Abstract
Tendon reconstruction using grafts often results in adhesions that limit joint flexion. These adhesions are precipitated by inflammation, fibrosis, and paucity of tendon differentiation signals during healing. To study this problem, we developed a mouse model in which the FDL tendon is reconstructed using a live autograft or a freeze-dried allograft and identified Gdf5 as a therapeutic target. Here we investigate the potential of rAAV-Gdf5 coated freeze-dried tendon allografts as “therapeutically-endowed” tissue engineering scaffolds to reduce adhesions. In reporter gene studies we demonstrate that rAAV-coated tendon allografts mediate efficient transduction of adjacent soft tissues, with expression peaking at 7-days. We also demonstrate that rAAV-Gdf5 vector significantly accelerates wound healing in an in vitro fibroblast scratch model, and when loaded onto freeze-dried FDL tendon allografts significantly improves the metatarsophalangeal joint flexion compared to rAAV-lacZ controls. Collectively, our data demonstrate the feasibility and efficacy of therapeutic tendon allograft processing as a novel paradigm in tissue engineering to address difficult clinical problems such as tendon adhesions.
Keywords: tendon, allograft, adhesions, recombinant adeno-associated virus, growth and differentiation factor 5
Introduction
Successful repair of ruptured flexor tendons, as measured by return of digital flexion function, is a great challenge to hand surgeons because the biological cascade of events during healing often causes the tendon proper to adhere indiscriminately to its surrounding tissue [1]. Clinical and experimental observations suggest that formation of adhesions is precipitated by injury to the tendon sheath, surgical manipulation, and immobilization [2,3]. This problem is most challenging to tendon injuries in the “no-man’s land” or Zone II, which in the past were not repaired due to their poor prognosis [4]. As an alternative to primary repair, the transplantation of a tendon graft allows the surgeon to place the graft junctures outside of the confines of the flexor sheath in zone II, where they can be attached distally in Zone I where no gliding motion takes place and proximally in Zone III to the FDP tendon. However, even simple surgical manipulation of live flexor tendon grafts can result in cellular necrosis and inflammation leading to adhesion [4]. Therefore, devitalized structures such as freeze-dried tendon allografts or tissue engineered biomaterial scaffolds are potentially attractive alternatives to live autografts in reconstructing the digital flexor mechanism.
Current tissue engineering strategies using synthetic biomaterial scaffolds have yet to yield clinically usable tendon substitutes. The appeal of these engineered scaffolds is that they can potentially be by impregnated with growth factors or genes for targeted and timed-release at the site of implantation to improve healing. However, many of these “manufactured” scaffolds do not match the mechanical strength of native tissue necessary for expedited return to function and they do not remodel in response to daily activity; rather they breakdown producing byproducts that induce inflammation and compromise the repair process [5]. As an alternative, naturally derived materials processed from animal tissue or produced using recombination technology maybe better tolerated when implanted. Arguably, the most suitable choice for a naturally-derived biomaterial scaffold for tendon tissue engineering would be one that is derived from “allogeneic” tendon tissue. Such scaffolds must meet several functional criteria. As aptly described by Whitlock et al., a naturally derived biomaterial scaffold from tendon tissue must be “amenable to host cell mediated remodeling”, “devoid of cellular material to minimize inflammatory potential”, and “distinguished by sufficient biomechanical integrity” [5].
To test this concept in a preclinical model of tendon adhesion, we recently developed a mouse distal flexor digitorum longus (FDL) tendon grafting model in which a 3 mm intercalary live autograft or freeze-dried allograft is implanted [6]. We demonstrated that both autografts and allografts experienced significant reductions in the range of motion (ROM) of the metatarsophalangeal (MTP) joint at 14 and 28 days which resolved 42 days after surgery. Interestingly, we also observed that the gene expression of the growth and differentiation factor 5 (Gdf5) was significantly increased in 28-day grafts, implicating this factor in the remodeling that leads to the functional improvements observed thereafter. We were intrigued by this observation since GDF-5 deficiency in mice significantly delays the healing of the Achilles tendon [7] and adenovirus-mediated Gdf5 gene expression in rat Achilles tendon injuries leads to increased strength [8]. Based on these findings and our previous success with rAAV-coated cortical bone grafts [9,10], we hypothesized that loading freeze-dried mouse FDL tendon allografts with recombinant adeno-associated vector (rAAV) expressing Gdf5 gene will improve the functional properties of the reconstructed tendon and abolish the fibrotic adhesions. In this study, we report that the remarkable hydrophilic capacity of freeze-dried tendon allografts can indeed be exploited for efficient loading of gene delivery vectors such as rAAV-Gdf5 to improve the functional outcome of flexor tendon reconstruction.
RESULTS
Processing Freeze-Dried FDL Tendon Allografts as Gene Delivery Scaffolds
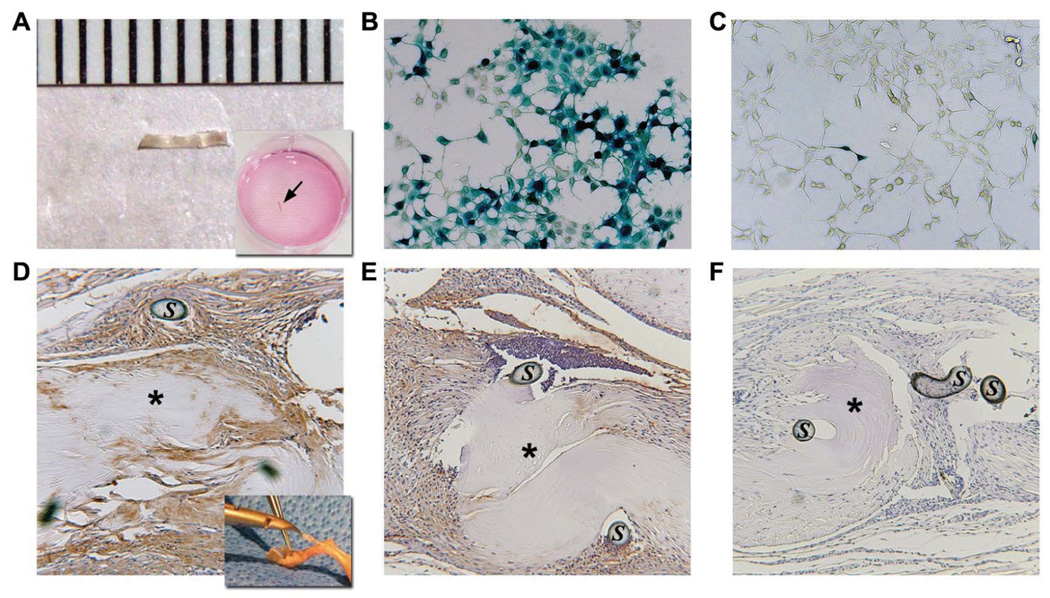
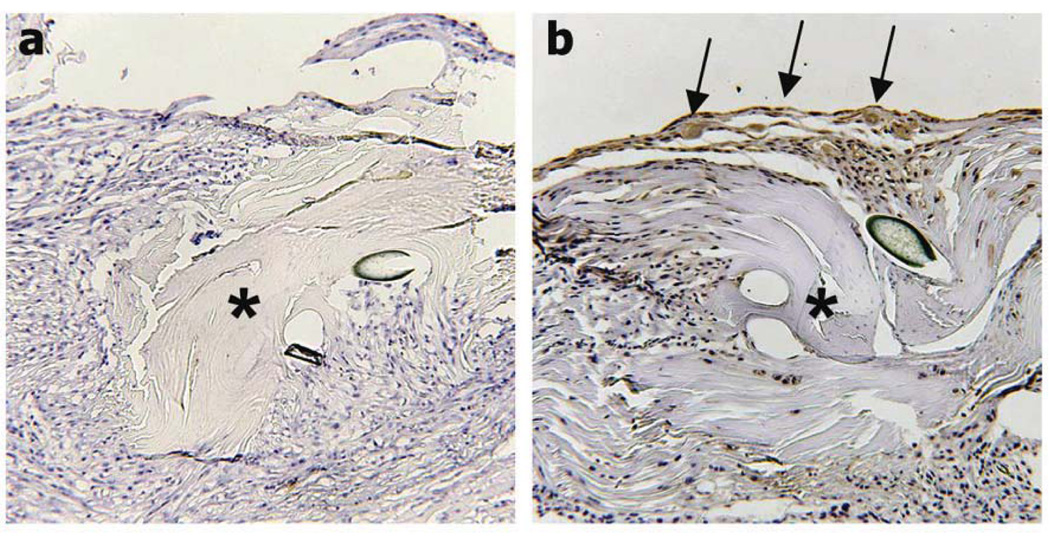
We have previously shown that 2 freeze-drying cycles produce no adverse effects on the mechanical properties of the tendon grafts [6]. Thus, pairs of freeze-dried FDL tendon grafts were reconstituted in a buffer containing 5×109 units of rAAV-lacZ and freeze-dried again. PCR analysis determined the rAAV retention efficiency to be ~10% (2.61×108 ± 1.44 ×108 genomes per graft, mean ± standard deviation; See Fig. S1 online). To assess the efficacy of cell transduction in vitro, rAAV-lacZ loaded FDL grafts were individually placed in culture wells containing 293 human embryonic kidney (HEK) cells (Fig. 1a). X-gal staining showed that large numbers of cells were transduced after 48-hour incubation with the rAAV-lacZ loaded FDL grafts (Fig. 1b), whereas control culture incubated with unloaded FDL grafts were negative (not shown). Furthermore, the random and attenuated β-galactosidase expression in regions of the wells away from the grafts indicates that the transduction is dependent upon diffusion of the virus after rehydration (Fig. 1c). To assess the transduction efficacy of rAAV-loaded FDL grafts in vivo, freeze-dried FDL tendon allografts loaded with rAAV-lacZ were implanted in mouse intercalary FDL tendon defects as previously described [6]. The mice were sacrificed at 7 and 14 days and the grafted tissues were harvested and fixed, paraffin-embedded, and processed for immunohistochemistry with antibodies specific to β-galactosidase. Although the grafts remained mostly acellular, they were surrounded by exuberant hyper-cellular fibrotic tissue that exhibited intense staining specific for β-galactosidase suggesting that the host cells in the peripheral tissue were transduced by the rAAV-lacZ vector as it slowly diffused out of the implanted graft, with more intense staining on day 7 versus day 14 (Fig. 1d,e).
Figure 1.
Transduction efficacy of freeze-dried tendon grafts in vitro and in vivo. 3mm freeze-dried mouse FDL tendon allografts (a) were loaded with 5×109 transducing units of rAAV-lacZ, and incubated on a confluent monolayer of HEK 293 cells for 48 hours (arrow). Representative micrographs of x-gal stained cultures show large numbers of LacZ+ cells proximal to the graft (b), and sparse staining in peripheral fields away from the graft (c). rAAV-lacZ loaded FDL allografts were also transplanted into FDL tendon defects of mice (n=4). Representative micrographs of one end of the rAAV-lacZ loaded FDL allografts stained with antibodies against β-galactosidase at 7 days (d) and 14 days (e) post-transplantation. Of note is the lack of viable cells and any staining in the freeze-dried allografts (asterisks) that are surrounded by hypercellular and intensely stained fibrotic tissue. (C) The specificity of the staining was verified by the absence of non-specific staining in negative controls (secondary antibody only – f). S indicates remnants of the repair suture.
Kinetics and Biodistribution of Tendon Allograft Mediated Gene Delivery
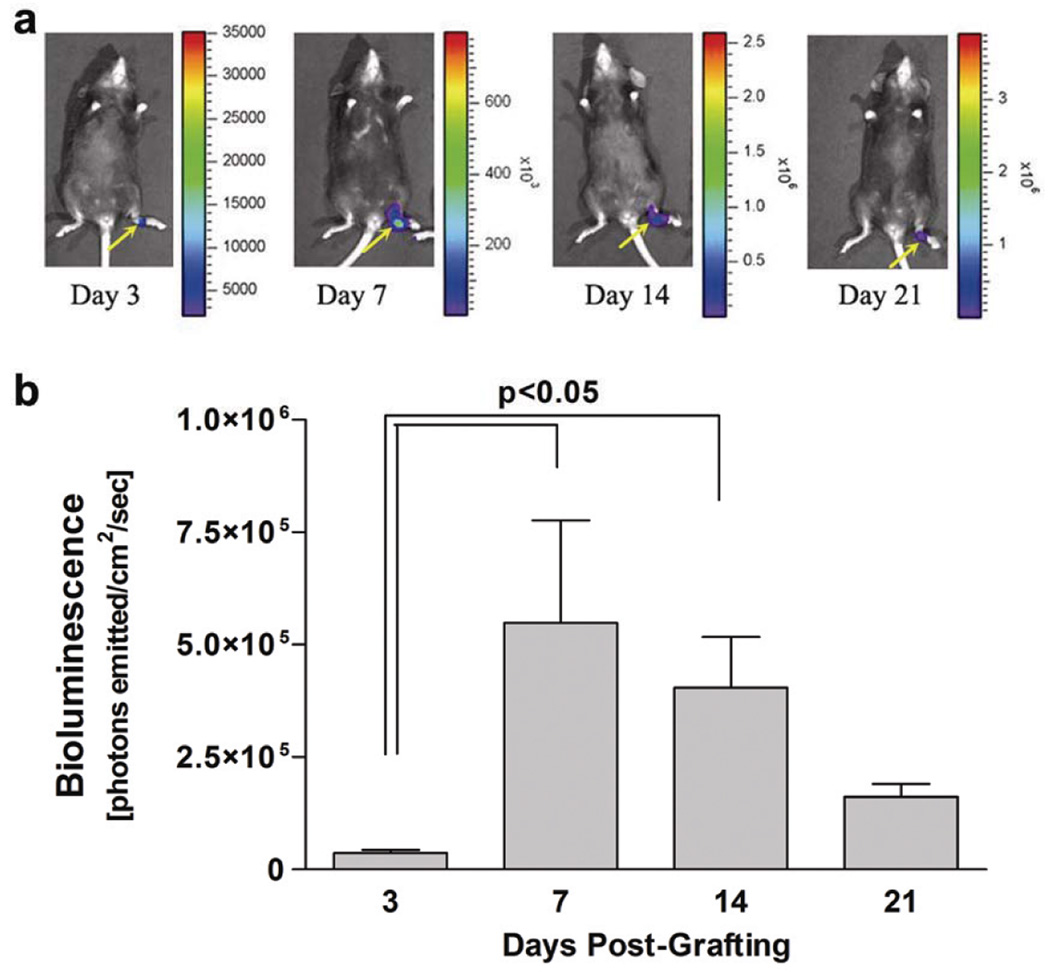
We next set out to determine the kinetics and biodistribution of the tendon allograft-mediated gene delivery in vivo. FDL tendon allografts (3 mm in length) were freeze-dried, then reconstituted in a PBS solution in a vial containing 5×109 particles of rAAV-Luc and freeze-dried until surgically implanted in FDL tendon defects. To assess transduction in vivo over time, the mice were imaged on days 3, 7, 14, and 21 post grafting (n=4 mice) using a real-time bioluminescent imaging (BLI) system. As hypothesized, the only detectable BLI signal was localized to the site of tendon grafting further supporting the efficacy of targeted gene delivery via processed tendon allografts. Furthermore, the transduction was transient as the BLI signal peaked at day 7 but persisted, albeit at declining levels, up to 21 days after implantation (Fig. 2b). More sensitive analysis (e.g. PCR) will be needed, however, to determine the reporter gene biodistribution in distant tissues and organs.
Figure 2.
Kinetics and biodistribution rAAV-mediated transduction via processed tendon allografts. Temporal bioluminescence images (BLI) of a representative mouse grafted with a freeze-dried FDL tendon allograft loaded with rAAV-Luc over 21 days show the localized biodistribution of rAAV-Luc transduction (heat map-yellow arrows) at the site of allograft implantation in the hind foot (a). Kinetics of in vivo rAAV transduction (b) based on average BLI signal intensity computed from measurements of total integrated light signal (photons emitted/cm2/sec) emitted from a standardized region of interest (ROI) in a standard 3 minute time interval (mean ± SEM; n=4).
Functional Verification of rAAV-Gdf5
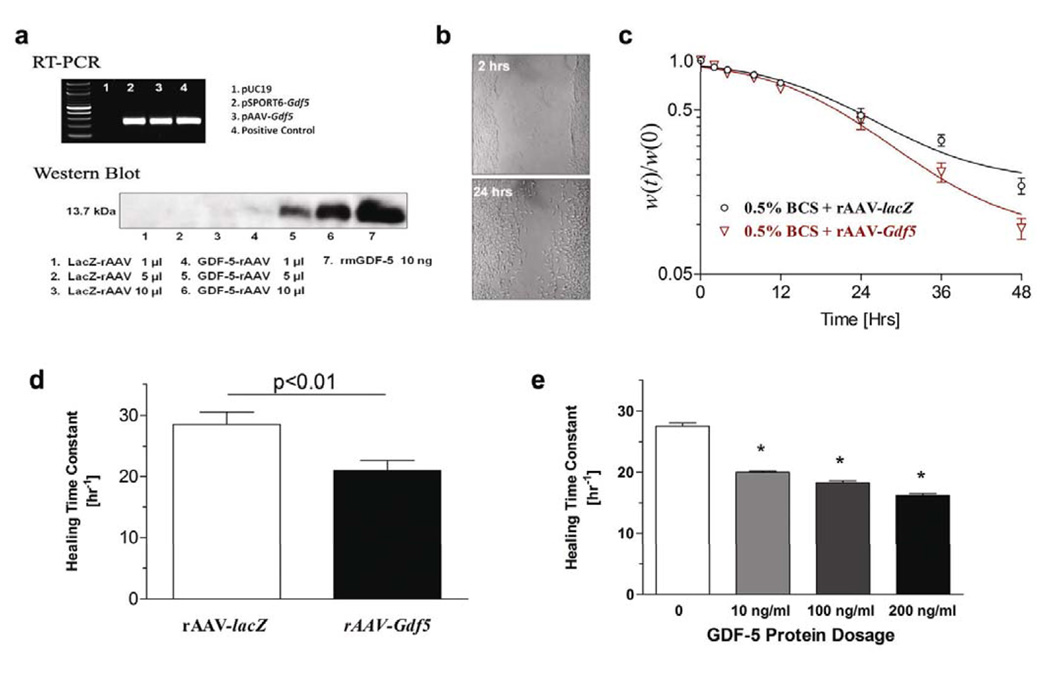
The cDNA for Gdf5 was PCR amplified and used to create a plasmid (pAAV-Gdf5) which was used to produce the rAAV through a helper virus-free method and purified as previously described [10]. To verify the specificity of the vector, we performed RT-PCR on pAAV-Gdf5 transfected HEK293 cells and demonstrated the predicted 485bp PCR product (Fig. 3a; Top). Western blots on culture supernatants from rAAV-Gdf5 infected HEK293 cells also demonstrated the predicted 13.7 kDa GDF-5 protein (Fig. 3a; Bottom). The effects of rAAV-Gdf5 gene delivery were evaluated in vitro using a standard microwound monolayer assay (Fig. 3b). These experiments demonstrated that infecting NIH 3T3 cells with rAAV-Gdf5 leads to accelerated wound healing compared to rAAV-lacZ treated controls (Fig. 3c). We further estimated the healing time constant which showed significant differences in of the healing rate of the rAAV-Gdf5 treated wells compared to controls (p<0.05; Fig. 3d). It is likely that the rAAV-Gdf5 effect in this experiment might be masked by the innate ability of the 3T3 cells to proliferate even under serum-deprived, control conditions. Real time PCR analysis indicated that the accelerated microwound healing rates were attributed to significant early induction in Cyclin D1 and β1-integrin mRNA expression suggesting a synergistic proliferation and migratory effect of rAAV-Gdf5 (not shown). In parallel experiments, we treated microwounded cultures of 3T3 cells with various concentrations of rmGFDF5 protein, and demonstrated a dose-dependent acceleration of healing with the treatment (Fig. 3e). Interestingly, the effects of rAAV-Gdf5 delivery on the microwound healing rate were comparable to the effects of bolus delivery of the GDF-5 protein to these cultures.
Figure 3.
Functional verification of the rAAV-Gdf5 vector. HEK293 cells were grown in 6-well plates and transfected with: 1) pUC19, 2) pSPORT-Gdf5, or pAAV-Gdf5, and 48hrs later total RNA was harvested from the cells. The mRNA was reverse transcribed and used as the template for PCR with Gdf5 specific primers. 4) The pSPORT-Gdf5 plasmid was used for template in the positive control. The ethidium bromide stained agarose gel shows the predicted 485bp PCR product (a – Top). HEK293 cells were grown in 6-well plates and infected with the indicated amount of rAAV-lacZ or rAAV-Gdf5 (5.0×107 particles per ml). After 48hrs of culture, the supernatants were collected and 30µl was used for Western blotting with GDF-5 specific antibodies. 10ng of recombinant murine GDF-5 was used as a positive control. Autoradiography of the Western blot reveals the predicted 13.7kDa GDF-5 protein (a – Bottom). Microwound monolayer assay: 80% confluent 3T3 cells were growth arrested for 24 hours then microwounded by passing a pipette tip across the culture well (b) and treated with 0.5% bovine calf serum (BCS) and 5.0×107 particles/ml of either rAAV-lacZ or rAAV-Gdf5. The average width of the defect was digitally measured over time and the wound width normalized to the time zero width [w(t)/w(0)] versus time was plotted(c). Healing time constants (τ) for the different treatments were computed and plotted as mean ± SEM (d). Note that higher τ values indicate slower wound healing rates. In a separate experiment, 3T3 cells grown to 80% confluence were microwounded and treated with 0.5% BCS and incremental doses of rmGDF-5. Data presented are mean ± SEM for the healing time constant (τ) for the different doses of the GDF-5 protein treatments (e). Asterisks indicate significant differences (p<0.01; n=6 per treatment) compared to untreated controls.
Gdf5 Targeted Gene Delivery for Freeze-Dried Flexor Tendon Allografts
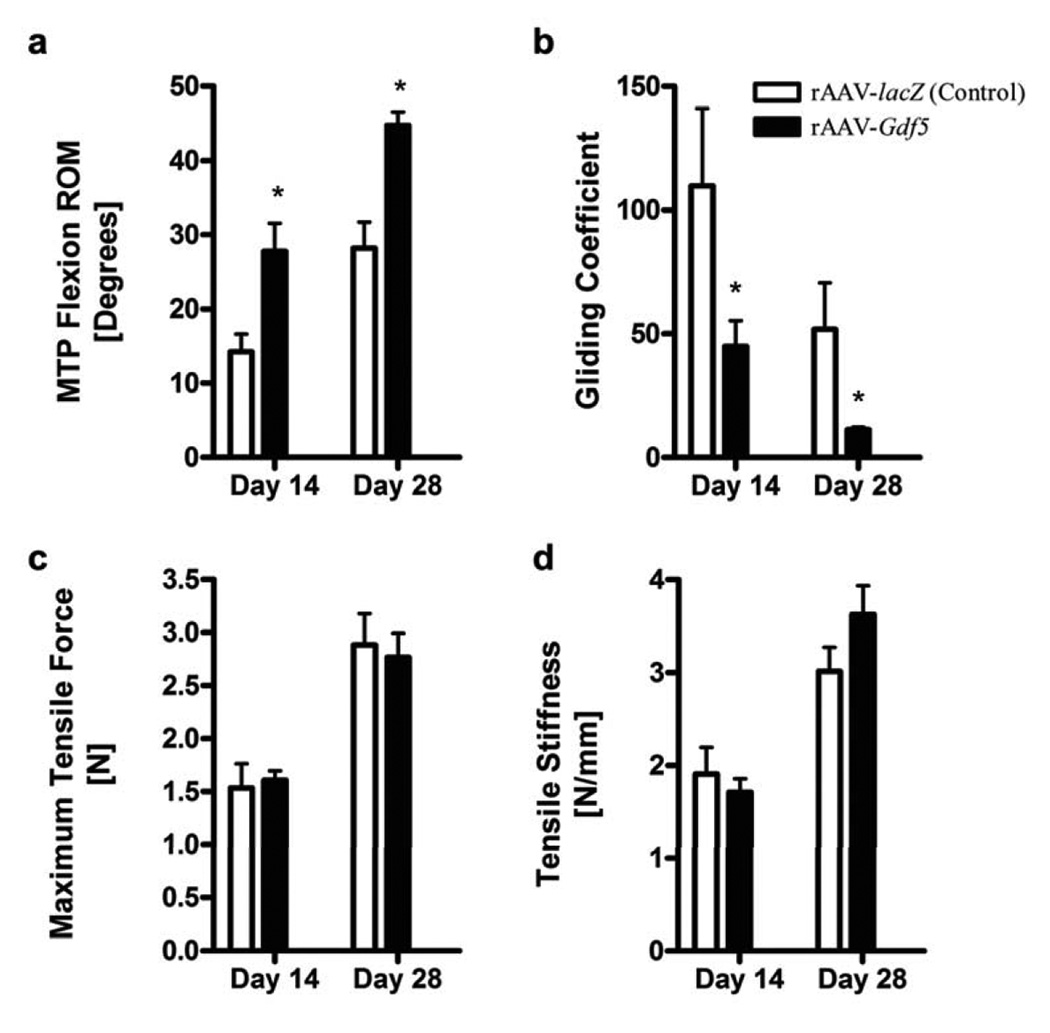
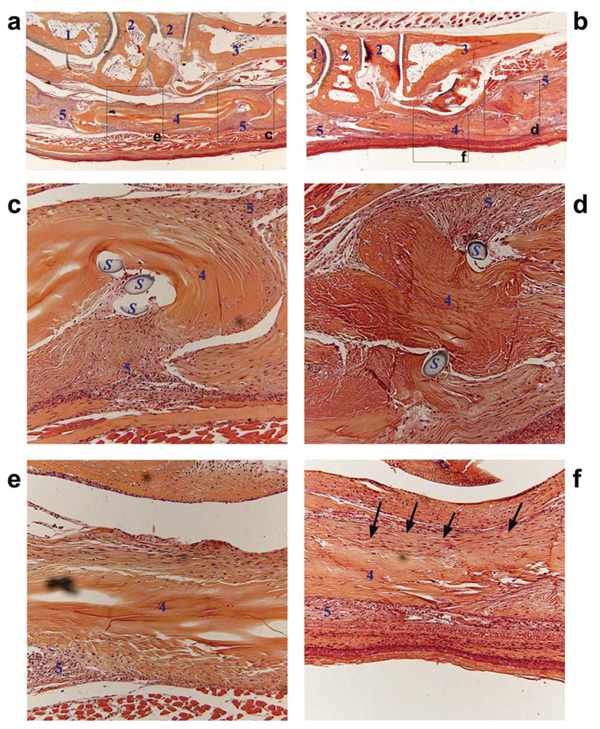
To investigate whether tendon allografts processed as delivery vehicles for therapeutic genes can reduce adhesions and improve the biomechanical properties of the grafted tendons, we performed experiments with FDL tendon allografts loaded with rAAV-lacZ (controls) or rAAV-Gdf5 (treated) in our murine model. MTP flexion tests (See Fig. S2 online) demonstrated that rAAV-Gdf5 loaded allografts had significantly improved range of joint flexion and decreased Gliding Coefficient compared to lacZ control (p<0.05; Fig. 4) at 14 and 28 days post operatively. The flexion function improved over time between 14 and 28 days for both treatments, but the improvement of the rAAV-Gdf5 loaded grafts were still significantly greater than the lacZ controls. There were also trends of increased tensile mechanical properties (maximum force and stiffness) over time, but there were no significant differences between the Gdf5 and lacZ treated grafts. Furthermore, tendons from mice sacrificed at 14 days were harvested and fixed, paraffin-embedded, and processed for immunohistochemistry with anti-mouse GDF-5 antibody. The data demonstrate positive anti-GDF-5 staining of host cells (arrows) surrounding the grafts loaded with rAAV-Gdf5 that is absent in the rAAV-lacZ loaded controls, further validating the efficacy of Gdf5 gene delivery (Fig. 5). Next we histologically examined the implanted allografts at 14 days post surgery (Fig. 6). Both Gdf5 treated and lacZ control allografts were surrounded by hypercellular fibrotic tissue at the junction with the host tendon, which could have contributed to impairment of gliding, and hence reduction in the flexion ROM (Fig. 6c,d). However, there were marked differences in the morphology of the middle segment of the grafts. Wherein the rAAV-Gdf5 treated graft was surrounded by organized tissue that resembles neotendon and integrates with the graft, which itself appears to have been repopulated by cells (Fig. 6f), the rAAV-lacZ control allograft was mostly acellular and was surrounded with disorganized and hypercellular fibrotic tissue (Fig. 6e). However, additional assays such as immunohistochemistry (for Collagen types I and III, for example) are needed to confirm these observations.
Figure 4.
rAAV-Gdf5 loading of freeze-dried allografts improves the MTP flexion range of motion and the gliding function of reconstructed FDL tendons while maintaining their biomechanical properties. Mice had their FDL tendons reconstructed with freeze dried allografts loaded with rAAV-Gdf5 (treated) or rAAV-lacZ (controls) and sacrificed at 14 and 28 days post-operatively (n=9 per treatment per time point). The operated hind feet were harvested and subjected to the MTP flexion test to determine the MTP joint flexion ROM (a) and the Gliding Coefficient (b). The tendons were then isolated and tested biomechanically to determine their breaking (maximum) tensile force (c) and their linear tensile stiffness (d). Data presented are mean ± SEM. Asterisks indicate significant differences compared to time-matched controls (p<0.05).
Figure 5.
rAAV-Gdf5 loading of freeze-dried allografts mediates de novo GDF-5 protein synthesis by the host cells at the periphery of the implanted allograft. Representative immunohistochemical sections of the rAAV-lacZ (a) or rAAV-Gdf5 (b) loaded FDL tendon allografts at 14 days post grafting and stained with anti-mouse GDF-5 antibody. Of note is the matrix-bound GDF-5 (positive staining indicated by arrows) presumably synthesized by the transduced host cells surrounding the rAAV-Gdf5 treated allografts (asterisk) that is absent in the rAAV-lacZ treated graft.
Figure 6.
rAAV-Gdf5 loading of freeze-dried allografts mediates cellular repopulation of the graft and remodeling of the fibrotic scar tissue. Representative histological sections of the rAAV-lacZ (a,c,e) or rAAV-Gdf5 (b,d,f) loaded FDL tendon allografts at 14 days post grafting and stained with Alcian Blue and Orange G. Micrographs at 4X (a and b) show the implanted grafts with their anatomical relationships to the surrounding tissue. Boxed regions are shown in the magnified micrographs (20X) that show the distal ends of both grafts (c and d) and the middle segment of the grafts (e and f). Tissues marked by numbers are: 1) Talus, 2) Tarsal bones, 3) Metatarsal bone, 4) FDL tendon allograft, and 5) fibrotic/inflammatory tissue. S indicates remnants of suture. Arrows in f indicate a remodeled tissue that appears to align and integrate with the rAAV-Gdf5 loaded allograft which also seems to have been repopulated with host cells compared to the mostly acellular rAAV-lacZ loaded allograft (e).
DISCUSSION
Tendon, ligament, and joint capsular injuries represent 45% of the almost 33 million musculoskeletal injuries each year in the United States and hand injuries account for 5–10% of annual emergency department visits nationwide [11]. While flexor tendon injuries might represent only a small fraction of these statistics, adhesion formation associated with tendon surgery in general is a much more widespread problem [12]. This problem is not only limited to primary repair, but has also been reported in response to tendon reconstruction using autografts or allografts [13] as an inevitable byproduct of the biological cascade of events in tendon healing. It is well established that tendon healing consists of three phases: inflammatory, proliferative, and remodeling. The inflammatory phase involves the recruitment of fibroblasts and macrophages to the site of injury and phagocytosis of the necrotic tissue. The second phase involves the proliferation of fibroblasts and the formation of a repair scar composed from immature and disorganized collagen matrix. Remodeling then follows in the final phase as the immature collagen fibers in the scar tissue become organized and align with the tendon fibers. While the etiology of adhesion formation has been clinically linked to this last phase [1], it is hypothesized that adhesions result from post-operative inflammation that extends to surrounding tissue [14]. Clinical and experimental strategies to abrogate the adhesion formation can be categorized into either mechanical or biochemical intervention protocols. Mechanical strategies include postoperative passive motion and rehabilitation protocols early after surgery [3,15]; optimized surgical techniques that involve minimally-traumatic manipulation of the tendon, graft, and surrounding tissues [16]; and the use of anti-adhesion surface coating of the graft as physical barriers against adhesion formation [12,17]. Biological and biochemical intervention strategies primarily rely upon growth factor delivery to accelerate the rate of tendon healing and remodeling [18,19]. While a number of growth factors [19–24] could potentially improve the repair of tendon, their effects on tendon adhesion have gone mostly unstudied. A rational design for a growth-factor delivery therapy should arguably be based on the natural history of gene expression of growth factors during the different phases of tendon repair, a thorough understanding of the molecular action of these factors, and a sustained delivery mechanism to maximize the factors’ therapeutic effects.
In our mouse FDL tendon grafting model, we previously observed that that Gdf5 mRNA levels were significantly increased in 28-day autografts [6]. This increase in Gdf5 levels was concomitant with the observed increases in Vegfa expression, which is consistent with other reports [25]. Interestingly, the timing of the peak in Gdf5 and Vegfa gene expression was coincidental with the marked improvements in MTP joint flexion observed thereafter [6]. Based on this observation, we hypothesized that GDF-5 plays an important role in the remodeling phase and that exogenous delivery of paracrine GDF-5 signals will accelerate remodeling and lead to functional improvements in joint flexion. GDF-5 belongs to the cartilage derived morphogenetic proteins (also known as CDMP-1, brachypodism (BP) or bone morphogentic protein 14 (BMP-14)). This TGF-β subfamily of proteins, which in addition to GDF-5 includes GDF-6 and GDF-7 (CDMP-2 and -3 or BMP-13 and -12), has been shown to be important for skeletal development in general and for tendon formation and repair in particular [26,27]. GDF-5 effects on cell recruitment, migration/adhesion, differentiation, proliferation, and angiogenesis in vitro and in vivo [7] as well as its effects on the ultrastructure of the collagen fibrils and the biomechanical properties of normal and repair tendon tissue have been reported [7,28]. However the exact mechanism of GDF-5 action in tendon repair has yet to be deciphered. It has been reported that GDF-5 binds with activin receptor-like kinase 3 (ALK-3) and/or ALK-6 (also termed BMP type IA and type IB receptors, respectively) [29,30] and this presumably activates the Smad signaling pathway. A recent study demonstrated that constitutive activation and nuclear translocation of Smad8 upregulated Scelraxis transcription factor and led to tendon formation in C3H10T1/2 cells (a murine multipotent cell line) which when implanted on a collagen sponge into a rat Achilles tendon gap tenotomy model led to formation of tendon like-tissue [31]. The hypothesis that GDF-5 might be the paracrine signal that leads to Smad8 activation remains an intriguing possibility despite some preliminary observations to the contrary [31].
Next we were faced with the choice of the GDF-5 delivery mechanism. The therapeutic window of bolus or topical delivery is not long as the signaling is almost instantly initiated and short-lived [32]. Alternatively, local transfer of genes that express the relevant healing factors may mediate sustained expression of these factors. The efficacy of different viral vector systems (including retrovirus and adenovirus) in mediating targeted and transient gene transfer in tendon repair has been demonstrated in vitro and in vivo [8,33]. In a recent study, direct injection of recombinant adenovirus vectors expressing green fluorescent protein (AdGFP) or BMP-13 (AdBMP-13 or AdGDF-6) into rabbit flexor tendons demonstrated a transient dose-dependent transgene expression up to 12 days in vivo [34]. These results are consistent with our data that demonstrated that rAAV loading of freeze-dried FDL allograft mediates targeted and transient gene expression by host cells at the implant site that peaks by 7 days but persists up to 21 days. Notwithstanding its known safety concerns that are abated by low dose vector delivery, rAAV-based gene therapy can potentially be a therapeutic option for musculoskeletal tissue (including tendon) reconstruction due to its localized and transient expression.
Finally, given our previous findings which suggested that freeze-dried tendon allografts appear to have been tolerated well in the host mouse and provided biomechanical scaffolding functions equivalent to those afforded by live autografts [6], we examined whether tendon allografts can serve as a therapeutic factors’ delivery scaffolds to mediate adhesion-free reconstruction of flexor tendon gap defects. Our data indeed show that despite the modest retention efficiency (~10%), freeze-dried FDL allografts loaded with rAAV-Gdf5 transduced local expression of the GDF-5 protein at 14 days that was associated with significantly improved range of flexion compared to rAAV-lacZ controls. While previous studies reported the presence of small foci of bone and fibrocartilage within ectopic tendon/ligament tissue in response to Ad-GDF-6 (or BMP-13) injections in athymic rats [35], we did not observe such untoward effects in our model.
Interestingly, while we observed beneficial functional effects of rAAV-Gdf5 on the grafted tendon, we did not observe significant effects on the biomechanical properties. Previous reports suggested that GDF-5 deficient mice displayed a delay in the accrual of biomechanical strength during the initial healing of the Achilles tendon compared to controls [7]. On the other hand, Dines et al (2007) reported in the rat model that lacerated Achilles tendons repaired with sutures coated with rhGDF-5 showed an increased rate of healing versus control repairs at 3 weeks [36]. In both studies, the mechanical properties of controls, GDF-5 deficient and GDF-5 augmented tendon repairs were equivalent at later time points. These results suggest that the effects of GDF-5 treatment might be temporally-sensitive and healing phase-dependent. Our results are not different than similar gene therapy based tendon repair studies, however. Rickert et al (2005) reported efficacious Adenovirus-mediated transfer of Gdf5 gene via injections of AdGDF-5 at the site of lacerations in the rat Achilles tendon that was not associated with any significant improvements in mechanical properties [8]. By contrast, Lou and co-workers (2001) previously reported that AdGDF-7 injections at the site of lacerations in chicken flexor digitorum profundus (FDP) tendon results in delayed but significant 1-fold improvements in mechanical properties at 6 weeks post-treatment [37]. It is therefore possible that other isoforms of the growth and differentiation factor such as GDF-7 have more potent effects on the mechanical properties of repairing tendon tissue. Other possibilities cannot be excluded. The lack of improvements in the mechanical properties in our rAAV-Gdf5 treated allografts compared to controls may be related to the dose of the treatment (number of rAAV particles transferred) which might have to be optimized in future studies, the efficiency of rAAV-mediated gene transfer, the absence of the interactions with in vivo forces in our model, and the observation that the transfected host cells resided in the external callus which resulted in remodeling of the fibrotic callus tissue, reduced adhesions, and improved the gliding function and not necessarily any remodeling of the graft tissue proper.
Based on these findings, we propose a simplified alternative paradigm in tissue engineering in which freeze-dried allograft tissue can be used to deliver cues to the host cells in situ to reprogram the repair response. Freeze-dried tendon allografts can provide these delivery functions with a number of desirable characteristics over synthetic and naturally derived biomaterials. Freeze-dried tendon allografts are biochemically unaltered as the lyophilization is purely a physical process that leads to dehydration of the tissue. They potentially have an indefinite shelf-life and will likely have less regulatory hurdles to clear en route to clinical applications since they can still be classified as allografts. Furthermore, freeze-dried tendon allografts have biomechanical properties equivalent to fresh or fresh frozen tendon tissue. Despite being devoid of live cells which privileges them against eliciting an immune response leading to graft rejection, they can be readily remodeled and populated by host cells upon implantation in vivo. Most importantly, freeze-dried tendon allografts have remarkable native hydrophilic properties that permit efficient reconstitution of the tissue in a physiologic solution containing therapeutic molecules. This concept could be applied to other tendon and ligament models including the Anterior Cruciate Ligament (ACL), Achilles Tendon (AT) and the supraspinatus “rotator cuff” tendon and could involve loading gene delivery vectors or recombinant or tissue-derived growth factors (See Fig. S3 online). Future development could also focus on differential processing (multiple genes and proteins) of composite allograft tissue (bone-tendon-bone) to address clinically challenging problems such as soft tissue insertion into bone. Furthermore, such technology can potentially be translatable to other musculoskeletal soft tissue models including articular cartilage and meniscus tissues.
MATERIALS & METHODS
rAAV preparation
rAAV-lacZ and rAAV-Luc stock solutions were purchased and the single stranded rAAV-Gdf5 vector (serotype 2), which was custom cloned from an existing plasmid (pAAV-Gdf5) containing a CMV promoter and the Gdf5 cDNA, was purified and titered at the Gene Therapy Center of the University of North Carolina, Chapel Hill.
Processing of tendon allografts
FDL tendon allografts were aseptically isolated, placed in sterile vials, frozen at −80°, and freeze-dried. To load the tendon grafts with rAAV vectors, pairs of tendons were soaked in a vial containing 50 µl of PBS solution containing 5×109 units of rAAV (lacZ, Luc, or Gdf5). After allowing the dehydrated grafts to uptake the solution for 1 hour, the grafts were snap-frozen and then freeze-dried and stored until used experimentally.
Real-time quantitative PCR assessment of rAAV retention in the allografts
rAAV-lacZ loaded FDL tendon grafts were digested in a buffer solution of proteinase K (10µg/ml) at 50°C for one hour and then at 95°C for 20 minutes to deactivate the enzyme. Samples from a serial dilution of digested virus at standard concentrations of 1010, 109, 108, 107, 106, 105, and 104 units were used to create a standard curve. Duplicate samples (2 µl) of each standard dilution along with samples from tendon digests were added to Real Time PCR Master Mix and allowed to react in a Rotor-Gene 2000 Real-Time DNA Detection System (Corbett Research, Sydney, Australia) for 40 cycles with a program of 94°C for 20 seconds, 61.6°C for 30 seconds, and 72°C for 30 seconds. The mean cycle threshold (Ct) values were used to calculate the rAAV content and retention efficiency in the tendon samples based on the standard curve.
Microwound Experiments
The microwond assay was performed as previously described by Hocking and Chang [38, 39]. Briefly, Mouse embryonic fibroblast (NIH3T3) cells were plated and allowed to grow to 80% confluence. The cells were serum deprived for 24 hours prior to creating wounds. Using a 100µl pipette tip wounds were created by scratching the pipette tip across the monolayer, resulting in wounds initially measuring 1.00mm (±0.20). Cells were then cultured with 0.5% bovine calf serum (BCS) and 5.0×107 particle units/ml of either rAAV-lacZ or rAAV-Gdf5. Digital photos of the microwound were taken at 0, 2, 4, 8, 12, 24, 36, and 48 hours. Using a custom Matlab program, the average width of each wound was measured at each time and normalized by the initial wound width (w(t)/w(0)). The data were fitted to the equation w(t)/w(0) = A/(B · exp(t/τ)+1) wherein τ represents the healing time constant such that wounds that heal faster would have a lower healing time constant.
Bioluminescent Imaging (BLI)
To demonstrate the efficacy of processed tendon allograft mediated gene delivery, freeze-dried allografts loaded with rAAV-Luc were implanted to reconstruct mouse FDL tendons as described below. Host cells transduced by this virus express the firefly luciferase gene. At each time point following implantation, we injected each mouse with the substrate D- Luciferin Potassium Salt (Xenogen), which when cleaved by the transduced luciferase enzyme emits light that can be captured using special camera system and software (IVIS 100 Bioluminescent Imaging System, Xenogen) and the bioluminescence intensity gradients can be represented by a heat map intensity (purple least intense and red most intense) computed from measurements of total integrated light signal (photons emitted/cm2/sec) emitted from a standardized region of interest (ROI) in a standard time interval (3 minute exposure).
Mouse FDL tendon grafting surgeries
Animal studies were conducted in compliance with principles and procedures approved by the University of Rochester Committee for Animal Resources. Surgeries were performed using aseptic technique under a 2X micro dissection magnifying lens. Briefly, a longitudinal plantar incision was made on the left hind foot. The distal FDL tendon of the C57Bl/6 mouse was isolated and transected on the plantar surface of the metatarsal bones. A 3 mm freeze-dried tendon allograft that had been previously harvested from a C57Bl/6 mouse donor and loaded with rAAV was reconstituted in PBS and sutured between the ends of host tendon using an 8-0 nylon suture in a horizontal mattress suture pattern (similar to a modified Kessler technique). The proximal tendon insertion into the flexor muscle was severed to eliminate early gliding in order to protect the repair during the early phases of healing and to induce adhesion formation. The skin was closed with 4-0 silk suture.
MTP joint flexion test
Immediately following sacrifice, the lower hind limb were disarticulated from the knee and the proximal FDL tendon along the tibia was released just proximal the tarsal tunnel without disrupting the skin at the ankle or foot. The proximal end of the tendon was secured between two square pieces of tape using a thin layer of cyanoacrylate as previously described 36. The lower hind limb was fixed in a custom apparatus where the tibia was rigidly gripped to prevent rotation. To standardize the neutral position, the toes were passively extended by the examiner and allowed to return to an unloaded position before a digital image was taken medially to determine the neutral position (zero load) of the MTP joint. The FDL tendon was incrementally loaded in the same anatomical direction as flexor muscle line of force using dead weights that were statically suspended from a hook and line passing through the proximal FDL tendon/tape composite. The dead weights were suspended for 30 seconds before the digital pictures were taken to avoid creep effects. With each increment of load, a digital image was taken to quantify the MTP flexion angle relative to the neutral position. The MTP joint flexion angles were measured from the digital images by 2 independent observers (PB and TD) using ImageJ software (http://rsb.info.nih.gov/ij/) and plotted versus the applied loads. The flexion data were fitted to a single-phase exponential association equation of the form: MTP Flexion Angle = β × [1 – exp(−m/α)]; where m is the applied load (Prism GraphPad 3.0, GraphPad Software, Inc., San Diego, CA). The curve fit was constrained to the maximum flexion angle (β) for normal tendons that was previously determined to be 75° for the maximum applied load. The constant α (Gliding Coefficient) governing the rate of rise of the flexion curve with loading was determined by nonlinear regression as a measure of the resistance to MTP flexion due to impaired gliding.
Biomechanical test
Following the MTP flexion test, the proximal extent of the FDL tendon was released at the tarsal tunnel, with dissection medially along the bone. Once the tendon was free from the tunnel, the calcaneus was removed, freeing the proximal end of the tendon for direct gripping in the mechanical test as described [40]. The distal bones of the foot were directly gripped in custom grips without disrupting the graft or the branching tendon insertion into the phalanges. The specimen was placed in sterile gauze soaked with saline to maintain adequate tissue hydration until tested. The FDL tendon was then mounted on the Instron 8841 DynaMight™ axial servohydraulic testing system (Instron Corporation, Norwood, MA) and tested using published protocols 36. The tendon was loaded in tension in displacement control at a rate of 30 mm/minute until failure. Force-displacement data were automatically logged and plotted and the maximum tensile force and stiffness were determined.
Histology and Immunohistochemistry
Grafted limbs were harvested by disarticulating the tibia from the knee joint. With the tibia perpendicular to the foot, the FDL tendon was kept in tension by passing a pin through the flexor muscles and the tibia. Tissues were then prepared for histology and analyzed using routine techniques. Briefly, the harvested limbs were fixed in 10% neutral buffered formalin, and decalcified in 10% EDTA at 4°C for 21 days. The decalcified tissues were dehydrated in a gradient of alcohols and then embedded en bloc in paraffin. Serial 3µm sagittal sections through the FDL tendon plane were prepared and stained with Alcian Blue and Orange G. For Immunohistochemistry the rAAV loaded tendon sections were stained with primary antibodies against β-galactosidase (PAb # GTX26646, GeneTex, Inc., San Antonio, TX) or against the murine GDF-5 (AF853, R&D Systems Inc., Minneapolis, MN). The tissue sections were then treated with appropriate biotin-conjugated secondary antibodies before developing with streptavidin-conjugated AEC chromogen (Zymed Laboratories Inc., San Francisco, CA).
Statistical Analysis
Data analysis including Analysis of Variance with Bonferroni post-hoc multiple comparisons (α=0.05) and the nonlinear regression analyses to estimate the Gliding Coefficient and from the MTP flexion data were performed using Prism GraphPad 4.0 statistical software.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Basile and Ms. Dadali share first authorship as they had equal contributions to the work. The Authors would like to thank Brendan Boyce for the valuable advice and stimulating discussions, Denise Hocking for advice on the in vitro microwound assay, Gwynne Bragdon for advice on surgeries, Krista Scorsone for technical assistance with the histology, David Reynolds for technical assistance with biomechanical testing, and Tony Chen for help with data analysis. This work was supported in part by research grants from the National Institutes of Health (PHS AR054041, AR051469, DE017096), Whitaker Foundation, the Danish Medical Research Council, the Musculoskeletal Transplant Foundation, the Orthopaedic Research Education Foundation (OREF), and DePuy J&J.
REFERENCES
- 1.Lilly SI, Messer TM. Complications after treatment of flexor tendon injuries. J Am Acad Orthop Surg. 2006;14:387–396. doi: 10.5435/00124635-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Gelberman RH, Manske PR. Factors influencing flexor tendon adhesions. Hand Clin. 1985;1:35–42. [PubMed] [Google Scholar]
- 3.Silva MJ, Boyer MI, Gelberman RH. Recent progress in flexor tendon healing. J Orthop Sci. 2002;7:508–514. doi: 10.1007/s007760200090. [DOI] [PubMed] [Google Scholar]
- 4.Leddy JP. Flexor Tendons - Acute Injuries. In: Green DP, editor. Operative Hand Surgery. Vol. 3. New York: Churchill Livingstone; 1988. pp. 1935–1968. [Google Scholar]
- 5.Whitlock PW, Smith TL, Poehling GG, Shilt JS, Van Dyke M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials. 2007;28:4321–4329. doi: 10.1016/j.biomaterials.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Hasslund S, Jacobson JA, Dadali T, Basile P, Ulrich-Vinther M, Soballe K, et al. Adhesions in a Murine Flexor Tendon Graft Model: Autograft versus Allograft Reconstruction. J Orthop Res. 2007 doi: 10.1002/jor.20531. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826–835. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 8.Rickert M, Wang H, Wieloch P, Lorenz H, Steck E, Sabo D, et al. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res. 2005;46:175–183. doi: 10.1080/03008200500237120. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–218. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Praemer A, Furner S, Rice D. Musculoskeletal Conditions in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 12.Meislin RJ, Wiseman DM, Alexander H, Cunningham T, Linsky C, Carlstedt C, et al. A biomechanical study of tendon adhesion reduction using a biodegradable barrier in a rabbit model. J Appl Biomater. 1990;1:13–19. doi: 10.1002/jab.770010104. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper MA, Laverty PH, Soiderer EE. Bilateral flexor tendon contracture following onychectomy in 2 cats. Can Vet J. 2005;46:244–246. [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80–85. doi: 10.1197/j.jht.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Hatano I, Suga T, Diao E, Peimer CA, Howard C. Adhesions from flexor tendon surgery: an animal study comparing surgical techniques. J Hand Surg [Am] 2000;25:252–259. doi: 10.1053/jhsu.2000.jhsu25a0252. [DOI] [PubMed] [Google Scholar]
- 17.Mentzel M, Hoss H, Keppler P, Ebinger T, Kinzl L, Wachter NJ, et al. The effectiveness of ADCON-T/N, a new anti-adhesion barrier gel, in fresh divisions of the flexor tendons in Zone II. J Hand Surg [Br] 2000;25:590–592. doi: 10.1054/jhsb.2000.0385. [DOI] [PubMed] [Google Scholar]
- 18.Towler DA, Gelberman RH. The alchemy of tendon repair: a primer for the (S)mad scientist. J Clin Invest. 2006;116:863–866. doi: 10.1172/JCI28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg [Am] 2004;29:551–563. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg [Am] 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang XT, Liu PY, Tang JB. Tendon healing in vitro: modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor beta but minimally affects expression of collagen genes. J Hand Surg [Am] 2005;30:222–229. doi: 10.1016/j.jhsa.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Abrahamsson SO, Lohmander S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. J Orthop Res. 1996;14:370–376. doi: 10.1002/jor.1100140305. [DOI] [PubMed] [Google Scholar]
- 24.Kashiwagi K, Mochizuki Y, Yasunaga Y, Ishida O, Deie M, Ochi M. Effects of transforming growth factor-beta 1 on the early stages of healing of the Achilles tendon in a rat model. Scand J Plast Reconstr Surg Hand Surg. 2004;38:193–197. doi: 10.1080/02844310410029110. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Q, Li X, Beck G, Balian G, Shen FH. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40:374–381. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Mikic B. Multiple effects of GDF-5 deficiency on skeletal tissues: implications for therapeutic bioengineering. Ann Biomed Eng. 2004;32:466–476. doi: 10.1023/b:abme.0000017549.57126.51. [DOI] [PubMed] [Google Scholar]
- 27.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark RT, Johnson TL, Schalet BJ, Davis L, Gaschen V, Hunziker EB, et al. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res. 2001;42:175–186. doi: 10.3109/03008200109005648. [DOI] [PubMed] [Google Scholar]
- 29.Schreuder H, Liesum A, Pohl J, Kruse M, Koyama M. Crystal structure of recombinant human growth and differentiation factor 5: evidence for interaction of the type I and type II receptor-binding sites. Biochem Biophys Res Commun. 2005;329:1076–1086. doi: 10.1016/j.bbrc.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 30.Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, et al. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci. 2001;114:1483–1489. doi: 10.1242/jcs.114.8.1483. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eming SA, Krieg T, Davidson JM. Gene transfer in tissue repair: status, challenges and future directions. Expert Opin Biol Ther. 2004;4:1373–1386. doi: 10.1517/14712598.4.9.1373. [DOI] [PubMed] [Google Scholar]
- 33.Gerich TG, Kang R, Fu FH, Robbins PD, Evans CH. Gene transfer to the patellar tendon. Knee Surg Sports Traumatol Arthrosc. 1997;5:118–123. doi: 10.1007/s001670050037. [DOI] [PubMed] [Google Scholar]
- 34.Mehta V, Kang Q, Luo J, He TC, Haydon RC, Mass DP. Characterization of adenovirus-mediated gene transfer in rabbit flexor tendons. J Hand Surg [Am] 2005;30:136–141. doi: 10.1016/j.jhsa.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Helm GA, Li JZ, Alden TD, Hudson SB, Beres EJ, Cunningham M, et al. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J.Neurosurg. 2001;95:298–307. doi: 10.3171/jns.2001.95.2.0298. [DOI] [PubMed] [Google Scholar]
- 36.Dines JS, Weber L, Razzano P, Prajapati R, Timmer M, Bowman S, et al. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg. 2007;16:S215–S221. doi: 10.1016/j.jse.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 38.Hocking DC, Chang CH. Fibronectin matrix polymerization regulates small airway epithelial cell migration. Am J Physiol Lung Cell Mol Physiol. 2003;285:L169–L179. doi: 10.1152/ajplung.00371.2002. [DOI] [PubMed] [Google Scholar]
- 39.Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 2000;28:499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- 40.Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res. 2001;19:365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.