Abstract
Pancreatic adenocarcinoma (PCA) is an almost invariably fatal disease. Recently, it has been shown by several groups as well as ours that IGF-1 receptor (IGF-1R) overexpression is related to higher proliferation, survival, angiogenesis, and highly invasive pancreatic tumors. Several studies have been carried out to understand the pathways that lead to growth factor mediated signaling, but the molecular mechanism of receptor overexpression remains mostly unknown. Treatment with neutralizing antibodies or a specific kinase inhibitor against IGF-1R could block the receptor expression in PCA cells. Furthermore, we also demonstrated that insulin receptor substrate (IRS)-2, but not IRS-1, is involved in regulation of IGF-1R expression which is most likely not transcriptional control. By blocking mTOR pathway with rapamycin as well as other biochemical analysis, we defined a unique regulation of IGF-1R expression mediated by protein kinase C δ (PKC δ) and mTOR pathway. Moreover, we demonstrated that the down regulation of IGF-1R expression due to IRS-2 siRNA can be compensated by overexpression of dominant active mutant of PKC δ suggesting that PKC δ is downstream of IGF-1R/IRS-2 axis. Overall, these findings suggest a novel regulatory role of IRS-2 on the expression of IGF-1R through PKC δ and mTOR in pancreatic cancer cells.
Keywords: IGF-1R, protein expression, PKC δ, mTOR, TSC2, pancreatic cancer
Introduction
Pancreatic cancer is associated with high morbidity. Due to late stage diagnoses, rapid tumor progression, and resistance to conventional chemotherapeutic agents, the fiveyear survival rate remains at less than 5% (1). A hallmark of tumor growth is the overexpression of growth factors and their receptors. Overexpression of insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) has been demonstrated in human pancreatic cancer tissue (2–5). While the structure and function of IGF-1R are the main focus of intensive investigations (6–8), less data are available on the mechanisms and causes for the observed dysregulation of IGF-1R protein expression (9–11). Defining the molecular mechanisms underlying the highly aggressive nature of pancreatic adenocarcinomas may provide more selective methods for an effective treatment.
IGF-1R is composed of an extracellular ligand-binding domain that controls¬ the activity of an intracellular tyrosine kinase (12–14). During ligand binding by either IGF-1, IGF-2, or insulin (at high concentrations), IGF-1R becomes tyrosine phosphorylated through an autophosphorylation reaction, an essential step in its activation cascade (15). Most intracellular signals are generated through cellular scaffold proteins. These scaffold proteins, including the insulin receptor substrates (IRS), bind to autophosphorylation sites and are themselves phosphorylated on multiple tyrosine residues by the activated receptor kinase (16, 17). IRS proteins do not contain intrinsic kinase activity but rather act at the interface between the cell surface receptor and intracellular signalling molecules. At least three IRS proteins occur in the human: IRS-1/Irs-1 and IRS-2/Irs-2, which are widely expressed, and IRS-4/Irs-4, which is limited to the thymus, brain, kidney and possibly beta cells of the pancreas (18). IRS-1 and IRS-2 are known to be overexpressed in pancreatic cancer cells (3, 19).
Given that tumorigenesis is a complex and mulifaceted process, tumor cells have a variety of cellular defects promoting uncontrolled growth. Germline inactivating mutations of either the TSC1 or TSC2 tumor suppressor protein are linked to Tuberous sclerosis complex (TSC), a genetic disorder that is characterized by the development of benign tumors called hamartomas in several tissues and organs including the central nervous system, skin, lungs, and kidneys (20, 21, 22, 23). Although there are very few reports of mutation of TSC1 and TSC2 genes in PCA, a recent study demonstrates that reduced expression of TSC2 might be involved in the progression of pancreatic cancer (24). The main target of TSC described so far is the mTOR, mammalian target of rapamycin (also known as rapamycin and FK506-binding protein target 1, RAFT1) pathways (25, 26). The atypical Ser/Thr kinase mTOR regulates the translation of key mRNA transcripts for proteins required for cell cycle progression. Rapamycin, a bacterial macrolide with antifungal, immunosuppressant, and antitumor activities is known to target mTOR. Rapamycin forms a complex with the cytosolic 12-kDa FK506 binding protein (FKBP12) and binds to mTOR, inducing a partial dephosphorylation and deactivation of p70S6 kinase, an enzyme critical for G1 to S transition (27). Furthermore, rapamycin inhibits the mitogen-stimulated phosphorylation of 4e-binding protein 1 (4E-BP-1) (28). Dephosphorylated 4E-BP-1 interacts with the translation initiation factor eIF-4E and thereby inhibits cap structure-dependent protein synthesis and cell growth (28, 29). Interestingly, AKT/PKB pathways are the main modulator of mTOR activation; however, recent experiments demonstrated that protein kinase-C δ (PKC δ) associates with RAFT1 and thereby regulates the phosphorylation of 4IE-BP1 and cap-dependent initiation of protein translation (30). PKC δ contains phospholipid-dependent serine/threonine kinase activity and plays a key role in cellular signal signaling (31).
In the present study, we demonstrated that IRS-2, but not IRS-1, is involved in the regulation of IGF-1R protein expression and found that the overexpression of IGF-1R in PCA is an autocrine expression loop and is mainly regulated at the translational level by a signaling pathway via mTOR (32). In order to define this pathway, we provided evidence that PKC δ plays a crucial role by regulating IGF-1R overexpression in PCA cells.
Materials and Methods
Cell culture and reagents
AsPC-1 and Su86.86 cells, purchased from ATCC, were cultured in RPMI-1640 with 20% or 10% FBS (Hyclone Laboratories), respectively, and 1% Penicillin-Streptomycin (Invitrogen Corporation). Serum starvation was performed with 0.1%FBS in RPMI 1640. Rapamycin was obtained from Sigma. TATFLAGVHL peptide (107–122) was described previously (33). IGF-1R kinase inhibitor, □Picropodophyllin (PPP, first inhibitor reported to discriminate between IGF-1R and Insulin Receptor) and Proteasome Inhibitor I (34) (Cat. No. 539160) were purchased form Calbiochem Inc.
Antibodies
The antibodies used were from the following sources: anti-IGF-IRα 1H7 (for blocking), anti-IGF-IRα 2C8 (for western blot detection), anti-IGF-IRα H60 (for immunoprecipitation), and anti-nPKC-δ, Santa Cruz Biotechnology; anti-mTOR, anti-phospho-mTOR, anti-phospho-FKHR, and anti-phospho-PKC-δ, Cell Signalling Technology; anti-IRS-2, Upstate Biotechnology; anti-flag-tag, anti-β-actin, normal rabbit serum, and rabbit-IgG, Sigma; anti-HA-tag, Boehringer-Mannheim.
Plasmids
HA-PH-PTB IRS-1 and HA-PH PTB IRS-2, both in pcDNA3, were described previously (35). In brief, IRS-1 and −2 proteins were lacking the tail of tyrosine phosphorylation sites; however, these truncated IRS-1 and −2 proteins retained the NH2-terminal pleckstrin homology (PH) and phosphotyrosine binding (PTB) domains. pZipNeo-17N Ras was as described (36). pGEFPC1-PKC δ (PKC δ KR; point mutation of lysine 376 to arginine) was a kind gift from R. Dutta. Dominant-negative T410A PKC-ζ (threonine 410 to alanine) was described previously (37). Dominant negative mutant of AKT (T308A, S473A, K179A) expression vector was a kind gift from K. Walsh (Tufts University).
Transient transfections
3–4 × 105 cells/well in a 6-well plate (western blot analysis), 2–3 × 106 cells/60-mm plate (real-time PCR), and 6–8 × 106 cells/100-mm plate (immunoprecipitation) were plated one day prior to transfection. Cell confluency was 85–95% for all experiments. Plasmids were transiently transfected using Effectene (Qiagen) according to the manufacturer's instructions at a 1:10 DNA to Effectene ratio. For all experiments, a sample containing the empty vector was run. The IRS1 siRNA (sc-29376) and IRS2 siRNA (sc-29378) were purchased from Santa Cruz Biotech.
Immunoprecipitation
AsPC-1 cells were washed twice with ice-cold PBS and lysed with radioimmune precipitation assay buffer (RIPA, Boston Bioproducts) containing 10 µg/ml leupeptin, 0.5% aprotinin, 2 mM pepstatin A, and 1 mM phenylmethylsulfonyl fluoride, incubated for 10 min on ice, and scraped. Lysates were centrifuged for 10 min at 14,000 rpm at 4 °C. In a total volume of 500 µl, 1 µg of antibody was added to equal amounts of protein from each sample protein lysate and incubated for 2 h or overnight for the IRS-2 antibody at 4°C while rocking. 50 µl of protein A-agarose beads (Amersham Biosciences) were then added to the samples and again incubated for 2 h under shaking conditions at 4°C. Samples were washed three times with RIPA buffer and proteins were separated by electrophoresis and visualized with a Western blot.
Western Blot
Protein samples were mixed with 2x loading dye (125 mM Tris-HCL, pH 6.8, 20% glycerol, 10% β-mercaptoethanol, 4% SDS, and 0.0025% bromophenol blue), boiled, and separated by SDS-polyacrylamide gel electrophoresis (PAGE) at 150V. Agarose beads with bound proteins were treated in the same way. Size-separated proteins were transferred onto a polyvinylidene difluoride membrane (PerkinElmer Life Sciences) at 300 mA for 1 h. For immunodetection, the membranes were blocked with 4% milk or BSA in PBS-T (phosphatebuffered saline and 0.1% Tween 20) and incubated with a primary antibody. After washing with PBS-T, the membrane was incubated with a peroxidase-linked secondary antibody. After a second round of washing, the reactive bands were detected with a chemiluminescent substrate (Bio-Rad).
RNA preparation and real time PCR
After washing AsPC-1 cells twice with ice cold PBS, total RNA was extracted according to the RNeasy Mini Kit protocol (Qiagen). For Taqman PCR, the sequences for forward, reverse, and Taqman middle primers for human IGF-1R and for human β-actin were taken from the PubMed GenBank™ and synthesized by Integrated DNA Technology. IGF-1R forward: 5′-CAT CGA CAT CCG CAA CGA-3′, IGF-1R reverse: 5′-CCC TCG ATC ACC GTG CA-3′; Taqman middle primer: 5′-TTC TCC AGG CGC TTC AGC TGC-3′. The middle primer had a 5′-TET reporter and a 3′-Tamra quencher. Each real-time PCR reaction was prepared with 0.5 µg of total RNA, 25 µl of reverse transcriptase-PCR Master Mix (Applied Biosystems), 1.25 µl of RNase inhibitor (Applied Biosystems), 50 nM forward primer, 50 nM reverse primer, and 100 nM middle primer. In all IGF-1R real-time PCR experiments, the β-actin amount was detected in parallel as a housekeeping gene for normalization. For reverse transcription, a 30-min incubation period at 48 °C was run before inactivating the reverse transcriptase at 95 °C for 10 min. 40 cycles at 95 °C for 15 s and 60 °C for 1 min were performed with an ABI Prism 7700 Sequence Detector (Applied Biosystems). CT (cycle threshold) values were measured, and the relative RNA amount was calculated as follows: Δ= CT(IGF-1R sample) – CT(β-actin sample). ΔΔ=Δ(transfected sample) – Δ(empty vector sample). The relative RNA amount in comparison with the empty vector = 2−ΔΔ. All experiments were repeated three times; and from each experiment, each reading was taken in triplicate.
Results
Autocrine protein expression loop of IGF-1R in human pancreatic cancer
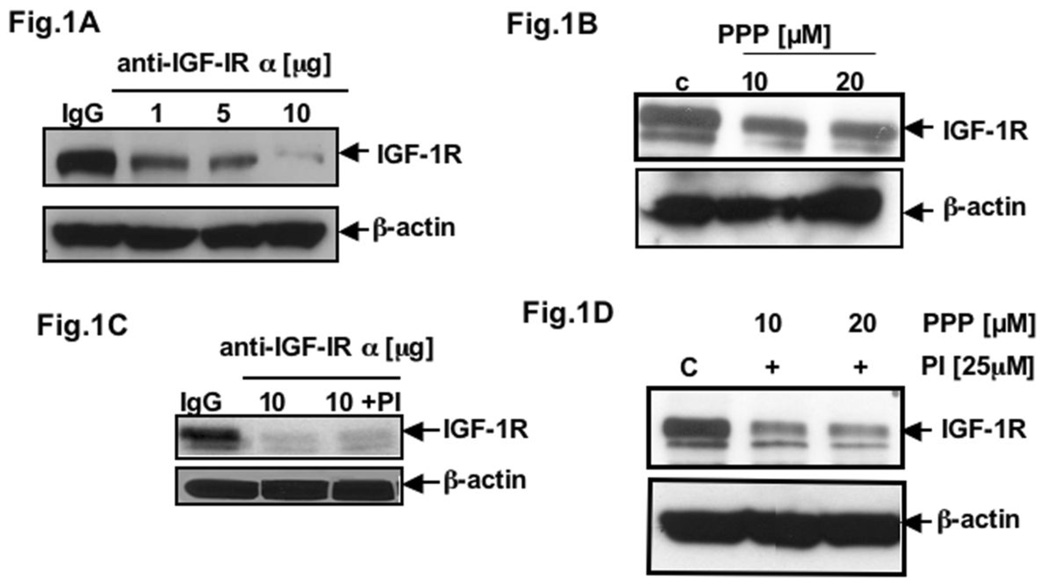
Herein, we would like to understand the molecular mechanisms of IGF-1R overexpression in pancreatic adenocarcinoma. Several reports indicate autocrine control of tumor cell growth by the IGF-1/IGF-1 receptor system in pancreatic cancer (3, 38). To examine whether IGF-1R contributes to the dysregulation of its own expression, we blocked IGF-1R function under serum-starved conditions by using the highly specific antibody (anti-IGF-1Rα 1H7) that binds to the ligand-binding α-subunit of IGF-1R. As a control, anti-β-actin was analyzed. After 24h treatment of AsPC-1 cells with 1.0, 5.0, and 10.0 µg of anti-IGF-IRα 1H7, we found a strong inhibition of IGF-1R protein expression by Western blot analysis. Control cells were treated with mouse IgG (Fig. 1A). To confirm the possibility that the receptor internalization is not responsible but rather IGF-1R kinase activity is, we used a potent IGF-1R tyrosine kinase inhibitor PPP to examine IGF-1R expression in PCA cells. Fig. 1B showed the inhibition of IGF-1R expression with increasing concentration of PPP treatment, which is in favor of an autocrine loop hypothesis that is accountable for IGF-1R overexpression via its kinase activation. The decrease in protein expression of IGF-1R, after treating the cells with anti-IGF-1Rα antibody, was also detected at 20 hr even in the presence of a proteasome inhibitor (34) (Fig. 1C) suggesting that the inhibitory effect IGF-1R expression is not due to activation of proteasome degradation pathways. Similarly, treatment of cells with PI couldn’t overcome the effect of PPP as well (Fig. 1D). Hence, our results indicate that in PCA cells IGF-1R is overexpressed due to its autocrine loop. We then examined the role of downstream molecules such as mTOR pathway in this regulation.
Fig. 1. Autocrine loop of IGF-1R in PCA.
Total cell lysates from AsPC-1 cells (A) treated with 1.0, 5.0 and 10.0 µg of anti-IGF-IRα 1H7 or mouse IgG (control); (B) treated with PPP (IGF-1R inhibitor) for different concentrations for 24h; (C) and (D) pretreated with protesome inhibitor (34) were resolved by SDS-PAGE and analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-IR). β-actin served as control protein.
Regulation of IGF-1R protein expression by mTOR
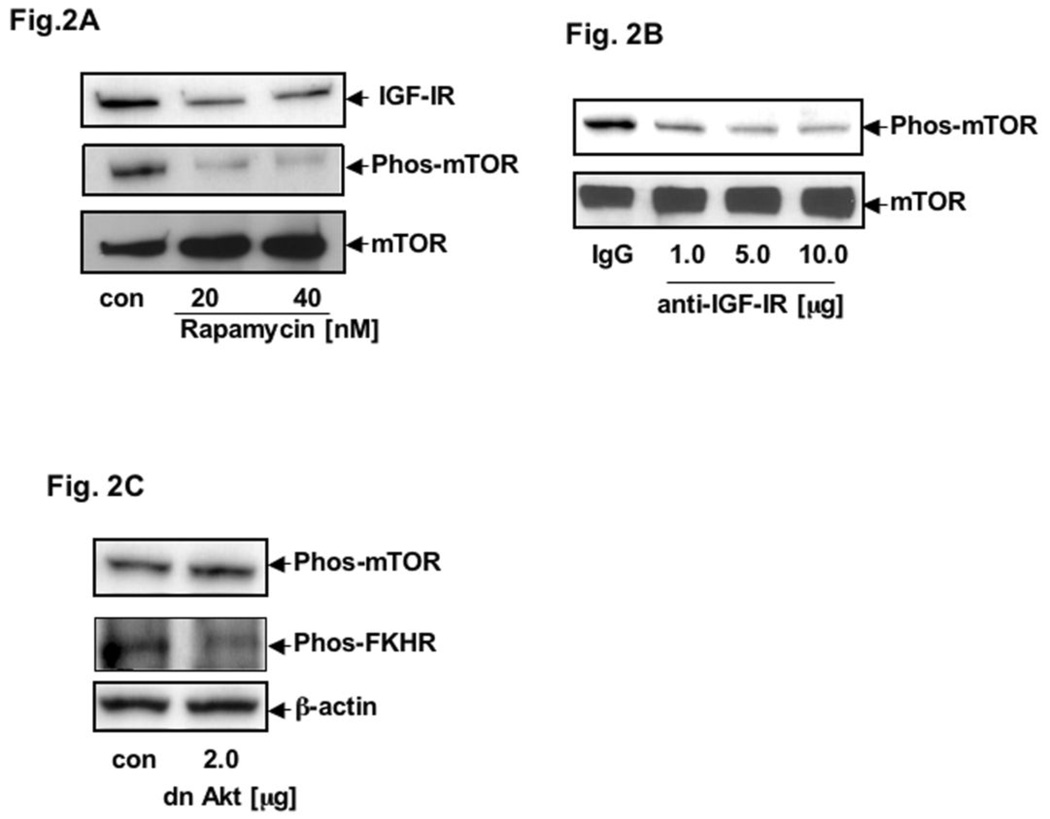
To further determine whether mTOR participates in the regulation of IGF-1R overexpression in PCA cells, we treated AsPC-1 cells with 20 and 40 nM rapamycin for 16 hr. To examine the effect of rapamycin on mTOR, we measured the phosphorylation level of mTOR by using anti-phospho-mTOR antibody; and, as expected, the phospho-mTOR level was inhibited (Fig. 2A). Moreover, as shown in Fig. 2A, we also observed that IGF-1R protein expression is downregulated indicating the role of mTOR in IGR-1R mediated overexpression. To further establish our hypothesis that mTOR participates in IGF-1R auto-regulation, we blocked IGF-1R function with 1.0, 5.0, and 10.0 ug of anti-IGF-IRα 1H7 for 24h and observed a decrease in mTOR phosphorylation (Fig. 2B). To link up between the mTOR and Akt pathway, we utilized expression vector containing a triple mutant (T308A, S473A, K179A) of Akt-1 (a kind gift from Dr. K. Walsh) to block AKT pathways. Notably, AsPC-1 cells transfected with an expressing vector of dominant-negative Akt mutant did not change the phosphorylation levels of mTOR; whereas, with its other downstream effector, FKHR, phosphorylation was inhibited (Fig. 2C). These results suggest that mTOR modulates IGF-1R expression by a pathway where AKT might not be involved. At this point, we thought other IGF-1R adapters and signaling molecules might be engaged in this pathway.
Fig. 2. mTOR-meditated IGF-1R protein expression in PCA.
(A) Total cell lysates from AsPC-1 cells untreated (control) or treated with 20 and 40 nM rapamycin were resolved by SDS-PAGE and analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-IR) and anti-phospho-mTOR (phos-mTOR). Total mTOR served as control protein. (B) Total cell lysates from AsPC-1 cells treated with anti-IGF-IRα 1H7 or mouse IgG (control) and analyzed by immunoblotting with anti-phospho-mTOR (pho-mTOR). (C) Total cell lysates from AsPC-1 cells transduced with vector expressing dominant-negative Akt (dn-Akt) or constitutively active Akt (control) and analyzed by immunoblotting with anti-phospho-mTOR (phos-mTOR) and anti-phospho-FKHR. β-actin served as control protein.
IRS-2 but not IRS-1 is involved in the translational regulation of IGF-1R expression
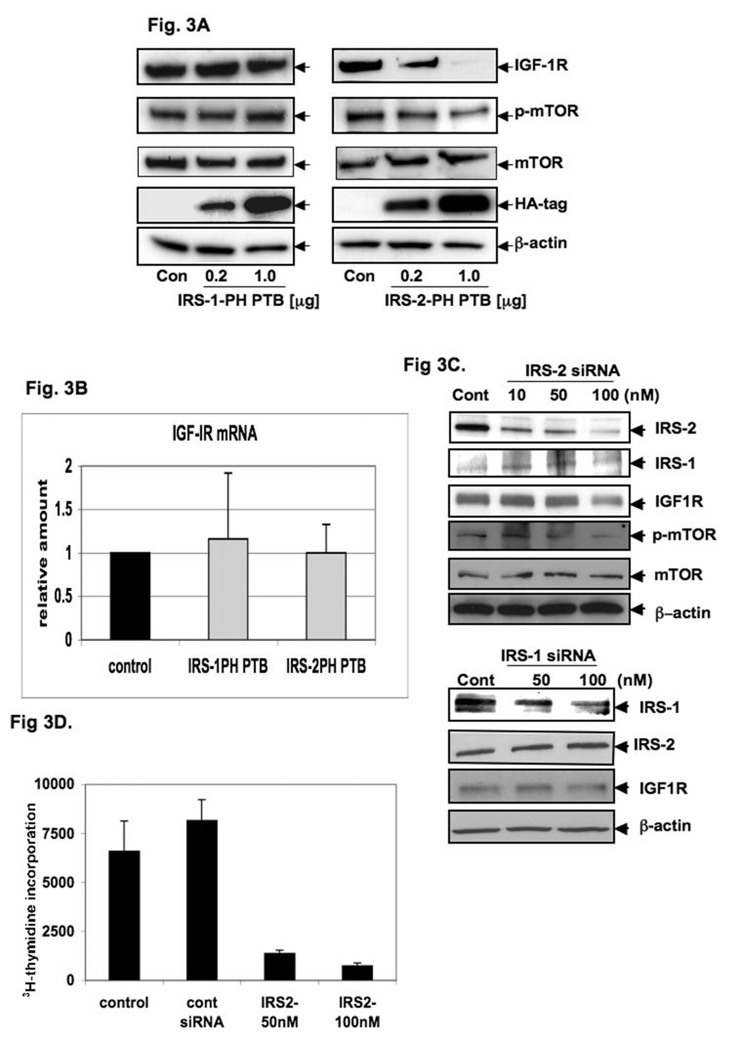
To determine whether IRS-1 or IRS-2 are involved in the downstream regulation of IGF-1R expression, we transiently transfected AsPC-1 cells with 0.2 and 1.0 µg HA-IRS-1-PH-PTB or HA-IRS-2-PH-PTB constructs. The PH-PTB constructs are composed of both the NH2-terminal pleckstrin homology (39) domain and the phosphotyrosine-binding (PTB) domain. The PH domain binds membrane phospholipids or acidic motifs of different proteins, and the PTB domain interacts with the IGF-1R receptor α-subunit. These constructs lack the COOH-terminal portions of both IRS proteins that enable them to interact with specific downstream SH-2 domain-containing proteins. Therefore, HA-tagged IRS-1-PH-PTB and IRS-2-PH-PTB constructs block the IRS-1 and IRS-2 function, respectively. As shown in Fig. 3A, the expression of HA-IRS-1-PH-PTB did not affect the IGF-1R protein level, whereas the expression of HA-IRS-2-PH-PTB (Fig. 3A) led to a significant reduction of IGF-1R protein expression in AsPC-1 cells. Furthermore, we examined whether phosphorylation of mTOR is modulated by IRS proteins. The HA-IRS-1-PH-PTB transfected AsPC-1 cells did not show any changes in the phosphorylation status of mTOR; whereas we found a strong reduction of phosphorylated sites in HA-IRS-2-PH-PTB transfected cells (Fig. 3A). To confirm the role of IRS-2 in the regulation of IGF-1R expression, we utilized a different pancreatic cancer cell line. SU86.86 cells were transiently transfected with 1.0 µg HA-IRS-2-PH-PTB were found to have a significant decrease in IGF-1R protein expression (data not shown). Similarly, we also found that in HA-IRS-2-PH-PTB expressing cells, there was no apparent change in the mRNA level of IGF-1R as compared to that of parental cells (Fig 3B).
Fig. 3. IRS-2, but not IRS-1, in IGF-1R overexpression in PCA.
Total cell lysates from AsPC-1 cells transiently transfected with 0.2 and 1.0 µg of expression vector containing either (A) IRS-1 PH-PTB or IRS-2 PH-PTB or empty vector (control) were analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-IR) and anti-phospho-mTOR (phos-mTOR). Anti-HA tag (HA-tag) was measured to monitor transfection efficiency, β-actin served as control protein. (B) IGF-1R mRNA expression was analyzed from total RNA extracted from AsPC-1 cells transiently transfected with 1.0 µg of expression vectors containing either IRS-1 PH-PTB or IRS-2 PH-PTB and empty vector (control) were analyzed by RT-PCR using beta-actin as control. (C) siRNA of IRS-2, but not IRS-1 inhibits IGF-1R protein expression. Total cell lysates from AsPC-1 cells transiently transfected with siRNA of IRS-2; siRNA of IRS-1 or control siRNA (control) (all purchased from Santa Cruz) analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-1R) EGFR and other proteins. siRNA of IRS proteins did not influence each other. β-actin served as control protein. (D) 3H-thymidine incorporation of AsPC-1 cells after treating with IRS-2 siRNA (50nM and 100 nM) which showed that significant inhibition of cell proliferation.
To confirm the role of IRS-2 in the regulation of IGF-1R, we have utilized the siRNA approach to block the expression of the IRS family of proteins. Figures 3C display that by blocking IRS-2 expression there are significant changes in protein expression levels of both families of proteins, whereas blocking IRS-1 has no apparent effect. Of importance, the level of IRS-1 is much less in both mRNA as well as protein level in most of the PCA cells. To confirm the importance of IRS-2 in PCA growth, we have performed 3H-thymidine incorporation of AsPC-1 cells after treating with IRS-2 siRNA (50nM and 100nM) in the presence of serum which showed significant inhibition of cell proliferation (Fig 3D). Overall, our data suggest that IRS-2 is involved in the regulation of IGF-1R expression through mTOR.
Protein Kinase C δ (PKC δ), mTOR and IGF-1R regulation
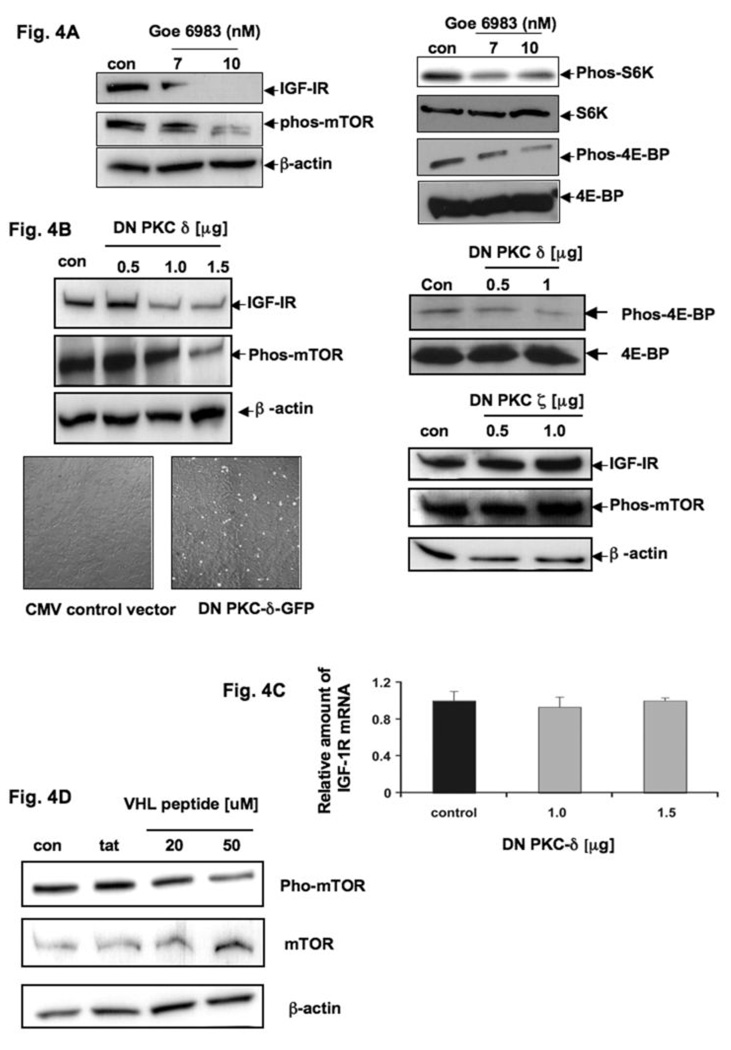
As IRS-2 only acts as an adapter molecule, we were now interested in downstream molecules that possess kinase activity. Others, as well as our group, showed that PKC δ is involved in the signaling pathways associated with IGF-1R activation (33, 34). Initially, to prove whether PKCs are involved in the regulation of IGF-1R expression, we treated AsPC-1 cells overnight with 7 and 10nM of the general PKC-inhibitor Goe6983 and examined IGF-1R protein expression and the phosphorylation status of mTOR by Western blot analysis. Up to 7nM, Goe6983 specifically blocks calcium-dependent PKCs, such as PKC α and β; and 10nM Goe6983 will block novel PKCs, including PKC δ. Whereas with higher concentrations, it blocks atypical PKCs, including PKC ζ. Compared to untreated AsPC-1 cells, we detected a strong reduction of IGF-1R protein levels as well as a reduction of mTOR phosphorylation and its related molecules such as S6-kinase and 4E-BP, beginning with 10nM Goe6983, indicating that novel and/or atypical PKCs may be involved in the regulation of IGF-1R expression (Fig. 4A). Moreover, rottlerin (a more specific inhibitor of PKC δ) treatment revealed a similar effect as shown with Goe6983 (data not shown).
Fig. 4. PKC δ controls IGF-1R overexpression in PCA.
(A) Total cell lysates from AsPC-1 cells untreated (control) or treated with 7 or 10 nM of the PKC inhibitor Goe6983 were resolved by SDS-PAGE and analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-1R) and anti-phospho mTOR (phos-mTOR). β-actin served as control protein. Up to a concentration of 7nM Goe6983 blocks specifically calcium-dependent PKC α and β. Up to 10nM Goe6983 also inhibits novel PKC’s, including PKC δ and in higher concentrations than 10nM to atypical PKC’s including PKC ζ. β-actin served as control protein. Same extracts were immunoblotted with anti-Phos-S6K, -S6K, -Phos-4E-BP and -4E-BP antibodies. (B) Total cell lysates from AsPC-1 cells transiently transfected with 0.5, 1.0 and 1.5 µg of dominant negative PKC δ (dn-PKC-δ) or empty vector (control) were analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-IR) and anti-phospho-mTOR (phos-mTOR). β-actin served as control protein. Same extracts were immunoblotted with anti-Phos-4E-BP and -4E-BP antibodies. (lower-panel) Total cell lysates from AsPC-1 cells transiently transfected with 0.5 and 1.0 µg of dominant negative PKC-ζ or empty vector (control) were resolved by SDS-PAGE and analyzed by immunoblotting with anti-IGF-1R 2C8 (IGF-IR) and anti-phospho-mTOR (phos-mTOR). β-actin served as loading control. (C) IGF-1R mRNA extracted from AsPC-1 cells transiently transfected with 1.0 and 1.5 µg of dominant negative PKC-δ (dn-PKC-δ) or empty vector (control) was analyzed by RT-PCR using beta-actin as control. (D) Total cell lysates from AsPC-1 cells untreated (control), 50 µM TATFLAG peptide (control) and treated with 20 and 50 µM of TATFLAGVHL-peptide, were resolved by SDS-PAGE and analyzed by immunoblotting with anti-phospho-mTOR (phos-mTOR) and anti-mTOR (mTOR). TATFLAGVHL peptide (107–122) binds directly to PKC-δ and blocks its kinase activity.
To further confirm the importance of PKC δ in this pathway, we transiently transfected PCA cells with 0.5, 1.0, and 1.5 µg of dominant-negative mutant of PKC δ and investigated IGF-1R protein expression as well as the phosphorylation levels of mTOR. As shown in Fig. 4B, we observed a significant inhibition of IGF-1R expression accompanied by a marked decrease of phosphorylated mTOR. We also confirmed a decrease of phosphorylation of 4E-BP after dominant negative mutant transfected cells (Fig. 4B) In contrast, with overexpression of dominant-negative mutant of PKC ζ, we were unable to show similar results indicating that PKC ζ is not involved this pathway (Fig. 4B). We also found that PKC δ regulates IGF-1R expression typically at the translational level, as there was no apparent change in the mRNA level of IGF-1R when cells were expressed with 1.0 and 1.5 µg of dominant negative mutant of PKC δ (Fig. 4C).
In a different approach to confirm these results, we treated AsPC-1 cells overnight with 20nM and 50nM TATFLAGVHL peptide (107–122) which directly binds to PKC δ and blocks its kinase activity (33). Untreated cells and cells treated with only TATFLAG peptide (33) served as controls. As expected, we detected a decrease of phosphorylated mTOR, confirming our hypothesis that mTOR phosphorylation is dependent upon PKC δ activity (Fig. 4D).
PKC δ is downstream of IGF-1R/IRS-2 axis to promote IGF-1R expression
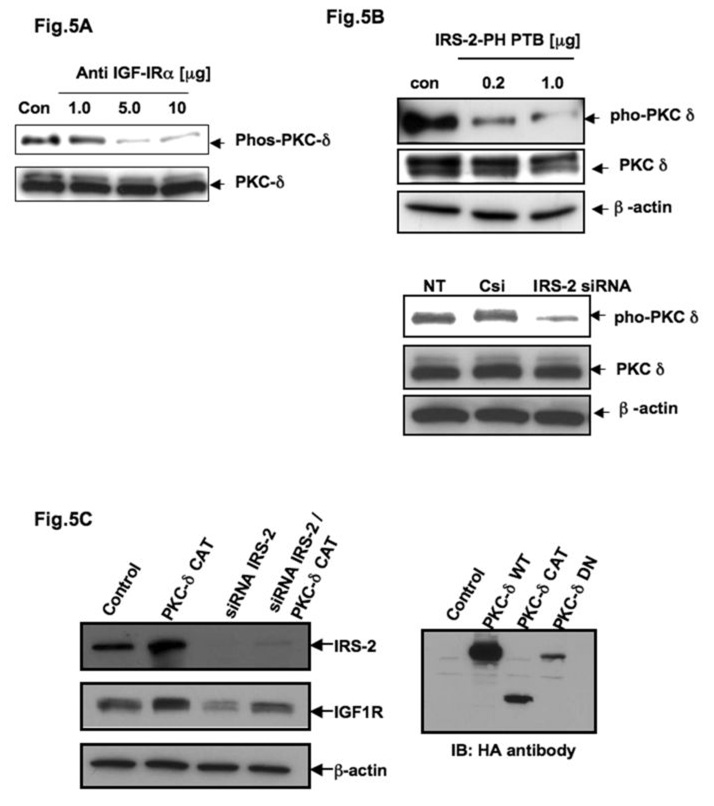
To prove whether the phosphorylation of PKC δ depends on IGF-1R activation, we treated AsPC-1 cells with 1.0, 5.0, and 10.0 µg of anti-IGF-IRα 1H7 for 24 h and examined the phosphorylation status of PKC δ with anti-phospho-PKC δ by Western blot analysis. Fig. 5A shows a marked decrease in the phosphorylation levels of PKC δ after blocking IGF-1R function, which clearly suggests that PKC δ is the downstream of IGF-1R. It also indicates that PKC δ may be a potential kinase of mTOR through the adapter IRS-2. We also observed a functional interaction between PKC δ and IGF-1R in AsPC-1 cells, as reported by other workers (34) in different cell lines (data not shown). To further establish the relationship of PKC δ within IGF-1R/IRS-2 axis, we observed that PKC δ phosphorylation is IRS-2 dependent since the IRS-2-PH-PTP mutant overexpressing cells and IRS-2 knock down cells showed less phosphorylation of PKC δ (Fig. 5B) as we observed in anti- IGF-IRα antibody treatment (Fig. 5A).
Fig. 5. Protein kinase-delta (PKC δ) is in the loop of IGF-1R expression in PCA.
(A) Total cell lysates from AsPC-1 cells treated with 1.0, 5.0 and 10.0 µg of anti-IGF-IRα 1H7 or mouse IgG (control) were analyzed by immunoblotting with anti-phospho-PKC-δ (phos-PKC δ) and anti-PKC δ (PKC δ). (B) Total cell lysates from AsPC-1 cells transiently transfected with 0.2 and 1.0 µg of IRS-2 PH PTB or empty vector (control) were immunoblotted with either anti-PKC-δ or anti-phospho-PKC-δ antibodies respectively. Total cell lysates from AsPC-1 cells transfected with 0.1 µM of IRS-2 siRNA or control siRNA were immunoblotted with either anti- PKC-δ or anti-phospho-PKC-δ antibodies respectively. β-actin served as loading control. (C) Total cell lysates from AsPC-1 cells transiently transfected with 0.1 µM of IRS-2 siRNA or control siRNA (control) with or without dominant active mutant (aa 334–674) of PKC δ (PKCδCAT) were resolved by SDS-PAGE and analyzed by immunoblotting with IRS-2 and IGF-1R antibodies. β-actin served as control protein.
Next we examined whether dominant active mutant (aa 334–674, CAT) of PKC δ (PKCδCAT) can rescue the IRS-2 null effect with respect to IGF-1R expression and mTOR poshphorylation. We then transfected expression vector of PKCδ CAT in IRS-2 siRNA expressing AsPC-1 cells and performed similar experiments as described earlier. Indeed, overexpression of PKCδCAT can override the IRS-2 null effect by expressing more IGF-1R as shown in Fig. 5C. Taken together our results suggest that IRS-2 is playing an important role as an adaptor of IGF-1R and PKC δ that might lead to mTOR activation and thus IGF-1R expression in PCA.
Discussion
Our results elucidate several important aspects of IGF-1R mediated signaling and may eventually suggest a novel mechanism of IGF-1R protein expression in pancreatic adenocarcinoma. Although the reasons for the highly aggressive nature of pancreatic cancer are not fully understood, it is already known that tumorigenesis is a complex process also characterized by an activation of oncogenes, specifically K-ras (40), and the loss of tumor suppressor p53 gene function (41) and PTEN mutation (42). Additionally, the recent data showed that reduced expression of TSC2 might be involved in the progression of pancreatic cancer (24). Previously, it has been shown by several groups that mTOR activation leads to the formation of a rapamycin-sensitive complex with Raptor (regulatory-associated protein of mTOR) followed by increases in mRNA translation via phosphorylation of two effector molecules: S6-kinase and eIF4E binding protein (4E-BP) (32, 43–47). Interestingly, TSC1 or TSC2 removal constitutively activates rapamycin-sensitive functions of mTOR independently of Akt (48). A similar situation might exist in PCA cells as well as in the absence of TSC. Our data indicate that mTOR signaling in the context of IGF-1R expression is independent of Akt whereas PKC δ might play an important role. There is increasing evidence that tumors with mutant p53 use a PI3K dependent but Akt independent pathway, while tumors with loss of p16 use a PI3K/Akt/ reactive oxygen species (ROS) dependent pathway. Future studies will reveal whether p53 and p16 mutation in pancreatic cancer can be the detrimental factors for selection of PKC δ vs. Akt/ROS inhibitors for therapeutics.
The overexpression and excessive activation of insulin-like growth factor-1 receptor (IGF-IR) are associated with malignant transformation, increased tumor aggressiveness, and protection from apoptosis (49, 50). Previously, we have shown that overexpressed IGF-1R promotes the proliferation and invasion in AsPC-1 cells (51). Though the mechanisms inducing aberrant IGF-1R expression are not yet clear, several reports indicate an autocrine control of tumor cell growth by the IGF-I/IGF-I receptor system in pancreatic cancer (3, 38). We have determined in our investigations that an autocrine IGF-1R protein expression loop is mediated by at least two different signaling pathways. A recent report showed that mTOR inhibition activates PI3-kinase/Akt by up-regulating IGF-1R signaling in acute myeloid leukemia mostly due to up-regulation IRS-2 (52). Although the authors didn’t describe the effect of IGF-1R expression in that regard, there might be a different regulation in AML vs. pancreatic cancer cells as well.
Further investigations to determine downstream molecules that mediate IGF-1R expression at the translational level revealed a crucial role for PKC δ. AsPC-1 cells transfected with dominant-negative PKC δ expressed markedly less IGF-1R protein while IGF-1R mRNA levels did not change. We found evidence for functional interactions between PKC δ and IGF-1R wherein IRS-2 plays a role like an adaptor in the loop. Li et al. showed that activated IGF-1R was able to phosphorylate purified PKC δ in vitro and stimulate its kinase activity (34). Our data also indicate that phosphorylation of PKC δ depends upon IGF-1R function. Furthermore, AsPC-1 cells transfected with dominant-negative PKC δ showed a decrease in phosphorylated mTOR suggesting that PKC δ acts upstream of mTOR, and mTOR phosphorylation is PKC δ dependent. Kumar et al. showed that PKC δ constitutively associates with mTOR (30); however, in our experimental conditions, we were unable to detect mTOR and PKC δ in same immunocomplexes reproducibly. Nonetheless, other PKCs like PKC-α, -β or -ζ are not involved in the regulation of IGF-1R protein expression.
Another important aspect of our findings is that IRS-2, but not IRS-1, is involved in this signaling pathway of IGF-1R regulation. An overexpression of IRS-2 and IRS-1 in pancreatic cancer has been shown by several groups (3, 19). Several reports indicate that IRS-1 and IRS-2 are not fully interchangeable and signaling intermediates for the biological effect of IGF-1 and insulin (53). In this report, we have shown that inhibition of IRS-2, but not IRS-1, function leads to a decrease of IGF-1R protein expression. Additionally, we have shown that the presence of IRS-2 is required to activate PKC-δ by IGF-IR. In conclusion, our results indicate that overexpression of IGF-1R in human pancreatic cancer cells are an autocrine mechanism and primarily regulated by a signaling pathway via mTOR. In this pathway, PKC-δ plays a key role by functionally downstream of IGF-1R/IRS-2 axis. Further studies are in progress that employ this unique pathway to develop a novel therapy of pancreatic adenocarcinoma where there is no therapy currently available for patients.
Acknowledgement
This work was partly supported by an American Cancer Society grant and grants from NIH CA78383 and HL70567 to DM. DM is an American Cancer Society Scholar.
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Bauer TW, Somcio RJ, Fan F, et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther. 2006;5:1676–1682. doi: 10.1158/1535-7163.MCT-05-0175. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007–2011. [PubMed] [Google Scholar]
- 4.Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2003;48:1972–1978. doi: 10.1023/a:1026122421369. [DOI] [PubMed] [Google Scholar]
- 5.Karna E, Surazynski A, Orlowski K, et al. Serum and tissue level of insulin-like growth factor-I (IGF-I) and IGF-I binding proteins as an index of pancreatitis and pancreatic cancer. Int J Exp Pathol. 2002;83:239–245. doi: 10.1046/j.1365-2613.2002.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–F126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 7.Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- 8.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair PN, De Armond DT, Adamo ML, Strodel WE, Freeman JW. Aberrant expression and activation of insulin-like growth factor-1 receptor (IGF-1R) are mediated by an induction of IGF-1R promoter activity and stabilization of IGF-1R mRNA and contributes to growth factor independence and increased survival of the pancreatic cancer cell line MIA PaCa-2. Oncogene. 2001;20:8203–8214. doi: 10.1038/sj.onc.1205044. [DOI] [PubMed] [Google Scholar]
- 10.Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–593. [PubMed] [Google Scholar]
- 11.Flossmann-Kast BB, Jehle PM, Hoeflich A, Adler G, Lutz MP. Src stimulates insulin-like growth factor I (IGF-I)-dependent cell proliferation by increasing IGF-I receptor number in human pancreatic carcinoma cells. Cancer Res. 1998;58:3551–3554. [PubMed] [Google Scholar]
- 12.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 13.Ebina Y, Ellis L, Jarnagin K, et al. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985;40:747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich A, Bell JR, Chen EY, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 15.White MF, Shoelson SE, Keutmann H, Kahn CR. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988;263:2969–2980. [PubMed] [Google Scholar]
- 16.Gibson SL, Ma Z, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 2007;6:631–637. doi: 10.4161/cc.6.6.3987. [DOI] [PubMed] [Google Scholar]
- 17.Myers MG, Jr, White MF. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–650. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- 18.Uchida T, Myers MG, Jr, White MF. IRS-4 mediates protein kinase B signaling during insulin stimulation without promoting antiapoptosis. Mol Cell Biol. 2000;20:126–138. doi: 10.1128/mcb.20.1.126-138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornmann M, Maruyama H, Bergmann U, et al. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998;58:4250–4254. [PubMed] [Google Scholar]
- 20.Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Hum Genet. 2000;107:97–114. doi: 10.1007/s004390000348. [DOI] [PubMed] [Google Scholar]
- 21.Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 22.Wienecke R, Maize JC, Jr, Shoarinejad F, et al. Co-localization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Oncogene. 1996;13:913–923. [PubMed] [Google Scholar]
- 23.Avruch J, Hara K, Lin Y, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka K, Fujimoto K, Ito D, et al. Expression and prognostic value of tuberous sclerosis complex 2 gene product tuberin in human pancreatic cancer. Surgery. 2005;138:450–455. doi: 10.1016/j.surg.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Astrinidis A, Henske EP. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene. 2005;24:7475–7481. doi: 10.1038/sj.onc.1209090. [DOI] [PubMed] [Google Scholar]
- 26.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Price DJ, Grove JR, Calvo V, Avruch J, Bierer BE. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- 28.Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. Embo J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 29.Rousseau D, Gingras AC, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- 30.Kumar V, Pandey P, Sabatini D, et al. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. Embo J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dekker LV, Parker PJ. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 32.Easton JB, Kurmasheva RT, Houghton PJ. IRS-1: auditing the effectiveness of mTOR inhibitors. Cancer Cell. 2006;9:153–155. doi: 10.1016/j.ccr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Datta K, Nambudripad R, Pal S, Zhou M, Cohen HT, Mukhopadhyay D. Inhibition of insulin-like growth factor-I-mediated cell signaling by the von Hippel-Lindau gene product in renal cancer. J Biol Chem. 2000;275:20700–20706. doi: 10.1074/jbc.M909970199. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Jiang YX, Zhang J, et al. Protein kinase C-delta is an important signaling molecule in insulin-like growth factor I receptor-mediated cell transformation. Mol Cell Biol. 1998;18:5888–5898. doi: 10.1128/mcb.18.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yenush L, Zanella C, Uchida T, Bernal D, White MF. The pleckstrin homology and phosphotyrosine binding domains of insulin receptor substrate 1 mediate inhibition of apoptosis by insulin. Mol Cell Biol. 1998;18:6784–6794. doi: 10.1128/mcb.18.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosravi-Far R, White MA, Westwick JK, et al. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou MM, Hou W, Johnson J, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 38.Ohmura E, Okada M, Onoda N, et al. Insulin-like growth factor I and transforming growth factor alpha as autocrine growth factors in human pancreatic cancer cell growth. Cancer Res. 1990;50:103–107. [PubMed] [Google Scholar]
- 39.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe M, Nobuta A, Tanaka J, Asaka M. An effect of K-ras gene mutation on epidermal growth factor receptor signal transduction in PANC-1 pancreatic carcinoma cells. Int J Cancer. 1996;67:264–268. doi: 10.1002/(SICI)1097-0215(19960717)67:2<264::AID-IJC18>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 41.Barton CM, Staddon SL, Hughes CM, et al. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991;64:1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Zhang Y, Arrazola P, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 44.Goncharova EA, Goncharov DA, Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 45.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and-2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 48.Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaleko M, Rutter WJ, Miller AD. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990;10:464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sell C, Baserga R, Rubin R. Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res. 1995;55:303–306. [PubMed] [Google Scholar]
- 51.Zeng H, Datta K, Neid M, Li J, Parangi S, Mukhopadhyay D. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochem Biophys Res Commun. 2003;302:46–55. doi: 10.1016/s0006-291x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 52.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 53.Giovannone B, Scaldaferri ML, Federici M, et al. Insulin receptor substrate (IRS) transduction system: distinct and overlapping signaling potential. Diabetes Metab Res Rev. 2000;16:434–441. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr159>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]