Summary
The study of regeneration would be aided greatly by systems that support large-scale genetic screens. Here we describe a non-surgical method for inducing tissue damage and regeneration in Drosophila larvae by inducing apoptosis in the wing imaginal disc in a spatially and temporally regulated manner. Tissue damage results in localized regenerative proliferation characterized by altered expression of patterning genes and growth regulators as well as a temporary loss of markers of cell fate commitment. Wingless and Myc are induced by tissue damage and are important for regenerative growth. Furthermore, ectopic Myc enhances regeneration when other growth drivers tested do not. As the animal matures, the ability to regenerate is lost and cannot be restored by activation of Wg or Myc. This system is conducive to forward genetic screens, enabling an unbiased search for genes that regulate both the extent of and the capacity for regeneration.
Introduction
Since the discovery of regeneration in hydra by Trembley in 1740, scientists have been fascinated by the remarkable capacity exhibited by some animals to regenerate damaged or missing portions of their bodies almost completely following injury (reviewed in Morgan, 1901). Among the different types of regeneration that have been studied, epimorphic regeneration refers to a phenomenon where tissue growth is central to the regenerative process and occurs from a localized population of progenitor cells referred to as a blastema. Historically, epimorphic regeneration was mostly studied in the context of limb regeneration in urodele amphibians such as salamanders and newts where differentiated cells near the amputation site appear capable of dedifferentiating and reentering the cell cycle to form a blastema (Brockes and Kumar, 2005). The blastema itself is then capable of patterning all the structures that are generated as a result of the regenerative growth. Epimorphic regeneration has also been observed in a variety of other situations such as heart and fin regeneration in zebrafish (Johnson and Weston, 1995; Poss et al., 2002), the regeneration of digit tips in mammals (Muller et al., 1999) as well as appendage regeneration in hemimetabolous insects (French et al., 1976). A central and unsolved question pertaining to epimorphic regeneration is why some animals respond to tissue damage by regenerative growth while damage to the same tissues in other animals results in wound healing and scarring.
Importantly, the capacity for regenerative growth appears to differ not just between different organisms, but also between different developmental stages of the same organism. For instance, in Xenopus tadpoles there is a brief developmental window during which regeneration of the tail normally does not occur (Beck et al., 2003) but can be induced by manipulating Notch and BMP-signaling. Similarly, human digits can regenerate in utero, but that ability is lost in early childhood (Yokoyama, 2008). Hence, a better understanding of the mechanisms that regulate the capacity for regenerative growth could point to strategies for promoting regeneration rather than scarring in damaged human tissues.
Studies of epimorphic regeneration have been hampered by an inability to conduct large-scale screens to identify genes that regulate regeneration, such as those that can be conducted in invertebrate model organisms. While adult Drosophila are incapable of regenerating damaged appendages, Hadorn and colleagues showed that Drosophila imaginal discs, the larval primordia of adult structures such as the wing and leg, are indeed capable of undergoing regenerative growth when implanted into the abdomens of adult females (Hadorn and Buck, 1962). These regenerating discs had a local zone of cell proliferation (Adler and MacQueen, 1984; Kiehle and Schubiger, 1985) similar to the blastema that is observed during limb regeneration in urodele amphibians. The technical difficulties associated with disc transplantation experiments have hindered the widespread use of this system as a way of studying regenerative growth. As an alternative, some studies have used genetic methods to induce transdetermination or changes in cell fate in the leg disc that result in the outgrowth of wing tissue (Maves and Schubiger, 1998; McClure et al., 2008). However, it is likely that there are differences between the regulation of growth in response to transdetermination and growth in response to tissue loss.
To simplify the study of imaginal disc regeneration, we have developed a novel, non-surgical method of inducing tissue ablation and regenerative growth in situ that can be incorporated into large-scale screens for genetic regulators of regenerative growth. Here we describe the characteristics of regenerative growth in imaginal discs and implicate a pathway involving Wingless and Myc in its regulation.
RESULTS
A non-surgical method for studying regenerative growth in Drosophila
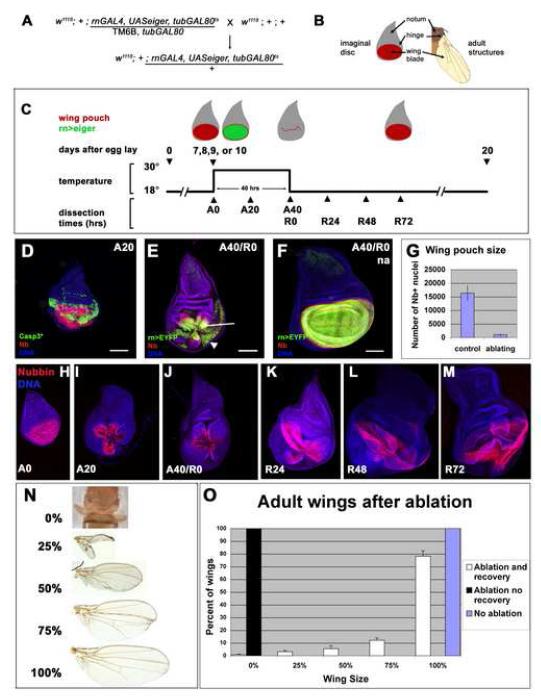
In order to target tissue ablation to the developing wing (Figure 1A-C), we used a GAL4 enhancer trap in the rotund locus (St Pierre et al., 2002), which is expressed in the primordium of most of the adult wing blade, the wing pouch (Figure 1B,F and Figure S1 A-D). Additionally, rnGAL4 is also expressed in the peripodial epithelium directly overlying the wing pouch (Figure S1 A-B). Lower levels of expression were observed in the pouch of the haltere disc, a ring in leg discs, and a small portion of the wing disc epithelium that gives rise to the adult notum (data not shown). rnGAL4 was used to drive expression of the pro-apoptotic gene eiger, which encodes the Drosophila ortholog of TNFα (Igaki et al., 2002; Moreno et al., 2002). Eiger induces apoptosis via its receptor, Wengen (Kanda et al., 2002), and downstream effectors including JNK and caspases . A transgene encoding a temperature-sensitive version of GAL80 (tubGAL80ts), which represses GAL4 function at 18°C but not at 30°C (McGuire et al., 2003), was used to switch eiger expression on and off. Transposons containing all three components of the system (rnGAL4, UASeiger and tubGAL80ts) were recombined onto a single third chromosome.
Figure 1. A novel genetic system induces ablation and regeneration.
(A) The genetic cross whose progeny undergo ablation in the wing imaginal disc. In specific experiments w1118;+;+ is replaced with other genotypes, such as w1118;+;UASEGFP in (E) and (F).
(B) Diagram showing regions of the wing-imaginal disc and the corresponding adult structures.
(C) The protocol used to study ablation and regeneration. Animals were raised at 18° and shifted to 30° for 40 hours during early third instar larval development. Unless otherwise specified, this shift began at 7d AEL. Larvae were returned to 18° and allowed to pupariate and eclose or were dissected at the time points noted during the Ablation (A) or Recovery (R) periods. Drawings not to scale.
(D) An antibody for cleaved Caspase 3 (green) marks dying tissue and debris at A20, which is no longer recognized by the wing pouch marker anti-Nubbin (red).
(E-G) Comparison of pouch size in ablated (E) and mock-ablated (F) wing discs. EGFP (green) marks the rnGAL4-expressing tissue that had not undergone apoptosis and remained in the epithelium (arrow) as well as debris from rnGAL4-expressing tissue that has undergone apoptosis and is found between the folds of the disc (arrowhead). Nb (red) marks the wing pouch. na = non ablating. (G) Quantification of extent of ablation as measured by number of Nb positive cells. Discs were from two separate experiments. Control n=2, Ablating n=10. Error bars mark one standard deviation.
(H-M) Size of the wing pouch, stained with anti-Nb, at different time points in the Ablation and Recovery periods.
(N) The range of adult wing sizes observed in adult animals that had undergone the ablation protocol compared to the size of a normal wing.
(O) Adult wing sizes observed under different experimental conditions. Ablation and recovery resulted in a range of wing sizes, n = 6 experiments, 356 wings. Error bars mark SEM.
All scale bars are 100μm.
To induce tissue ablation and regenerative growth, larvae of the appropriate genotype (Figure 1A) were initially maintained at 18°C to prevent the expression of UASeiger. During the early third instar of larval development, the cultures were shifted to 30°C for 40 hr, denoted as ablation times A0-A40 (Figure 1C). Unless otherwise noted, this shift occurred on day 7 after egg laying (AEL), when most larvae had just completed their transition to the third larval instar and when rnGAL4 is expressed in the wing pouch (Figure S1C,E). After 20 hr at 30°C, extensive apoptosis was observed in the wing pouch as assessed by staining with an antibody that detects activated Caspase as well as by TUNEL (Figure 1D, data not shown). At the end of the 40 hr at 30°C the size of the wing pouch, as measured by the number of Nubbin-positive cells (Ng et al., 1995), was approximately 6% that of mock ablated discs (Figure 1E-G). Moreover, of 52 discs examined, all showed similar reductions in the amount of rnGAL4, UASEYFP-expressing tissue (data not shown). Thus, UASeiger efficiently induced ablation of most of the wing pouch tissue. A band of Nb-expressing cells was always observed around the ablated region, consistent with the observation that rnGAL4 is expressed in a domain that is a few cell diameters smaller than the Nb-expressing wing pouch (Figure S1D). The presence of cells that express UASEYFP in the rim of surviving cells in the wing pouch at the end of the 40 hr ablation (Figure 1E) indicated that at least some rnGAL4 expressing cells had survived to this point despite expressing Eiger. If the cultures were maintained at 30°C after the 40 hr ablation, the adult flies that eclosed completely lacked wings, presumably because the regenerating tissue was continually ablated (Figure 1O).
In order to examine the regenerative capacity of wing discs, the larvae were shifted down to 18°C after 40 hr at 30°C to stop expression of UASeiger. In the first 72 hr after the return to 18°C, denoted recovery time points R0-R72, a progressive increase in the size of the wing pouch was observed as assessed by staining with anti-Nb (Figures 1H-M). The regenerating wing pouch looked more crumpled than wild type and was characterized by many folds of tissue. Despite this, most adult flies eclosed with recognizable wings, a proportion of which appeared relatively normal in size and shape (Figure 1N). The distribution of adult wings in a typical experiment is shown in Figure 1O. Thus we have developed a system that consistently and reproducibly ablated the presumptive wing, allowing for the observation of extensive regenerative growth.
Regenerative growth is characterized by localized cell proliferation and has systemic consequences
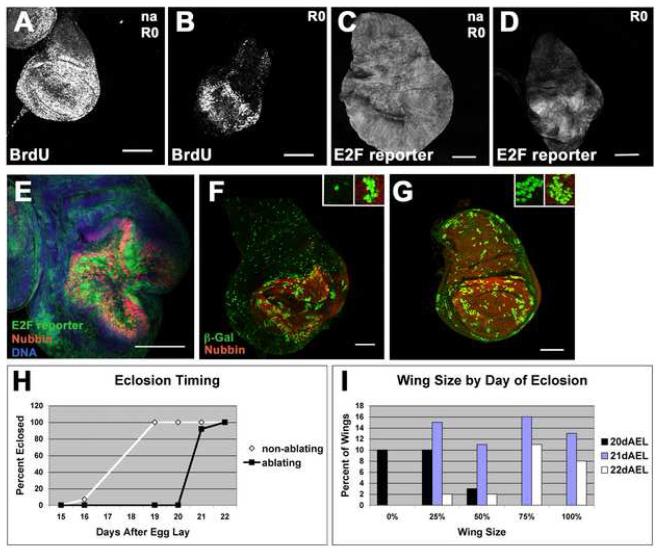
Previous studies of imaginal disc regeneration using surgery and transplantation demonstrated that regenerative growth occurs as localized cell proliferation at the wound edges (Abbott et al., 1981; O’Brochta and Bryant, 1987), similar to the regeneration blastema that is observed during vertebrate limb regeneration (Brockes and Kumar, 2005). We visualized cell proliferation by BrdU incorporation and a reporter, PCNA-GFP (Thacker et al., 2003), that senses the activity of the E2F transcription factor. Both BrdU incorporation and PCNA-GFP expression were indeed observed in the regenerating wing pouch consistent with the presence of a blastema (Figures 2A-D). Expression of these markers for proliferation was greatly reduced in the hinge and notum of the regenerating wing disc even when compared to mock-ablated control discs indicating a suppression of cell proliferation in undamaged regions of the disc. This phenomenon has been observed previously following transplantation (Sustar and Schubiger, 2005). Most of the cells that expressed high levels of the E2F reporter also expressed the wing pouch marker Nb (Figure 2E) demonstrating that the blastema was localized in the wing pouch. Evidence of increased localized cell proliferation was observed even when the cultures had been at 30°C for only 20 hr, while ablation was still occurring (Figure S1F-G). However new growth that occurred while the larva was at 30°C was likely eliminated by continued expression of UASeiger. Localized cell proliferation continued up to 72 hr after the return to 18°C (Figure S1H) when the area of the wing pouch approximated that observed in late third-instar discs from wild-type larvae.
Figure 2. Regenerative growth is localized at the site of ablation.
(A-B) BrdU incorporation marked cells in S phase in non-ablating (A) and ablated (B) wing discs at R0.
(C-D) E2F reporter (PCNA-GFP) marked cells that had cycled through S-phase in non-ablating (C) and ablated (D) discs.
(E) The E2F reporter (green) marked proliferating cells largely within the Nubbin-expressing (red) wing pouch at R0.
(F) lacZ-marked clones induced at R0 and visualized at R72. Insets show a clone from the notum (left) and a clone from the pouch (right).
(G) lacZ-marked clones in a non-ablating disc induced 72 hours before dissection at late wandering stage. Insets show a clone from the notum (left) and a clone from the pouch (right).
(H) Time to eclosion for animals with non-ablating and ablated wing discs in one representative experiment (n = 62 wings).
(I) Comparison of wing sizes on animals with ablated discs that eclosed on successive days in the same experiment as H. All scale bars are 100μm.
In order to visualize the relative rates of cell proliferation in a regenerating wing imaginal disc, we induced β-galactosidase-marked clones (Struhl and Basler, 1993) at the beginning of the recovery period (R0) and examined them 72 hr later (Figure 2F). In the notum and hinge regions of the disc, β-galactosidase was mostly detected in isolated cells or in two-cell clones. In contrast, in the region of the regenerating pouch, many clones consisting of >10 cells were observed. Marked clones allowed to grow for 72 hours just prior to pupariation in a normal disc kept at 18°C throughout development were similarly sized in all parts of the wing disc (Figure 2G). The reduced proliferation in the hinge and notum of regenerating wing discs was likely not due to the discs having reached their final size, as mock-ablated discs were actively proliferating at the time of clone induction (Figure 2A,C). Thus, regenerative growth is characterized by localized cell proliferation in the wing pouch and reduced cell proliferation in undamaged portions of the same imaginal disc.
In order to examine the origin of the cells that contribute to the regenerated wing pouch, we used the FLP-out technique to label cells that had expressed rnGAL4 and hence UASeiger (Duffy et al., 1998; Struhl and Basler, 1993). These experiments showed that both surviving eiger-expressing cells as well as cells outside the rn-expressing region contributed to the regenerating pouch (Figure S2 A, B).
In addition to its effect on cell proliferation in other parts of the disc, tissue ablation and regeneration delayed the onset of pupariation and eclosion by 4 to 6 days at 18°C (Figure 2H). Mock ablated animals pupariated around R24 (data not shown), when ablated discs were only partially regenerated (Figure 1K) indicating that the delay in pupariation is necessary for complete regeneration. Classical studies have shown that tissue damage induced in a variety of ways extends the larval phase of development by an unknown mechanism (Hussey et al., 1927; Simpson et al., 1980). This delay in pupariation has been shown to correlate with the extent of tissue damage and of regenerative growth. Moreover, we observed that the flies with the most complete adult wings were typically the latest to eclose (Figure 2I). Since there was little variation in the extent of ablation, this observation suggests that the extent of the developmental delay correlates with the amount of regenerative growth.
Tissue damage induces changes in cell fate commitment and the expression of growth regulators
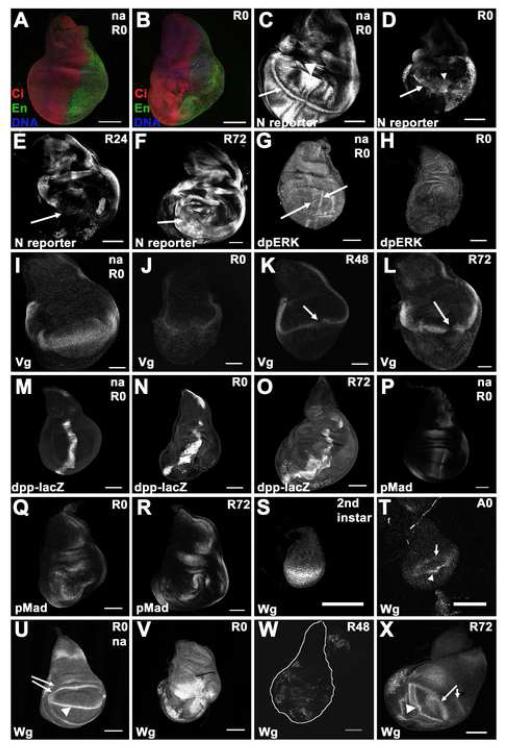
Growth of the wing imaginal disc is normally orchestrated by the expression of genes at the anteroposterior (AP) compartment boundary (reviewed by Affolter and Basler, 2007) and the dorsovental (DV) compartment boundary (Ng et al., 1996; Zecca and Struhl, 2007), which are established early in development. These growth promoting factors include the morphogen Dpp at the AP boundary (Affolter and Basler, 2007) and the transcription factor Vestigial (Vg) and morphogen Wingless (Wg) at the DV boundary (Couso et al., 1993; Ng et al., 1996; Williams et al., 1994; Zecca and Struhl, 2007). Because disc compartment boundaries establish the expression patterns of these growth regulators during normal development, we looked for clear compartment boundaries in regenerating discs. Examination of wing discs at the beginning of the recovery period showed that the anterior marker Cubitus interruptus (Motzny and Holmgren, 1995) and the posterior marker Engrailed (Patel et al., 1989) both stained the appropriate compartments (Figure 3A, B) with no evidence of cells expressing both markers or neither marker. Thus, either AP compartment integrity either remained intact during tissue damage and regeneration or was re-established immediately upon extrusion of the apoptotic tissue and wound closure.
Figure 3. Wing disc patterning is altered during ablation and regeneration.
(A-B) Ci (red) and En (green) marked the anterior and posterior compartments, respectively in non-ablating (A) and ablated discs (B) at R0. (C-F) E(Spl) Mβ-CD2 as a reporter for Notch signaling. (C) Non-ablating disc at R0. A stripe of N signaling was seen at the DV boundary (arrow) and N signaling was seen in the presumptive intervein regions (arrowhead). (D) Ablated disc at R0. (E) Ablated disc at R24. (F) Ablated disc at R72. All images recorded with the same gain.
(G-H) di-phosphorylated ERK as a measure of RTK signaling. (G) Non-ablating disc at R0. dpERK was seen in the presumptive wing veins (arrows). (H) Ablated disc at R0. (I-L) Vestigial expression. (I) Non-ablating disc at R0. (J) Ablated disc at R0. (K) Ablated disc at R48. (L) Ablated disc at R72. Arrows mark discontinuity in expression.
(M-O) dpp-lacZ expression. (M) Non-ablating disc at R0. (N) Ablated disc at R0. (O) Ablated disc at R72.
(P-R) Phosphorylated Mad as a measure of Dpp signaling. (P) Non-ablating disc at R0. (Q) Ablated disc at R0. (R) Ablated disc at R72.
(S-X) Wingless expression. (S) Wingless in a second instar disc was expressed throughout the pouch. (T) Wingless in an early third instar disc (A0) was expressed in a stripe at the DV boundary (arrowhead) and a ring around the pouch (arrow). (U) Non-ablating disc at T0. (V) Ablated disc at T0. (W) Ablated disc at R48. (X) Ablated disc at T72.
All scale bars are 100μm.
The DV compartment boundary is marked by a stripe of Notch (N) activity that is initiated by the apposition of ventral Delta- expressing cells with dorsal Fringe- and Serrate-expressing cells (Panin et al., 1997). As assessed by the expression of the E(spl)Mβ-CD2 reporter (de Celis et al., 1998), a stripe of N activity remained at the DV boundary at the beginning of, and throughout, the recovery period (Figure 3C-F). While this stripe of N signaling was weaker than normal, it was evident in all discs. Since most of the tissue from the middle of the pouch had been ablated, these observations suggest that N signaling near the DV boundary was re-established quickly after ablation by the apposition of dorsal cells with ventral cells.
These experiments also revealed that not all cell fate commitments were maintained in regenerating discs. The N reporter is also expressed in the presumptive intervein regions during third instar development (de Celis et al., 1997), where it extends to the periphery of the wing pouch (Figure 3C) and is seen as early as A0 (data not shown). Reporter activity in the intervein regions was only rarely evident in the remaining wing pouch at the end of ablation, was absent in the regenerating wing pouch at R24, and reappeared at R72 (Figure 3D-F). To confirm the loss of vein/intervein patterning, we visualized prospective veins with an antibody that recognizes diphosphorylated, activated MAPK (anti-dpERK) (Figure 3G) (Gabay et al., 1997). In discs that had undergone ablation, these presumptive wing veins were no longer evident in the remaining wing pouch (Figure 3H). Thus, expression of vein and intervein markers was absent in the surviving cells at the periphery of the wing pouch suggesting that these cells had lost commitment to these cell fates.
We examined whether the growth regulators that shape normal disc growth were still expressed and whether this expression was still associated with the compartment boundaries. Upon ablation, the normal stripe of Vg that crosses the wing disc became weak and diffuse (Figure 3I-J). The typical stripe of expression was almost completely re-established in some discs by 48 hours after the end of ablation (Figure 3K) yet in others discs was still incomplete at R72 (Figure 3L). Thus, Vg expression was eventually re-established throughout the DV boundary, but not before a significant amount of regenerative growth had occurred.
In normal wing discs Dpp expression is found at the AP boundary and can be visualized with a dpp-lacZ reporter (Tabata and Kornberg, 1994). Whether imaginal disc damage can induce ectopic Dpp expression has been unclear (Brook et al., 1993; Mattila et al., 2004). We observed expanded expression of the dpp-lacZ reporter in regenerating discs (Figure 3 M-O). Thus while Dpp expression was still restricted to the middle of the disc it had expanded well beyond the AP boundary. In wild-type third-instar discs Dpp signaling, as visualized by phospho-Mad staining, has two peaks of intensity on either side of the Dpp-producing cells near the AP boundary, with gradients of staining intensity observed laterally (Figure 3P) (Tanimoto et al., 2000). In regenerating discs, peaks of phospho-Mad staining were not confined to the vicinity of the AP boundary, but were observed within and surrounding the area of ablation and regrowth (Figure 3 Q-R). At R72 the peak of pMad staining returned to the AP boundary, but the normal double gradient flanking the boundary was not apparent. Thus, the domains of Dpp expression and signaling expanded in the regenerating wing pouch, with most regenerative growth preceding the re-establishment of a wild-type pattern of Dpp signaling.
In second instar larvae, Wingless (Wg) is expressed throughout the wing pouch (Figure 3S). In third instar wing discs, Wg is normally expressed along the DV boundary and in two concentric circles of expression at the border of and outside the wing pouch, a pattern that is established before the time tissue ablation is induced in these experiments (A0) (Figure 3T-U). We found that Wg expression was strongly upregulated by the end of the 40 hr ablation and remained upregulated during the first 24 hr of recovery (Figure 3V and data not shown). Interestingly, in each ablated disc Wg expression coincided with the remaining wing pouch, similar to the pattern of Wg expression in second-instar discs (Figures 3S, 5O). After 48 hr of recovery, many discs lacked significant Wg expression, suggesting the existence of a phase in between the pouch-wide expression induced by ablation and the re-establishment of normal third instar patterning (Figure 3W). Expression resembling the normal third instar pattern was restored after 72 hr of regenerative growth (Figure 3X). Thus Wg was strongly expressed in regenerating discs, and this expression coincided with the dimensions of the regenerating pouch and not with the DV boundary.
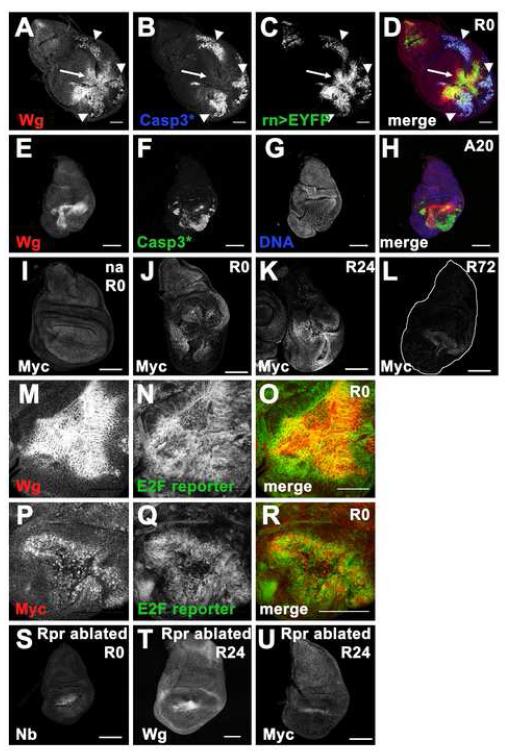
Figure 5. Wg and Myc are unpregulated in regenerating wing discs.
(A-D) Wg (using Anti-Wg antibody) expression at A40/R0. At time R0, low Wg expression was associated with cellular debris (arrowheads), high Wg expression was seen among cells that remained in the disc epithelium (arrow). (A) Wg (B) cleaved Caspase 3 (C) rn>EYFP (D) merge.
(E-G) Wg expression at A20. Apoptotic tissue is not associated with Wg. (E) Wg (F) cleaved Caspase 3 (G) DNA (H) merge.
(I-K) Myc expression (using anti-Myc antibody) in a non-ablating disc at R0 (I) an ablated disc at R0 (J) an ablated disc at R24 (K) and an ablated disc at R72 (L).
(M-O) High Wg expression was associated with cells showing high levels of the E2F reporter PCNA-GFP. (M) Wg (N) E2F reporter (O) merge
(P-Q) Elevated levels of Myc coincided with elevated levels of the E2F reporter in an ablated disc at R0. (P) Myc (Q) E2F reporter (R) merge.
(S-U) Wing discs ablated with rnGAL4, UASreaper. (S) Nubbin, (T) Wg and (U) Myc. All scale bars are 100μm.
A loss of capacity for regenerative growth occurs during the third larval instar
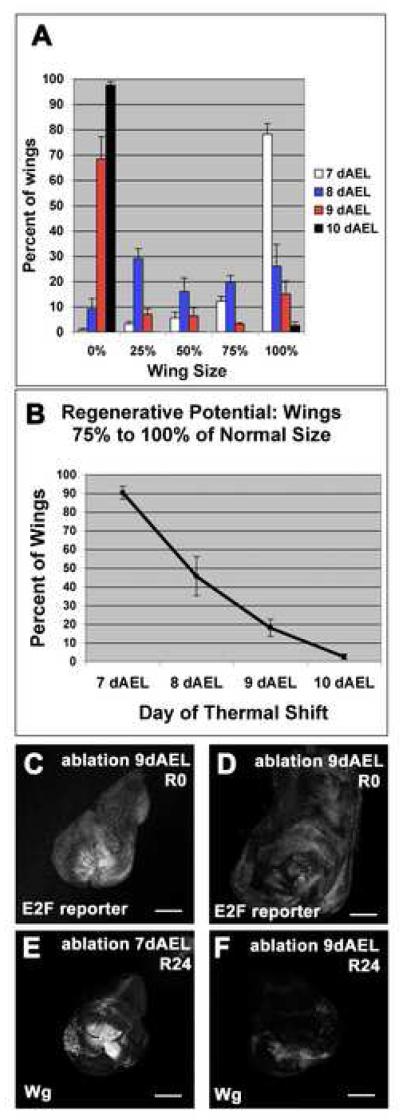
To address whether the capacity for regenerative growth changes during development, ablations were initiated on days 7, 8, 9 or 10 AEL, which together correspond to the third larval instar at 18°C. When the ablations were initiated on day 7, 90.3% of wings were scored as 75-100% in size. In ablations initiated on days 8, 9 and 10 the corresponding values were 45.6%, 18% and 2.5% respectively (Figure 4A, B). Moreover, when ablation was induced at 10dAEL the ablating animals failed to delay entry into pupariation (data not shown), perhaps because regenerative growth is required for induction of delay or the animals had committed irreversibly to metamorphosis. Thus there appeared to be a marked decrease in the extent of regenerative growth between days 7-10 AEL.
Figure 4. Regeneration only occurred during a specific developmental window.
(A) Adult wing sizes observed after ablations beginning on 7, 8, 9, or 10 dAEL. 7dAEL n = 6 experiments, 356 wings. 8dAEL n = 5 experiments 178 wings. 9dAEL n = 3 experiments 119 wings. 10dAEL n = 4 experiments 158 wings.
(B) The number of wings 75 to 100% of normal wing size as a measure of regenerative potential. Numbers of experiments and wings are the same as in panel (A).
(C-D) E2F reporter (PCNA-GFP) in discs that had undergone ablation at 9dAEL.
(E-F) Wg expression at R24 in a disc that had undergone ablation at 7dEAL (E), and a disc that had undergone ablation at 9dAEL (F).
Error bars=SEM.
The decrease in regenerative capacity in later larval development may reflect a decreased capacity of the mature disc epithelium for regenerative growth, or an inability to delay pupariation and thus to allow regeneration to occur prior to metamorphosis. We compared discs that were shifted to 30°C on day 7 to those that were shifted on day 9. Day 7-ablated discs examined at time R0 showed high levels of expression of the E2F reporter PCNA-GFP throughout the regenerating wing pouch (Figure 2D). In contrast, in day 9-ablated discs, upregulation of E2F reporter expression occurred in a smaller area of the wing pouch, or was absent altogether (Figure 4 C-D). Similarly, for the discs ablated on day 7, a robust upregulation of Wg was consistently observed 24 hr after the return to 18°C while discs ablated at day 9 showed a weak and variable expression of Wg at R24 (Figure 4 E-F). Thus older discs appeared less capable of displaying the molecular changes that correlate with regenerative growth, implying that their reduced capacity to regenerate is, at least in part, a result of changes in the disc itself.
Wg functions upstream of Myc in regenerating discs
A role for Wg in promoting disc growth is consistent with observations that reducing Wg function during the second instar of larval development results in decreased adult wing size (Couso et al., 1993). However, manipulations of Wg signaling in clones of cells in the third instar of larval development have yielded conflicting results with one study indicating that Wg signaling restricts growth (Johnston and Sanders, 2003) while others show that Wg promotes growth (Giraldez and Cohen, 2003). A recent study provides compelling evidence for a pathway where Wg promotes growth by a double repression mechanism: Wg signaling inhibits N activity, which in turn inhibits the expression of the growth-promoters Myc and bantam (Herranz et al., 2008). Here we present evidence that a similar mechanism is likely to operate during regenerative growth in the wing pouch.
Our studies have revealed that Wg expression is upregulated in regenerating discs. Wg upregulation occurs when cells undergoing apoptosis are prevented from dying by the expression of the caspase inhibitor p35 (Huh et al., 2004; Perez-Garijo et al., 2004; Ryoo et al., 2004). We did observe that Wg was associated with dying cells and debris that remained in the folds of the wing disc. However, the strong Wg expression noted in the regenerating wing pouch was not associated with apoptotic tissue (Figure 5A-D). Moreover, even as ablation was occurring (time A20), most apoptotic cells were not expressing Wg, while the cells surrounding the apoptotic tissue already had increased Wg expression (Figure 5 E-H). Indeed, elevated Wg expression was found in cells that expressed the E2F reporter indicating that Wg is expressed in the proliferating wing pouch cells (Figure 5M-O, Figure 2E). Interestingly, N activity was largely absent in the regenerating pouch and reduced at the DV boundary (Figure 3E), consistent with the notion that Wg signaling inhibits N activation. We also observed elevated levels of Myc expression in the remaining wing pouch, with levels returning to normal by R72 (Figure 5I-L). Moreover, elevated Myc expression co-localized with the E2F reporter indicating that Myc protein levels were elevated in the proliferating cells (Figure 5P-R).
Wg and Myc expression were also elevated in wing discs that had been ablated using UASreaper (White et al., 1994) instead of UASeiger (Figure 5S-U, Figure S3 A). Differences in the area of Wg expression likely reflect differences in the ablation efficiency of the two transgenes. In addition, Wg and Myc expression were upregulated when the wing disc was damaged in situ with a pinch through the cuticle (Figure S3 B-C), and others have observed Wg upregulation in leg disc fragments following transplantation (Gibson and Schubiger, 1999; McClure et al., 2008). Therefore the expression of Wg and Myc is observed in tissue regenerating after damage by a variety of methods and likely reactivates a pathway that promotes growth during normal disc development.
If indeed Wg promotes regenerative growth by downregulating N activity, thereby allowing the expression of Myc, then manipulations of upstream components of this pathway should affect the expression or activity of downstream components and potentially impact regeneration. Since rnGAL4 and tubGAL80ts were being used in our ablation system, expression of UAS-driven transgenes is limited to the rnGAL4-expressing cells at the time that they also express UASeiger. In our ablation protocol, some of the rnGAL4-expressing cells, which are marked by EYFP in Figures 1E and 5D, survived ablation and would express the UAS-regulated transgenes. Despite these limitations the UAS-driven transgenes have the advantage that, unlike mutations in myc or wg, they do not affect the overall rate of development.
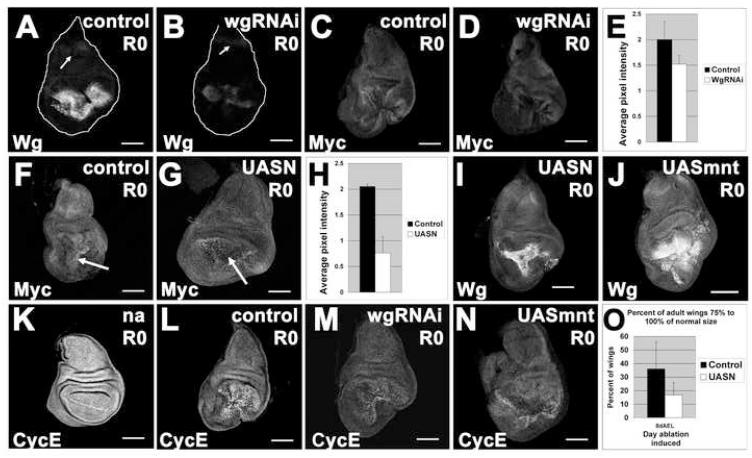
UASwgRNAi (Dietzl et al., 2007) reduced the level of Wg expression in the regenerating disc at R0 (Figure 6A-B). This reduction was temporary, as Wg expression was re-established by R24 (data not shown). In these discs, Myc expression was reduced (Figure 6C-D, quantified in Figure 6E) consistent with a role for Wg in activating Myc expression. Similarly, overexpression of a full-length (wild-type) Notch (UASNFL) (Zecchini et al., 1999) also reduced Myc protein levels consistent with a role for N in restricting Myc expression (Figure 6F-G, quantified in Figure 6H). In contrast, UASNFL did not impair Wg upregulation in regenerating discs (Figure 6I). Overexpression of Mnt was used to inhibit Myc function in the wing pouch (Loo et al., 2005) by antagonizing the expression of Myc target genes (Orian et al., 2005). UASmnt also did not abrogate Wg upregulation (Figure 6J). Taken together, these results are consistent with the ‘double-repression’ mechanism where Wg alleviates the inhibition of Myc expression by N and therefore promotes growth (Herranz et al., 2008).
Figure 6. Wg promotes regenerative growth through Myc and Cyclin E.
(A-B) Wg protein levels at R0 in a control ablated disc (A) and an ablated disc expressing UASwgRNAi (B). Arrows point to comparable Wg levels in the notum.
(C-E) Myc levels were reduced in ablated discs at R0 that expressed UASwgRNAi. (C) control (D) UASwgRNAi (E) Quantification of anti-Myc staining in pouch region, n=3 discs for each.
(F-H) Myc levels were reduced in the pouch of an ablated disc at R0 expressing UASNFL. (F) control (G) UASNFL. H) Quantification of anti-Myc staining, n = 3 discs for each.
(I-J) Wg expression in ablated discs expressing UASNFL (I) or UASmnt (J).
(K-L) Cyclin E protein in a non ablating disc (K), ablated disc (L), ablated disc expressing UASwgRNAi (M), and ablated disc expressing UASmnt (N).
(O) Expression of UASNFL in discs in which ablation was induced at 8dAEL led to reduced adult wing sizes. n = 4 experiments, 132 wings (UASNFL) and 194 wings (control).
All scale bars are 100μm. Error bars = SEM.
We also examined the effects of these transgenes on regeneration. Levels of Cyclin E, which functions downstream of Myc (Johnston et al., 1999), were elevated in the regenerating wing pouch compared to the notum and hinge (Figure 6K-L), consistent with a suppression of cell proliferation in these regions (Figure 2A-D). We used this localized elevation of Cyclin E expression as a sensitive measure of regenerative proliferation in the disc. UASwgRNAi expression reduced the area showing upregulated Cyclin E protein levels in the wing pouch (Figure 6M) and caused a slight reduction in the size of the regenerated adult wings (data not shown). Expression of UASmnt led to an absence of elevated Cyclin E expression in the center of the regenerating pouch (Figure 6N), as did expression of UASmycRNAi (Figure S4). In addition, expression of UASmnt led to a slight reduction in adult wing size (data not shown). Thus UASwgRNAi, UASmnt, and UASmycRNAi reduce regenerative proliferation, at least as assessed by Cyclin E levels in the wing pouch, but had only minor effects on adult wing size, perhaps because their effects were temporary and they only delayed the onset of regenerative growth or because the remaining Wg expression and Myc activity were sufficient.
In contrast, UASNFL, which reduced Myc levels effectively, induced a reduction in the adult wing sizes observed after ablation and regeneration (Figure 6O). The greater effect observed with UASNFL on adult wing size compared to UASwgRNAi or UASmnt might be due to the relative efficacy of each of these transgenes, perdurance of the N protein, or Wg- and Myc-independent effects of N signaling.
Expression of Myc enhances regenerative growth
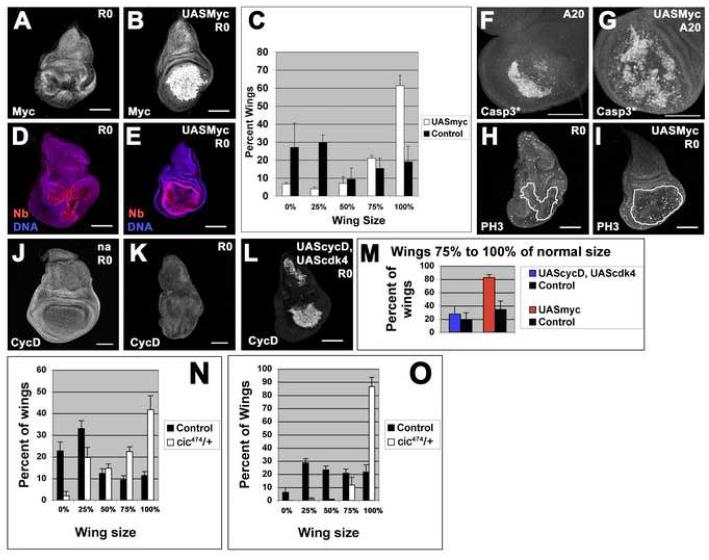
Since Myc appears to regulate regenerative growth, we wondered whether Myc or other growth promoters could be used to enhance regeneration. Expression of UASmyc in the regenerating wing pouch resulted in an obvious increase in the proportion of adult wings that had regenerated more completely (Figure 7A-C). Importantly, the completely regenerated wings were not larger in size and did not have larger cells when compared to control wing discs that had undergone ablation and regeneration without Myc overexpression (data not shown). Thus this enhanced regenerative growth was still constrained by the mechanisms that determine organ size. In regenerating discs that expressed Myc, the remaining Nb-positive pouch was larger than in control ablated discs even at the end of the 40 hr ablation period (Figure 7D-E). Extensive areas of the wing pouch were undergoing apoptosis, indicating that Myc did not protect against Eiger-induced programmed cell death (Figure 7F-G). Consistent with this observation, UASmyc also did not prevent ablation to any extent at later stages of development when regeneration did not occur (see below). At R0, regenerating discs that overexpressed Myc contained many more phospho-histone H3-expressing cells than the wing pouches of control regenerating discs (Figure 7 H-I) indicating that dying cells were being replaced more quickly. This increase in regenerative proliferation even during the time of ablation likely prevented the wing pouch from reducing in area to the same extent as the control ablating discs.
Figure 7. Myc potentiates regenerative growth.
(A-B) Myc protein levels at R0 in an ablated disc (A) and an ablated disc expressing UASmyc (B).
(C) Expression of UASmyc in ablated discs resulted in an increase in adult wing sizes. n = 3 experiments, 240 wings (UASmyc) and 78 wings (control).
(D-E) Nb marked the wing pouch at R0 in an ablated disc (D), and an ablated disc expressing UASmyc (E).
(F-G) Cleaved caspase 3 at R0 as a marker for tissue undergoing apoptosis in an ablated disc (F) and an ablated disc expressing UASmyc (G).
(H-I) Phospho-Histone H3 at R0 as a marker for mitotic cells in an ablated disc (H) and an ablated disc expressing UASmyc (I). Lines denote location of remaining Nubbin staining tissue.
(J-L) Cyclin D expression at R0 in a non-ablating disc (J) an ablated disc (K) and an ablated disc expressing UAScycD, UAScdk4 (L).
(M) Percent of adult wings 75% to 100% of normal size was increased slightly when UAScycD, UAScdk4 was expressed (n = 5 experiments, UAScycD, UAScdk4 = 175 wings, control = 190 wings), and increased considerably when UASmyc was expressed (n same as panel C).
(N-O) Heterozygosity for cic474 resulted in an increase in adult wing sizes when ablation was induced by UASeiger (N) (n = 3 experiments, cic474 = 205 wings, control = 252 wings) or UASreaper (O) (n = 4 experiments, cic474 = 178 wings, control = 378 wings).
All scale bars are 100μm. Error bars = SEM.
We compared the effects of Myc with the overexpression of other growth-promoting genes. In contrast to Myc, Cyclin D protein levels did not appear elevated in the regenerating pouch suggesting that this pathway is not activated to the same extent in regenerating discs (Figure 7 J-K). Furthermore, overexpression of Cyclin D and Cdk4 (UAScycD, UAScdk4) (Datar et al., 2000) in regenerating discs resulted in only a minor increase in adult wing size compared to overexpression of Myc (Figure 7 L-M). Also, increased expression of either Unpaired (UASupd), which is the ligand for the JAK/STAT pathway (Bach et al., 2003), or Rheb (UASrheb) (Saucedo et al., 2003; Stocker et al., 2003; Zhang et al., 2003), which activates TOR-mediated growth, did not promote regeneration as measured by adult wing size (data not shown, Figure S5). Therefore, Myc was able to enhance regenerative growth significantly when these other growth activators tested had little or no effect.
Importantly, overexpression of Myc in older discs that underwent ablation at 10dAEL did not lead to regeneration as assessed by adult wing size, nor did overexpression of Wg or activation of Wg signaling (data not shown, Figure S6). Thus Myc appears capable of promoting regenerative growth in a disc that still has regenerative capacity but is incapable of restoring regenerative capacity to more mature discs. Furthermore, reversal of the reduction in Wg expression and signaling in mature damaged discs was also not sufficient to induce regeneration as measured by adult wing size.
The extent of regeneration can be modified by mutations in other genes
To examine the feasibility of using this system to identify genes that can influence regenerative growth, we conducted two types of experiments to test whether loss-of-function mutations, when heterozygous, could modify the extent of regenerative growth. First we tested several mutations on the right arm of chromosome 3 that had been identified in a previous screen in our laboratory for negative regulators of tissue growth (Tapon et al., 2001). The parent chromosome used in the original screen served as the isogenic control. We found that heterozygosity for a chromosome bearing a mutation in capicua (cic474), a negative regulator of growth that functions downstream of the Ras pathway (Tseng et al., 2007), led to a significant increase in adult wing size when ablation was induced with either UASeiger on 8dAEL or UASreaper on 7dAEL (Figure 7 N-O). The extent of ablation was not affected during the experiment (data not shown) indicating that this mutant chromosome influenced the extent of regenerative growth. A different allele of capicua had a more moderate effect on adult wing size (data not shown). In contrast, mutation of Tsc1, which encodes a negative regulator of Tor signaling did not affect the extent of regeneration (Figure S5). Second, we tested some of the lines containing chromosomal deficiencies on 3R from the DrosDel collection (Ryder et al., 2007) that have been generated in an isogenic background for their ability to dominantly modify the extent of regeneration. Four of six deficiencies tested showed no effect thus highlighting the reproducibility of the phenotype. One deletion was scored as an enhancer and another scored as a suppressor (Figure S7). While regeneration in response to ablation by both Eiger and Reaper is modified by the same genetic mutations (Figure 7 and data not shown), the more direct induction of apoptosis by Reaper may be preferable in a genetic screen to minimize the isolation of mutations that affect ablation rather than regeneration. Thus, this method of inducing tissue damage and regeneration is amenable to unbiased genetic screens for novel genes that regulate regenerative growth.
DISCUSSION
The ablation/regeneration system that we have developed has several advantages over traditional methods that use surgical ablation and transplantation. First and foremost, this approach is far less labor-intensive and therefore allows for the examination of large numbers of regenerating discs in any experiment. Second, since the extent of ablation is defined by the expression of the pro-apoptotic gene under the control of tissue-specific regulatory elements, rather than a surgical instrument, the extent of ablation is relatively reproducible. Furthermore, by using different GAL4 driver lines, this method can be adapted to ablate tissue in other imaginal discs or in other organs during development. Finally, this process occurs entirely in vivo and hence allows an examination of disc-specific as well as systemic factors that regulate regeneration. Taken together, the advantages of this method will facilitate screens for genes that regulate regenerative growth.
The data presented here suggest that regeneration in response to surgical or apoptotic tissue damage occurs via at least one common mechanism, the upregulation of Wg and Myc. In addition, JNK activity is normally upregulated after injury and plays an important role in wound healing and regeneration in response to surgical damage (Bosch et al., 2008; Bosch et al., 2005; Mattila et al., 2005). While Eiger activity has been shown to induce JNK signaling (Igaki et al., 2002), we have also observed elevated JNK signaling in discs damaged by expression of UASreaper (data not shown). Therefore activation of JNK in response to tissue damage is also likely a universal characteristic of regenerating discs and not unique to discs damaged by expression of UASeiger. However, by activating JNK directly, Eiger may facilitate the initiation of tissue repair. In addition to activating JNK, surgical damage to discs leads to the upregulation of other molecules involved in wound healing such as Mmp1 (McClure et al., 2008). We have observed that Mmp1 is similarly expressed in discs regenerating in response to tissue ablation by Eiger (data not shown). Thus regeneration after tissue damage may involve several common mechanisms irrespective of the method by which tissue ablation occurs.
The expression of Wg is markedly elevated during regeneration, with the highest levels observed in proliferating cells that are close to the site of ablation. In addition to Drosophila imaginal discs, the upregulation of specific Wnt-family genes is observed in regenerating tissue in a wide variety of organisms including planaria (Gurley et al., 2008; Petersen and Reddien, 2008), Xenopus and zebrafish, as well as mammalian liver and skeletal muscle (Stoick-Cooper et al., 2007). Thus Wnts may have an important function in regenerative growth that is evolutionarily conserved. Recent studies have shown that Wnt-family proteins can maintain several types of stem cells in a self-renewing state (Nusse, 2008). Thus, increased expression of Wg may also antagonize differentiation signals in imaginal disc cells and allow the regenerating tissue to maintain a capacity for proliferation that is normally observed in even younger discs.
Our studies have revealed a likely role for Myc in driving regenerative growth. Others have observed that overexpression of CyclinD and Cdk4 is more effective than Myc at increasing wing size during normal development (de la Cova et al., 2004). Thus the regulation of growth during regeneration may differ from the regulation of growth during normal development. Consistent with this idea is the observation that in surgically cut discs, cell size and the proliferation kinetics of the regenerating tissue are different from those that are observed at any time during normal disc development (Sustar and Schubiger, 2005). The ability of Myc to potentiate regenerative growth may relate to Myc’s role in promoting cell plasticity, which was observed when the overexpression of c-myc together with three other genes (oct3/4, sox2 and klf4) converted cultured adult fibroblasts into pluripotent stem cells (Takahashi and Yamanaka, 2006). Indeed, our observations that some cell fate commitment and stage-appropriate patterning are abrogated in regenerating discs are consistent with such a role for Myc. These findings also raise the possibility that the ability of Wnt proteins to promote self-renewal in mammalian stem cells may be mediated, at least in part, by Myc.
A fundamental and unsolved question that underlies much of the research on regeneration is why the same tissues from different species or from different stages of development in the same species show a markedly different capacity for regenerative growth. In a similar vein, we have demonstrated that ablation of the wing pouch elicits a robust regenerative response until the middle of the third larval instar. The loss of the ability to regenerate likely reflects developmentally regulated changes in the disc epithelium that interfere with the activation of the gene expression programs that are required for regenerative growth. This block in regeneration may be the result of changes in the properties of the disc cells themselves or the result of changes in humoral factors that occur prior to the onset of metamorphosis. Indeed, these scenarios are not mutually exclusive as recent work has shown that Wg expression can be repressed by Ecdysone signaling (Mitchell et al., 2008). The inability to elicit regeneration in older discs either by overexpressing Myc or by activating Wg signaling indicates that additional growth regulators may have an important role in permitting or preventing regeneration. Indeed, some of these regulators may be unique to mature discs and would not have been identified in previous studies of the growth that occurs during normal development. Thus the development of the system described here can facilitate large-scale unbiased genetic screens for novel regulators of regenerative growth.
Experimental Procedures
Fly stocks and transgenes
See supplemental methods.
Ablation protocol
w1118; +; rnGal4, UASeiger, tubGal80ts / TM6B females were crossed to w1118;+;+ males or males harboring specified mutations or transgenes. Eggs were collected at room temperature in four-hour intervals on grape juice plates and subsequently maintained at 18°C. 48 hours later, 50 newly hatched larvae were picked and placed into each vial, which contained food prepared according to the recipe provided by the Bloomington stock center. The surface of the food was slightly churned and supplemented with yeast paste. Vials were maintained at 18° until the time noted for ablation induction. Vials were then placed in a 30° circulating water bath for 40 hours, then cooled in an ice-water bath for 60 seconds and returned to 18°. To induce ablation using UASreaper the protocol was the same, except larvae were maintained at 30°C for only 24 hr. Mock-ablated discs were rnGAL4, UASEYFP / tubGAL80ts or the siblings of the ablating animals, which were + / TM6B, tubGAL80. These two genotypes behaved identically.
Wing discs of rnGAL4, UASEYFP larvae were physically damaged in situ as previously described (Pastor-Pareja et al., 2008). The discs were pinched at 5 dAEL at 25°C, and allowed to recover for 24 hours at 25°C before dissection, fixation, and staining.
Clone induction
“Flip-out” clones were induced using hsFLP and act<stop<lacZ (Struhl and Basler, 1993) with a ten minute heat shock at 37°C at A40 for ablating discs, or 72 hours before larval wandering for control discs, followed by a 60 second immersion in ice water. Discs were dissected, fixed and stained after 72 hr at 18°C. Lineage tracing of rn-expresing cells was performed using rnGAL4, UASFLP and act<stop<lacZ in the background of the ablation system described above.
Immunohistochemistry and quantification of data from images
See supplemental methods.
Supplementary Material
Acknowledgements
We are grateful to K. Basler, R. Duronio, R. Eisenman, E. Hafen, L. Johnston and G. Struhl for fly stocks, to S. Carroll, S. Cohen, N. Dyson, R. Eisenman, R. Holmgren and T. Orr-Weaver for antibodies, to G. Schubiger for advice and discussions and to D. Bilder, T. Reis, S. Siegrist and J. Treisman for comments on the manuscript. IH is funded by the NIH (GM085576), RKSB was funded by the NIH (5F32GM072252) and now by the American Heart Association (0725135Y). MW is funded by a training grant from the California Institute for Regenerative Medicine and HK was funded by the Japan Society for the Promotion of Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LC, Karpen GH, Schubiger G. Compartmental restrictions and blastema formation during pattern regulation in Drosophila imaginal leg discs. Dev Biol. 1981;87:64–75. doi: 10.1016/0012-1606(81)90061-0. [DOI] [PubMed] [Google Scholar]
- Adler PN, MacQueen M. Cell proliferation and DNA replication in the imaginal wing disc of Drosophila melanogaster. Dev Biol. 1984;103:28–37. doi: 10.1016/0012-1606(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–674. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- Bach EA, Vincent S, Zeidler MP, Perrimon N. A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics. 2003;165:1149–1166. doi: 10.1093/genetics/165.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Bosch M, Baguna J, Serras F. Origin and proliferation of blastema cells during regeneration of Drosophila wing imaginal discs. Int J Dev Biol. 2008;52:1043–1050. doi: 10.1387/ijdb.082608mb. [DOI] [PubMed] [Google Scholar]
- Bosch M, Serras F, Martin-Blanco E, Baguna J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol. 2005;280:73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Brook WJ, Ostafichuk LM, Piorecky J, Wilkinson MD, Hodgetts DJ, Russell MA. Gene expression during imaginal disc regeneration detected using enhancer-sensitive P-elements. Development. 1993;117:1287–1297. doi: 10.1242/dev.117.4.1287. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Datar SA, Jacobs HW, de la Cruz AF, Lehner CF, Edgar BA. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. Embo J. 2000;19:4543–4554. doi: 10.1093/emboj/19.17.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis JF, Bray S, Garcia-Bellido A. Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development. 1997;124:1919–1928. doi: 10.1242/dev.124.10.1919. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signalling mediates segmentation of the Drosophila leg. Development. 1998;125:4617–4626. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Duffy JB, Harrison DA, Perrimon N. Identifying loci required for follicular patterning using directed mosaics. Development. 1998;125:2263–2271. doi: 10.1242/dev.125.12.2263. [DOI] [PubMed] [Google Scholar]
- French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Schubiger G. Hedgehog is required for activation of engrailed during regeneration of fragmented Drosophila imaginal discs. Development. 1999;126:1591–1599. doi: 10.1242/dev.126.8.1591. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Cohen SM. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development. 2003;130:6533–6543. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Alvarado A. Sanchez. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn E, Buck D. Über entwicklungsleistungen transplantierter teilstücke von flügel-imaginalscheiben von Drosophila melanogaster. Rev Suisse Zool. 1962;69:302–310. [Google Scholar]
- Herranz H, Perez L, Martin FA, Milan M. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. Embo J. 2008;27:1633–1645. doi: 10.1038/emboj.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hussey RG, Thompson WR, Calhoun ET. The influence of X-rays on the development of Drosophila larvae. Science. 1927;66:65–66. doi: 10.1126/science.66.1698.65. [DOI] [PubMed] [Google Scholar]
- Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. Embo J. 2002;21:3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Weston JA. Temperature-sensitive mutations that cause stage-specific defects in Zebrafish fin regeneration. Genetics. 1995;141:1583–1595. doi: 10.1093/genetics/141.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol. 2003;5:827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J Biol Chem. 2002;277:28372–28375. doi: 10.1074/jbc.C200324200. [DOI] [PubMed] [Google Scholar]
- Kiehle CP, Schubiger G. Cell proliferation changes during pattern regulation in imaginal leg discs of Drosophila melanogaster. Dev Biol. 1985;109:336–346. doi: 10.1016/0012-1606(85)90460-9. [DOI] [PubMed] [Google Scholar]
- Loo LW, Secombe J, Little JT, Carlos LS, Yost C, Cheng PF, Flynn EM, Edgar BA, Eisenman RN. The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol Cell Biol. 2005;25:7078–7091. doi: 10.1128/MCB.25.16.7078-7091.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J, Omelyanchuk L, Kyttala S, Turunen H, Nokkala S. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int J Dev Biol. 2005;49:391–399. doi: 10.1387/ijdb.052006jm. [DOI] [PubMed] [Google Scholar]
- Mattila J, Omelyanchuk L, Nokkala S. Dynamics of decapentaplegic expression during regeneration of the Drosophila melanogaster wing imaginal disc. Int J Dev Biol. 2004;48:343–347. doi: 10.1387/ijdb.041847jm. [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G. A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling. Development. 1998;125:115–124. doi: 10.1242/dev.125.1.115. [DOI] [PubMed] [Google Scholar]
- McClure KD, Sustar A, Schubiger G. Three genes control the timing, the site and the size of blastema formation in Drosophila. Dev Biol. 2008;319:68–77. doi: 10.1016/j.ydbio.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Cranna N, Richardson H, Quinn L. The Ecdysone-inducible zinc-finger transcription factor Crol regulates Wg transcription and cell cycle progression in Drosophila. Development. 2008;135:2707–2716. doi: 10.1242/dev.021766. [DOI] [PubMed] [Google Scholar]
- Moreno E, Yan M, Basler K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol. 2002;12:1263–1268. doi: 10.1016/s0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Morgan TH. Regeneration. The MacMillan Company; New York: 1901. [Google Scholar]
- Motzny CK, Holmgren R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev. 1995;52:137–150. doi: 10.1016/0925-4773(95)00397-j. [DOI] [PubMed] [Google Scholar]
- Muller TL, Ngo-Muller V, Reginelli A, Taylor G, Anderson R, Muneoka K. Regeneration in higher vertebrates: limb buds and digit tips. Semin Cell Dev Biol. 1999;10:405–413. doi: 10.1006/scdb.1999.0327. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Cohen SM. Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development. 1995;121:589–599. doi: 10.1242/dev.121.2.589. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Vincent JP, Wu J, Cohen SM. Specification of the wing by localized expression of wingless protein. Nature. 1996;381:316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- O’Brochta DA, Bryant PJ. Distribution of S-phase cells during the regeneration of Drosophila imaginal wing discs. Dev Biol. 1987;119:137–142. doi: 10.1016/0012-1606(87)90215-6. [DOI] [PubMed] [Google Scholar]
- Orian A, Grewal SS, Knoepfler PS, Edgar BA, Parkhurst SM, Eisenman RN. Genomic binding and transcriptional regulation by the Drosophila Myc and Mnt transcription factors. Cold Spring Harb Symp Quant Biol. 2005;70:299–307. doi: 10.1101/sqb.2005.70.019. [DOI] [PubMed] [Google Scholar]
- Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–912. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1:144–154. doi: 10.1242/dmm.000950. discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Ryder E, Ashburner M, Bautista-Llacer R, Drummond J, Webster J, Johnson G, Morley T, Chan YS, Blows F, Coulson D, et al. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics. 2007;177:615–629. doi: 10.1534/genetics.107.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- Simpson P, Berreur P, Berreur-Bonnenfant J. The initiation of pupariation in Drosophila: dependence on growth of the imaginal discs. J Embryol Exp Morphol. 1980;57:155–165. [PubMed] [Google Scholar]
- Pierre SE, Galindo MI, Couso JP, Thor S. Control of Drosophila imaginal disc development by rotund and roughened eye: differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development. 2002;129:1273–1281. doi: 10.1242/dev.129.5.1273. [DOI] [PubMed] [Google Scholar]
- Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sustar A, Schubiger G. A transient cell cycle shift in Drosophila imaginal disc cells precedes multipotency. Cell. 2005;120:383–393. doi: 10.1016/j.cell.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Itoh S, ten Dijke P, Tabata T. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol Cell. 2000;5:59–71. doi: 10.1016/s1097-2765(00)80403-7. [DOI] [PubMed] [Google Scholar]
- Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Thacker SA, Bonnette PC, Duronio RJ. The contribution of E2F-regulated transcription to Drosophila PCNA gene function. Curr Biol. 2003;13:53–58. doi: 10.1016/s0960-9822(02)01400-8. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Tapon N, Kanda H, Cigizoglu S, Edelmann L, Pellock B, White K, Hariharan IK. Capicua regulates cell proliferation downstream of the receptor tyrosine kinase/ras signaling pathway. Curr Biol. 2007;17:728–733. doi: 10.1016/j.cub.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Vorwerk K, Carroll SB. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature. 1994;368:299–305. doi: 10.1038/368299a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama H. Initiation of limb regeneration: the critical steps for regenerative capacity. Dev Growth Differ. 2008;50:13–22. doi: 10.1111/j.1440-169X.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G. Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development. 2007;134:3001–3010. doi: 10.1242/dev.006411. [DOI] [PubMed] [Google Scholar]
- Zecchini V, Brennan K, Martinez-Arias A. An activity of Notch regulates JNK signalling and affects dorsal closure in Drosophila. Curr Biol. 1999;9:460–469. doi: 10.1016/s0960-9822(99)80211-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.