heritage Affiliate Clinically Applied Research Award. EDM was supported by the PJ Schafer Memorial Cardiovascular Research Award at Johns Hopkins.
Disclosures: EDM has served as a consultant to Abbott pharmaceuticals.
References
- 1.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Muntner P, Chen J, Sutton-Tyrrell K, He J. Relation of inflammation to peripheral arterial disease in the national health and nutrition examination survey, 1999–2002. Am J Cardiol. 2005;96:1579–1583. doi: 10.1016/j.amjcard.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 5.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 6.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O′Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:H1751–H1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 8.Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation. 2000;102:2867–2872. doi: 10.1161/01.cir.102.23.2867. [DOI] [PubMed] [Google Scholar]
- 9.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 10.Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, Girija G. Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease. Eur J Epidemiol. 2001;17:567–571. doi: 10.1023/a:1014559600042. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D′Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 13.Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease--a review. Eur Heart J. 2004;25:1776–1787. doi: 10.1016/j.ehj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D′Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 15.National Health and Nutrition Examination Survey laboratory Procedures Manual. National Center for Health Statistics. 2001 Centers for Disease Control and Prevention. [Google Scholar]
- 16.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 17.Menke A, Muntner P, Wildman RP, Dreisbach AW, Raggi P. Relation of borderline peripheral arterial disease to cardiovascular disease risk. Am J Cardiol. 2006;98:1226–1230. doi: 10.1016/j.amjcard.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 21.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 22.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–646. [PubMed] [Google Scholar]
- 23.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71:134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 25.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 26.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 27.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 28.O′Connell TD, Simpson RU. Immunochemical identification of the 1,25-dihydroxyvitamin D3 receptor protein in human heart. Cell Biol Int. 1996;20:621–624. doi: 10.1006/cbir.1996.0081. [DOI] [PubMed] [Google Scholar]
- 29.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 30.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 32.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 33.Kasuga H, Hosogane N, Matsuoka K, Mori I, Sakura Y, Shimakawa K, Shinki T, Suda T, Taketomi S. Characterization of transgenic rats constitutively expressing vitamin D-24-hydroxylase gene. Biochem Biophys Res Commun. 2002;297:1332–1338. doi: 10.1016/s0006-291x(02)02254-4. [DOI] [PubMed] [Google Scholar]
- 34.Kunitomo M, Kinoshita K, Bando Y. Experimental atherosclerosis in rats fed a vitamin D, cholesterol-rich diet. J Pharmacobiodyn. 1981;4:718–723. doi: 10.1248/bpb1978.4.718. [DOI] [PubMed] [Google Scholar]
- 35.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 36.Fahrleitner-Pammer A, Obernosterer A, Pilger E, Dobnig H, Dimai HP, Leb G, Kudlacek S, Obermayer-Pietsch BM. Hypovitaminosis D, impaired bone turnover and low bone mass are common in patients with peripheral arterial disease. Osteoporos Int. 2005;16:319–324. doi: 10.1007/s00198-004-1693-3. [DOI] [PubMed] [Google Scholar]
- 37.Fahrleitner A, Dobnig H, Obernosterer A, Pilger E, Leb G, Weber K, Kudlacek S, Obermayer-Pietsch BM. Vitamin D deficiency and secondary hyperparathyroidism are common complications in patients with peripheral arterial disease. J Gen Intern Med. 2002;17:663–669. doi: 10.1046/j.1525-1497.2002.11033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]