Abstract
Myeloid Derived Suppressor Cells (MDSCs) are a heterogeneous population of myeloid cells that inhibit T cell activity and contribute to the immune suppression characteristic of most tumors. We discovered that bone marrow (BM) progenitor cells from the Muc1 knockout (KO) mice differentiated into CD11b+Gr1+ MDSCs in vitro under GM-CSF and IL-4 signaling. MUC1 is a tumor-associated mucin and its cytoplasmic tail (MUC1-CT) can regulate beta-catenin to promote oncogenesis. Given the importance of beta-catenin in hematopoiesis, we hypothesized that the MUC1 regulation of beta-catenin is important for MDSC development. Our current study shows that the aberrant development of BM progenitors into CD11b+Gr1+ MDSCs is dependent on the down regulation of beta-catenin levels that occurs in the absence of Muc1. In light of this, KO mice showed enhanced EL4 tumor growth and were able to better tolerate allogeneic BM185 tumor growth, with an accumulation of CD11b+Gr1+ cells in the blood and tumor draining lymph nodes. WT mice were able to similarly tolerate allogeneic tumor growth when they were injected with CD11b+Gr1+ cells from tumor-bearing KO mice, suggesting that tolerance of allogeneic tumors is dependent on MDSC-mediated immune suppression. This further delineates the ability of Muc1 to control MDSC development which could directly impact tumorigenesis. Knowledge of the biology by which Muc1 regulates the development of myeloid progenitors into MDSCs would also be very useful in enhancing the efficacy of cancer vaccines in the face of tumor immune suppression.
INTRODUCTION
The immune suppressive tumor microenvironment is a hallmark of cancer and a major obstacle to immune therapy. Myeloid Derived Suppressor Cells (MDSCs) are known to contribute to the immune suppression seen in cancer via mechanisms like IFN-γ-mediated production of nitric oxide (1), Th2-mediated arginase 1 pathway (2), ROS-mediated killing (2) or development of Foxp3+ T regulatory cells (3, 4). MDSCs, identified in mice by the markers CD11b and Gr1, are unable to develop into mature myeloid cells in response to tumor-derived cytokines like VEGF, GM-CSF and IL-1β (5).
We discovered that BM progenitor cells from the Muc1 knockout (KO) mice developed into CD11b+Gr1+ MDSCs in vitro under GM-CSF and IL-4 signaling. MUC1 is a tumor-associated mucin that is over expressed and aberrantly glycosylated in cancer and its role as an oncogeneic signaling protein has been extensively studied in epithelial cancer. MUC1 is expressed to a lesser extent on hematopoietic cells (6–12) but its role in these cells have not been as well defined; however, MUC1 has been shown to be important in T cell signaling (6, 7). Our current study shows that development of BM progenitors into CD11b+Gr1+ MDSCs is dependent on down regulation of beta-catenin levels, which can be regulated by Muc1.
The MUC1 cytoplasmic tail (MUC1-CT) has been shown to interact with beta-catenin in epithelial cancer and promote oncogenesis. Nuclear translocation of the MUC1-CT-beta-catenin complex allows MUC1 to influence the transcriptional regulatory activity of beta-catenin, driving tumor growth and invasiveness (13). The MUC1-CT can also compete with E-cadherin for binding to beta-catenin at adherens junctions, promoting the metastatic invasiveness of the tumor cell (14). In vitro knockdown of MUC1 can down regulate beta-catenin levels (15) and reduce cellular invasiveness associated with increased cytoplasmic localization of beta-catenin (16). Similarly, over expression of MUC1-CT is associated with increased stability and nuclear localization of beta-catenin (17). The importance of the Wnt/beta-catenin signaling cascade is, however, not solely restricted to its oncogeneic effects, as constitutive Wnt-beta-catenin activation in the BM can result in hematopoietic stem cell and multi-lineage defects (18, 19). In the absence of Wnt signaling, beta-catenin exists as part of a destruction complex where it is subsequently phosphorylated by casein kinase 1 and GSK3β for targeted ubiquitin-mediated degradation (20). Ligation of the Wnt receptor complex inhibits the activity of this beta-catenin destruction complex (21) and beta-catenin can translocate to the nucleus where it associates with the DNA binding proteins of the T cell factor/lymphoid enhancer factor family to initiate transcription.
In our study, we show that aberrant differentiation of MDSCs from Muc1 KO myeloid progenitors is dependent on the down regulation of beta-catenin in these cells. Given the central role that beta-catenin plays in hematopoiesis and its regulation by MUC1 in cancer, it is not surprising that the lack of Muc1 in myeloid progenitors from KO mice could promote beta-catenin down regulation and allow for aberrant MDSC differentiation in response to GM-CSF and IL-4 signaling. This translated into better growth of subcutaneously implanted EL4 lymphoma cells in the KO mice. Most intriguingly, KO mice were also able to better tolerate allogeneic tumor growth with an accumulation of CD11b+Gr1+ cells in the blood and tumor draining lymph nodes. Adoptive transfer of CD11b+Gr1+ cells from the BM of KO mice bearing EL4 tumors allowed for allogeneic tumor growth in WT mice. Our findings indicate a novel role of Muc1 in regulating the development of myeloid progenitors into CD11b+Gr1+ MDSCs, which would be very useful in enhancing the therapeutic efficacy of cancer vaccines in face of tumor immune suppression.
MATERIALS AND METHODS
Mice
Muc1 C57BL/6 KO mice were generated using homologous recombination as previously described (22).
BM lineage depletion
BM flushed from the tibia and femurs of WT and KO mice was subjected to magnetic activated cell sorting against a panel of antibodies directed against lineage-committed antigens (Miltenyi Biotec, Auburn, CA). Hematopoietic stem and progenitor (Lin−) cells were obtained from the negative flow through, while the positive fraction contained lineage-committed cells (Lin+). Lin− cells were plated at 2×105 cells/ml while Lin+ cells were plated at 106 cells/ml in DMEM with 10% FCS, 1% penicillin-streptomycin, and 1% Glutamax. The same doses of GM-CSF (20ng/ml) and IL-4 (20ng/ml) (both from BD Pharmingen, San Diego, CA) were used throughout.
BM transplant
WT female mice were given 11 Gy irradiation split into two doses, separated by 3 hours. After irradiation, 20 × 106 male donor KO or WT BM were injected into female irradiated recipients via tail vein and chimerism was monitored after 30 days using PCR analysis of DNA from peripheral blood mononuclear cells (PBMCs) for presence of the Y chromosome gene product in the female irradiated recipient mice (23). Percentage of chimerism was established using a standard made from the same PCR reaction of PBMC DNA that contains varying mixed ratios of male and female DNA.
Flow cytometry
106 cells were isolated, washed once with PBS and stained in 1x PBS with 0.5% FCS using the following antibodies at 1μg/ml: anti-CD11b FITC, anti-Gr1 (LY6C/G) PE, anti-LY6C FITC and anti-LY6G FITC(all BD Pharmingen, San Diego, CA) and anti-F4/80 APC (eBioscience, San Diego, CA). Acquisition was performed on a Dako Cyan flow cytometer and analysis was done on Summit 4.3. At least 20 000 events were isolated.
Subcellular fractionation
Subcellular fractionation was performed using an adapted protocol (18, 24). Cells were resuspended in buffer A (10 mM HEPES pH 7.5, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA and 0.1% Nonidet-P40) on ice. Lysate was then spun at 6000 rpm, 1min, 4°C and the supernatant collected as the cytosolic fraction. The pellet was washed twice in ice cold PBS before sonication in buffer B (20mM HEPES ph 7.9, 25% glycerol, 400mM NaCl, 1.5mM MgCl2, 0.2mM EDTA, 0.5mM DTT) to obtain the nuclear fraction which was then spun at 13 000 rpm for 20min, 4°C.
Allogeneic mixed lymphocyte reaction (MLR)
Dendritic cells (DCs) were derived from BM cells cultured for 5 days with GM-CSF and IL-4 at 106 cells/ml in DMEM with 10% FCS, 1% penicillin-streptomycin and 1% Glutamax. LPS (1μg/ml) was added on day 4 to obtain mature DCs on day 5. They were then incubated with allogeneic T cells at a 1:10 ratio for 5 days, with 3H thymidine added on day 4 to measure the extent of proliferation. T cells were sorted from the spleen using magnetic CD4 and CD8 beads (Miltenyi Biotec, Auburn, CA).
Tumor study and adoptive transfer experiments
Six week old female WT and KO mice were subcutaneously injected with 104 EL4 lymphoma cells. For the allogeneic tumor study, 6 week old female WT and KO mice were subcutaneously injected with 5×106 allogeneic BALB/c BM185 lymphoma cells. CD11b+Gr1+ cells (106) from the BM of WT and KO mice bearing EL4 tumors were sorted by flow cytometry and i.v. injected into WT mice implanted with 5×106 BM185 cells. Adoptive transfer of CD11b+Gr1+ cells to WT mice was performed 4 days post implantation of BM185 cells and then given every 7 days thereafter for 3 weeks. All mice were palpated every 3 days after 10 days post injection for tumor growth as measured by (length × width2)/2 (22). When tumors reached more than 10% of mouse weight, all mice were sacrificed for endpoint analysis.
RESULTS
Aberrant differentiation of CD11b+Gr1+ cells from BM progenitors under GM-CSF and IL-4 signaling is a result of beta-catenin down regulation in the absence of Muc1
Expression of Muc1 in the BM in wildtype C57BL/6 mice is low compared to epithelial tissues like kidney (Figure 1A). The Muc1 KO mouse was therefore used as a model to study hematopoiesis in the BM in the absence of Muc1. The Muc1 KO mouse has been extensively shown to lack Muc1 expression in epithelial tissues (25–30). We used the kidney as a positive control to demonstrate the Muc1 null levels in the KO mouse using qRT-PCR, where the BM of the KO mouse also showed similar null expression of Muc1 (Figure 1A).
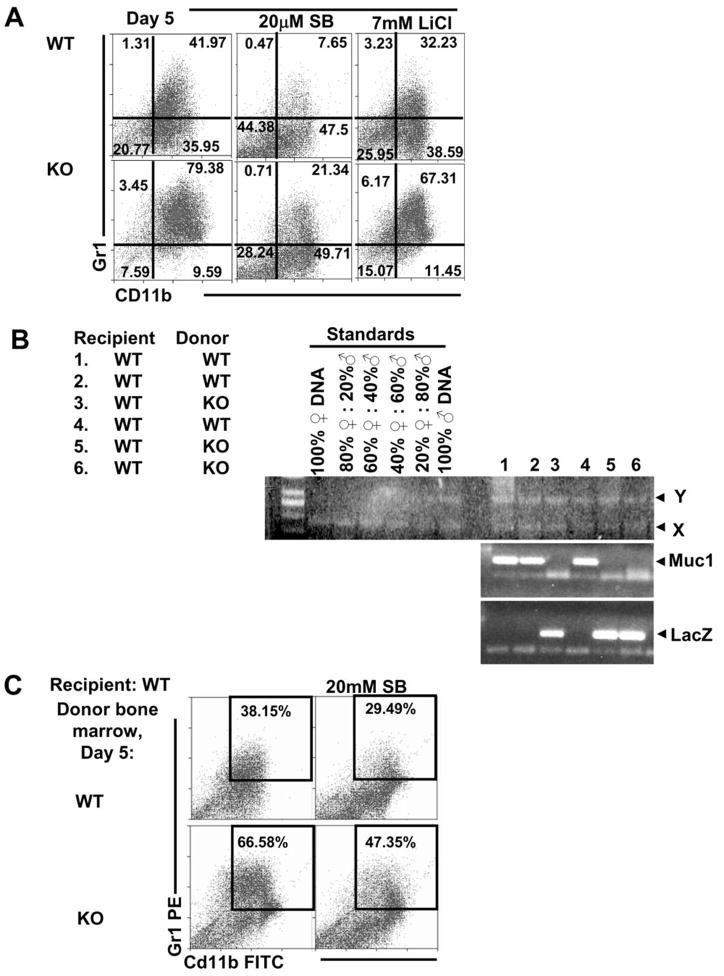
Figure 1. KO Lin− BM cells proliferate better in vitro with GM-CSF and IL-4.
(A) Muc1 mRNA expression was analyzed by generating a relative quantification graph from 4 independent experiments, using the ΔCT from the WT BM as a calibrator (set at 1). (B and C) Whole, Lin− and Lin+ BM (n=7) were cultured for 5 days with GM-CSF and IL-4 after which cell numbers were counted and computed as X fold change over the original plating concentration. Cells did not grow in vitro without IL-4 and GM-CSF. Phase contrast microscopic pictures of these cells were taken. (D) Cytosolic (C) and nuclear (N) fractions from day 5 WT and KO Lin− cells were analyzed for beta-catenin via western blot. Lamin B and IκKα were analyzed as nuclear and cytosolic markers, respectively. SB (5–20μM) was added at day 0 of culture with GM-CSF and IL-4. Data shown are representative of 3 independent experiments.
In vitro culture of Muc1 KO BM cells for 5 days with IL-4 and GM-CSF resulted in an increase in the proliferation of cells with a small and round morphology (Figure 1B) as compared to similarly cultured WT BM. This observation was also mirrored in the KO Lin− BM that were similarly cultured, but not in KO Lin+ BM, (Figure 1C), suggesting that the increased proliferation seen in the whole BM culture was due to a lack of Muc1 in the Lin− cells. Beta-catenin is regulated by MUC1 in epithelial tissue, and its role in hematopoiesis made it logical to analyze the levels of beta-catenin in relation to the increased proliferation of KO Lin− BM as observed in Figure 1C. Western blot analysis of beta catenin levels in Lin− KO BM cultured for 5 days in IL-4 and GM-CSF showed a reduction of beta-catenin in the cytosolic (C) and nuclear (N) fractions as compared to Lin− WT BM (Figure 1D). The levels of beta-catenin mRNA in these cells were unchanged (data not shown) suggesting that reduction of beta-catenin in cultured Lin− KO BM was a degradation dependent process. Beta-catenin degradation is triggered by its phosphorylation by GSK3β. SB-415286 (SB), a GSK3β inhibitor (31), was able to reverse the reduction of beta-catenin in KO BM cells in a dose dependent manner (5–20μM) with 20μM SB restoring the cytosolic levels of beta-catenin in cultured KO BM cells to that seen in cultured WT BM cells (Figure 1D).
The increased cell proliferation in the KO Lin− BM culture appeared to be due to an expansion of a CD11b+Gr1+ population from 42% in the WT Lin− BM culture to 79% in the KO Lin− BM culture (Figure 2A). This expansion of CD11b+Gr1+ cells could be reduced in both WT and KO cultures upon addition of 20μM SB to 7.65% and 21.34% respectively. Addition of 7 mM LiCl, another GSK3β inhibitor (18), also similarly reduced the expansion of the CD11b+Gr1+ population in both WT and KO cultures to 32.23% and 67.31% respectively. These GSK3β inhibitors that protected beta-catenin from degradation were able to significantly reverse the increase in CD11b+Gr1+ cells (Supplemental Figure 1).
Figure 2. Increased cell proliferation from KO Lin− BM cells was due to expansion of the CD11b+Gr1+ phenotype.
(A) After 5 days of culture with GM-CSF and IL-4, cells were analyzed for CD11b and Gr1 expression using flow cytometry. Expansion of CD11b+Gr1+ cells could be reversed with GSK3β inhibitors, SB or LiCl, that were added on Day 0 of culture together with GM-CSF and IL-4. Data shown are representative of 3 independent experiments. (B) Lethally irradiated WT mice were transplanted with KO BM. Chimerism was determined via presence of the Y chromosome gene product and appropriate levels of Muc1 or LacZ gene product depending on donor BM. Faint bands below the Muc1 or LacZ gene product are primer dimers. (C) Lin− BM from WT mice transplanted with KO donor BM showed an increase in the population of CD11b+Gr1+ cells upon culture with GM-CSF and IL-4 using flow cytometry.
In order to determine if the in vitro expansion of the CD11b+Gr1+ population is dependent on the lack of Muc1-mediated signaling from peripheral tissues outside of the BM, we transplanted female WT mice with BM from male WT or KO mice. This will enable us to study the in vitro expansion of CD11b+Gr1+ cells from KO BM that was derived from a WT mouse. All female transplant mice displayed successful hematopoietic engraftment based on presence of the Y chromosome gene product and either the Muc1 (for the WT mouse) or LacZ gene product (for the KO mouse), depending on donor phenotype, in PBMCs (Figure 2B). Lin− BM isolated from WT mice transplanted with KO BM also showed a similar increase in CD11b+Gr1+ cells when cultured for 5 days with GM-CSF and IL-4, compared to Lin− BM from WT mice transplanted with WT BM (Figure 2C).
CD11b+Gr1+ cells that expanded from Muc1 KO BM in vitro suppressed in vitro T cell proliferation
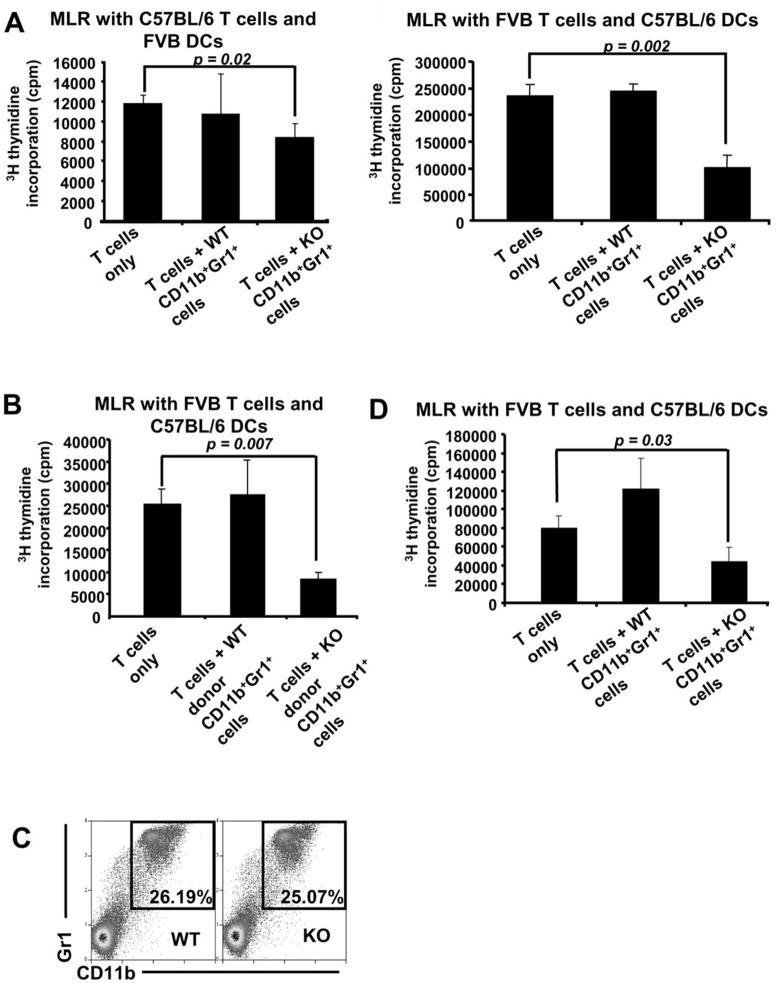
In order to determine if the expansion of the CD11b+Gr1+ phenotype cultured from Muc1 lacking Lin− BM was suppressive, we sorted CD11b+Gr1+ cells from both WT and KO Lin− BM cells that were cultured for 5 days with IL-4 and GM-CSF. These CD11b+Gr1+ cells were incubated in an allogeneic MLR consisting of spleen-derived T cells and LPS-stimulated DCs (Figure 3A). We also sorted CD11b+Gr1+ cells from day 5 Lin− BM cultures of WT mice transplanted with either WT or KO BM and incubated these cells in a MLR (Figure 3B). Indeed, in all MLRs, CD11b+Gr1+ cells from the KO Lin− BM culture were able to better suppress T cell proliferation while the corresponding CD11b+Gr1+ cells from the WT Lin− BM culture showed no suppression of T cell proliferation. This suggests that the CD11b+Gr1+ cells derived from KO Lin− BM belong to the MDSC population. Interestingly, although the levels of CD11b+Gr1+ cells in unstimulated BM of WT and KO mice were the same (Figure 3C), CD11b+Gr1+ cells sorted from unstimulated KO BM were able to suppress T cell proliferation more effectively as compared to WT (Figure 3D).
Figure 3. The CD11b+Gr1+ KO cells obtained after 5 days of Lin− KO BM culture in vitro with GM-CSF and IL-4 are suppressive.
Day 5 CD11b+Gr1+ cells from WT and KO Lin− BM were sorted by flow cytometry and incubated in an MLR with (A) left, C57BL/6 T cells and FVB DCs and right, FVB T cells and C57BL/6 DCs. (B) CD11b+Gr1+ cells from day 5 Lin−BM of WT mice transplanted with either WT or KO BM were sorted by flow cytometry and incubated in an MLR with C57BL/6 DCs and FVB T cells. In all MLRs, 5×103 DCs and 105 T cells were incubated with or without 5×103 CD11b+Gr1+ cells from day 5 cultures of either WT or KO Lin− BM. (C) Similar levels of CD11b+Gr1+ cells in freshly isolated WT and KO BM were detected via flow cytometry. (D) Freshly isolated CD11b+Gr1+ cells from the BM of both WT and KO mice were sorted by flow cytometry and incubated in an MLR with FVB T cells and C57BL/6 DCs. All data shown are representative of 3 independent experiments.
Muc1 KO mice show enhanced EL4 tumor growth
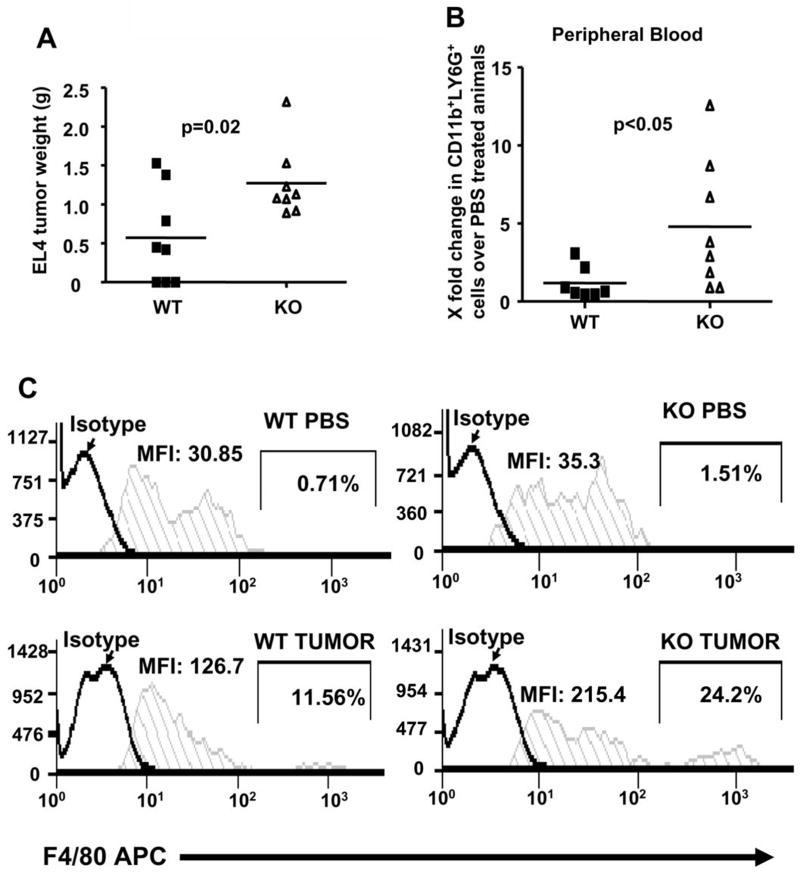
The development of MDSCs from KO BM progenitors in vitro under GM-CSF and IL-4 signaling prompted us to ask if we could observe a similar expansion of MDSCs in a tumor model using the KO mice. Subcutaneous implantation of 104 EL4 lymphoma cells resulted in significantly higher end point tumor weights for KO mice as compared to WT (Figure 4A). We saw a statistically significant increase in the number of CD11b+LY6G+ cells in the PBMCs of KO tumor bearing mice (Figure 4B) accompanied by a trend of increased accumulation of CD11b+Gr1+ cells in the BM, PBMCs, tumor draining lymph nodes and spleen (Supplemental Figure 2) of tumor bearing KO mice. A comparison of WT and KO mice bearing tumors of similar weight (1g) also showed an increase in CD11b+Gr1+F4/80+ cells in the PBMCs of KO tumor bearing mice (Figure 4C).
Figure 4. KO mice developed larger EL4 tumors.
(A) WT and KO mice (n=8) were subcutaneously injected with 104 EL4 cells. PBMCs from the sacrificed mice at endpoints were analyzed for expression of CD11b, LY6G, Gr1 and F4/80 via flow cytometry. Tumor bearing KO mice had a greater increase in the levels of (B) CD11b+LY6G+ cells and (C) CD11b+Gr1+F4/80+ cells in PBMCs, as compared to WT tumor bearing mice. X fold change is computed by dividing the number of CD11b+LY6G+ cells in tumor bearing mice over the number of CD11b+LY6G+ cells isolated from corresponding PBS-injected controls. CD11b+Gr1+ cells from PBMCs of WT and KO mice with similar tumor weight (~1g) and their PBS-injected controls were analyzed for F4/80 expression. Both the mean fluorescence intensities (MFI) and percentages of CD11b+Gr1+ cells that expressed high amounts of F4/80 were shown. Significance was computed using student’s t-test.
Muc1 KO mice are able to tolerate allogeneic BM185 tumor growth more effectively than WT mice
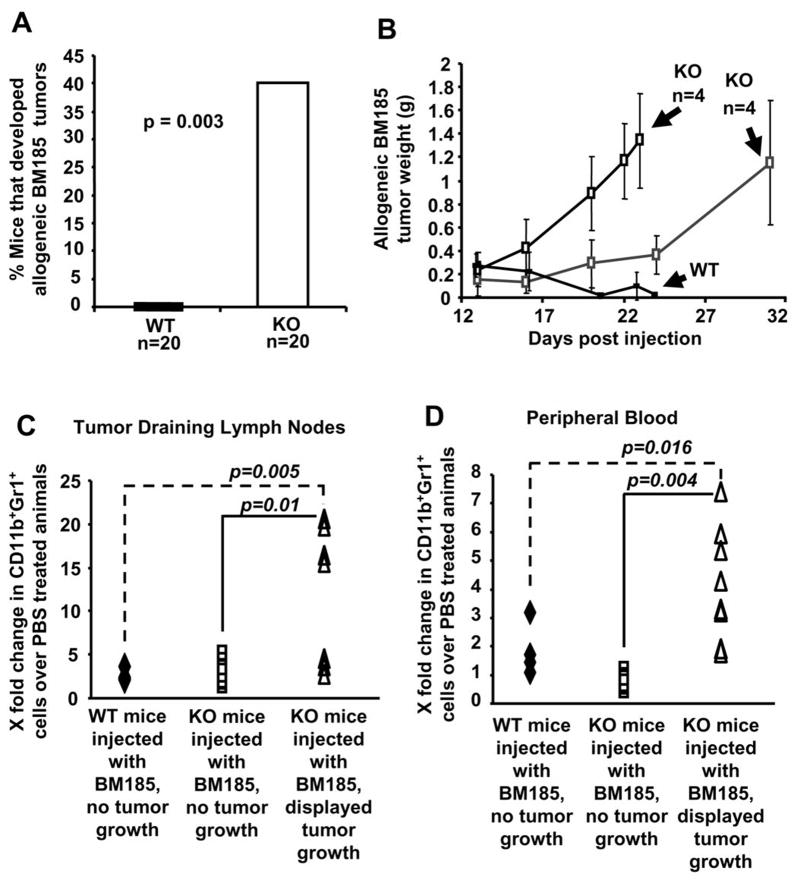
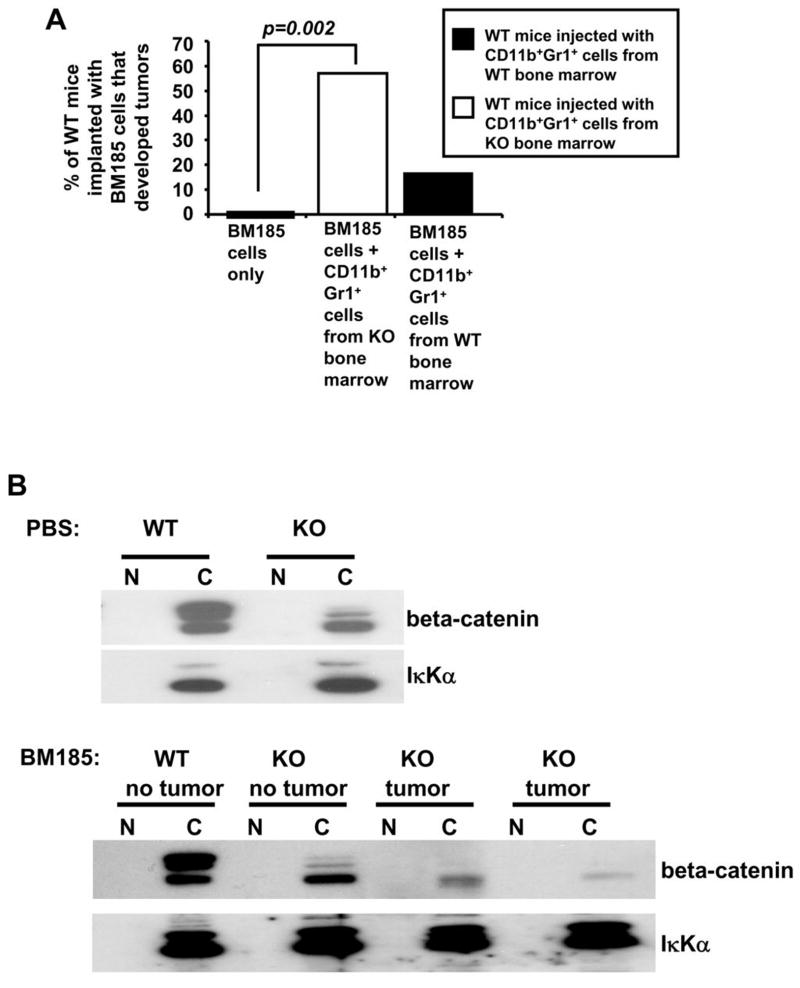
The preferential development of the CD11b+LY6C+ and CD11b+Gr1+F4/80+ myeloid population (Figure 4B and 4C) in the EL4 tumor bearing KO mice could account for the differences in EL4 tumor growth between WT and KO mice (Figure 4A). Given the development of different populations of myeloid suppressive cells in response to EL4 tumor growth in both WT and KO syngeneic mice that allowed for better EL4 tumor growth in KO mice, we also studied the ability of WT and KO mice to reject allogeneic tumor growth. We hypothesized that inoculation of an initial allogeneic tumor burden would generate sufficient levels of CD11b+Gr1+ MDSCs in the KO mice which could hinder the allogeneic rejection of these cells. We subcutaneously implanted 5×106 BALB/c BM185 lymphoma cells into the C57BL/6 WT and KO mice in order to analyze their ability to reject the establishment of an allogeneic tumor. Amazingly, 40% of the injected KO mice tolerated the growth of allogeneic BM185 cells (Figure 5A). The allogeneic tumors that grew in the KO mice showed two phases of tumor growth, with tumors reaching more than 1g at 23 days in 4 mice and at 31 days in 4 mice, while all WT mice rejected the BM185 cells (Figure 5B). While there was no significant increase of CD11b+Gr1+ cells in the BM and spleen of KO mice bearing allogeneic tumors (data not shown), we noticed a significant increase of CD11b+Gr1+ cells in the tumor draining lymph nodes (Figure 5C) and PBMCs (Figure 5D) of KO mice bearing allogeneic tumors. While our results suggest that this could be a result of MDSC-mediated immune suppression, it could also be indicative of a preexisting impaired immune response in the KO mice, even though there appears to be no significant difference in the blood cell counts for WT and KO mice (Supplemental Figure 3). We i.v. injected 106 CD11b+Gr1+ cells from the BM of either WT or KO mice bearing EL4 tumors, into WT mice that had been subcutaneously implanted with 5 × 106 BM185 cells 4 days earlier. 57% of WT mice that received adoptive transfer of KO CD11b+Gr1+ cells showed tumor growth while only 17% of WT mice that received adoptive transfer of WT CD11b+Gr1+ cells showed tumor growth. All WT mice implanted with BM185 cells that did not receive any adoptive transfer of CD11b+Gr1+ cells showed complete rejection of the allogeneic BM185 tumor cells (Figure 6A).
Figure 5. KO mice can better tolerate allogeneic tumor formation.
(A) WT and KO mice (n=20) were injected subcutaneously with 5×106 BM185 cells. Significance was computed using Fischer’s exact test. (B) Palpation growth curves showed BM185 tumor growth in KO mice and allogeneic rejection in WT mice. Increased amount of CD11b+Gr1+ cells are observed in (C) tumor draining lymph nodes and (D) PBMCs of tumor bearing KO mice, using flow cytometry. X fold change is computed by dividing the number of CD11b+Gr1+ cells in mice injected with BM185 cells over the number of CD11b+Gr1+ cells isolated from corresponding PBS-injected mice. Significance was computed using Wilcoxon Rank Sum pair wise comparison.
Figure 6.
(A) WT mice were subcutaneously implanted with BM185 tumor cells and i.v injected with CD11b+Gr1+ cells from either the BM of KO mice bearing EL4 tumors (n=7) (open box) or WT mice bearing EL4 tumors (n=6) (filled box), or without any injections of CD11b+Gr1+ cells (n=20). (B) Cytosolic (C) and nuclear fractions (N) of Lin− cell lysates were obtained from WT and KO mice injected with BM185 cells (as well as their corresponding PBS controls) and analyzed for beta–catenin and IκKα (cytosolic marker). Lin− BM cells isolated from these mice were too few for detection of nuclear protein. Data shown are representative of 3 independent experiments.
Reduction of beta-catenin in BM cytosolic fractions of allogeneic tumor bearing KO mice
The accumulation of CD11b+Gr1+ cells in the in tumor draining lymph nodes and PBMCs of KO mice bearing allogeneic tumors prompted us to similarly analyze the beta-catenin levels of both WT and KO mice that have been implanted with BM185 cells. Indeed, KO mice that could tolerate allogeneic BM185 tumor growth displayed the lowest amounts of cytosolic beta-catenin levels (Figure 6B).
DISCUSSION
Muc1 in the BM can act as a signaling molecule involved in controlling the expansion of MDSCs from BM progenitors
MUC1 is present at low levels in hematopoietic cells in comparison to epithelial tissue; however, this has not compromised its role as a signal transducer as evidenced in T cell signaling (6, 7). In this study, we discovered that a lack of Muc1 in BM progenitors resulted in their aberrant expansion into CD11b+Gr1+ MDSCs under GM-CSF and IL-4 signaling. Beta-catenin stabilization also appeared to be essential for MDSC expansion in vitro as stabilization of beta-catenin levels with GSK3β inhibitors reduced the expansion of CD11b+Gr1+ cells. MUC1 is known to interact with beta-catenin (14, 15, 32, 33) and silencing of MUC1 has been shown to reduce beta-catenin levels in epithelial cells (15), drawing a link between MUC1 and beta-catenin stability. This observation was paralleled in our studies when a lack of Muc1 in BM progenitor cells increased the susceptibility of beta-catenin to degradation in culture with GM-CSF and IL-4. Similarly, in KO mice bearing allogeneic BM185 tumors with increased levels of CD11b+Gr1+ cells in the tumor draining lymph nodes and PBMCs, we also observed a greater reduction in beta-catenin cytosolic levels of Lin− BM from these mice, as compared to WT mice or KO mice that did not develop any allogeneic tumors. Taken together, our results demonstrate the dependency of beta-catenin regulation and stability on Muc1 in MDSC differentiation.
The Muc1/beta-catenin regulatory axis in the BM can regulate MDSC accumulation in a tumor model
MDSCs are important in contributing to the immune tolerogenic tumor microenvironment (4, 34). However, their differentiation process is still relatively unknown, although the SHIP, STAT and the S100 family of proteins, have been shown to be involved (35–38). The increased tumor growth and increased levels of CD11b+Gr1+F480+ and CD11b+LY6G+ cells in KO mice implanted with EL4 lymphoma cells underscore our in vitro data that development of a suppressive CD11b+Gr1+ myeloid population is enhanced in the absence of Muc1. Classic allogeneic rejection is usually a T cell-mediated process and the ability of KO mice to tolerate allogeneic BM185 tumor growth more effectively suggests that this is dependent on MDSC-mediated immune suppression of T cells. We also do not exclude the possibility that inherent defective T cell or DC function could also contribute to allogeneic tolerance of the BM185 lymphoma cells by the KO mice, and studies to further define these possible scenarios are currently underway. However, adoptive transfer of CD11b+Gr1+ cells from KO mice bearing EL4 tumors allowed WT mice to tolerate allogeneic BM185 tumor growth as effectively as the KO mice, further emphasizing that this is a process highly dependent on MDSC-mediated immune suppression.
KO mice injected with PBS showed lower cytosolic levels of beta-catenin in the Lin− BM as compared to their WT counterparts (Figure 6B), but levels of CD11b+Gr1+ cells in the BM, spleen and blood of these two groups of mice are similar (data not shown). However, the CD11b+Gr1+ cells from the KO BM were suppressive in an allogeneic MLR, suggesting that the lower beta-catenin basal levels in the KO BM could result in an inherent tolerogeneic capacity of these mice for allogeneic tumor growth. Further reduction of beta-catenin levels in this process, as enhanced by the lack of Muc1 in the KO BM, would promote the development and accumulation of CD11b+Gr1+ MDSCs into the PBMCs and tumor draining lymph nodes, thus allowing for allogeneic tumor growth in the KO mice.
In our model, the loss of beta-catenin in the cytosolic compartment in response to tumor implantation could also be a mechanism by which KO myeloid progenitors lose their adherence to the BM stroma and mobilize into the periphery or tumor microenvironment as immature MDSCs. Although MUC1 has been shown to promote the cancer phenotype via oncogeneic activation of the Wnt/beta-catenin pathway, this is the first time that Muc1 has been shown to be involved in hematopoiesis. Therefore, the regulation of beta-catenin levels by Muc1 as a mechanism for controlling CD11b+Gr1+ myeloid expansion has significant impact with regards to the development pathway of CD11b+Gr1+ MDSCs
The accumulation of CD11b+Gr1+ MDSCs in the KO mice with allogeneic tumor growth can also be due to tumor-derived factors promoting abnormal myelopoiesis in myeloid progenitors lacking Muc1 as previously seen in our in vitro data. This would corroborate our in vitro studies with GSK3β inhibitors that suggest that the elevation of the levels of CD11b+Gr1+ MDSCs in response to cytokines like GM-CSF and IL-4 is a result of beta-catenin degradation in the absence of Muc1 in myeloid progenitors.
GM-CSF is a tumor-derived factor that when produced at uncontrolled amounts, can promote tumor growth via the generation of CD11b+Gr1+ MDSCs (39, 40), by directly activating myeloid progenitors (41) or inducing granulocyte-macrophage specific differentiation in early lymphoid progenitors (42). We obtained similar data in our experiments with Lin− WT and KO BM using GM-CSF only; however, results were more pronounced when GM-CSF and IL-4 were used together to stimulate myeloid differentiation (data not shown). This is unsurprising, given that IL-4 Rα is a functional marker for MDSCs (43, 44). SCF is another tumor-derived factor that acts directly on the c-kit receptor of myeloid progenitors to stimulate proliferation and differentiation, resulting in MDSC expansion (3). Addition of PGE2 to Lin− mouse BM cultured in vitro with GM-CSF and IL-4 resulted in an increase in the amount of suppressive CD11b+Gr1+ cells after 5 days (5), an effect that was mimicked in our study without any addition of PGE2, but with a lack of Muc1, giving a mechanistic insight into the differentiation process of MDSCs from their progenitors. While we have only looked at the response of myeloid progenitors to GM-CSF and IL-4 in this pilot study, it is possible that these myeloid progenitors that lack Muc1 would also be able to respond similarly to other cytokines that promote myeloid progenitor differentiation, such as SCF or PGE2.
We have previously shown that Muc1 is important for tumorigenesis in various mouse models (22, 45–48). This would appear to be at odds with our current data suggesting that a lack of Muc1 results in aberrant development of CD11b+Gr1+ MDSCs that are a hallmark of the immunosuppressive tumor microenvironment. While the ability of Muc1 to regulate the myelopoiesis of myeloid progenitors into CD11b+Gr1+ MDSCs is a departure from its current role as a cancer-associated mucin in literature, this suggests that the function of Muc1 can be diversely regulated in a tissue (e.g., epithelial vs hematopoietic) or differentiation specific manner by a vast array of post translational modifications that have not yet been fully studied nor exploited in therapy, indicating further complexities in the function of this mucin. MUC1 is expressed on human hematopoietic stem cells (HSCs) (12) and its expression on human lymphoma cell lines has been shown to be cyclical (Shanmugam and Gendler) suggesting that mechanisms exist to downregulate Muc1 expression on hematopoietic cells in a cancer model. MUC1 could therefore be downregulated on HSCs in cancer, thus promoting the MDSC accumulation that is frequently observed. Although we are unsure of the exact mechanisms by which this can occur, studies to further delineate this process are currently underway.
Our observations with the Muc1 KO mouse indicate for the first time that Muc1 might be acting as part of a regulatory mechanism that prevents aberrant differentiation and proliferation of myeloid progenitors into CD11b+Gr1+ MDSCs via stabilization of beta-catenin. This establishes Muc1 and its regulation of beta-catenin stability as a critical mechanistic link between cytokine imbalance and the myelopoiesis of MDSCs from its progenitors, which could be useful for therapeutic design against various immunological malignancies. Also, as MUC1 is targeted by many biologic therapies aimed at carcinomas, understanding the possible effects of down regulation of MUC1 on other hematopoietic cells like the myeloid progenitors in our study, is key to learning how best to optimize treatment strategies. In light of this, our findings not only redefine the cancer-associated role of MUC1 in current literature, they also highlight the need to further understand MUC1 function in a tissue specific manner in context with disease state.
Supplementary Material
Acknowledgments
This work was funded by NIH/NCI RO1 CA64389 (SJG) and The Mayo Foundation.
References
- 1.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 2.Bronte V, Serafini P, De Santo C, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 3.Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Ren J, Kufe D. Interaction of human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochem Biophys Res Commun. 2004;315:471–6. doi: 10.1016/j.bbrc.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee P, Tinder TL, Basu GD, Gendler SJ. MUC1 (CD227) interacts with lck tyrosine kinase in Jurkat lymphoma cells and normal T cells. J Leukoc Biol. 2005;77:90–9. doi: 10.1189/jlb.0604333. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T Cells - Implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998;58:4079–81. [PubMed] [Google Scholar]
- 9.Treon SP, Mollick JA, Urashima M, et al. MUC1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999;93:1287–98. [PubMed] [Google Scholar]
- 10.Kruger W, Kroger N, Zander AR. MUC1 expression in hemopoietic tissues. J Hematother Stem Cell Res. 2000;9:409–10. doi: 10.1089/152581600419044. [DOI] [PubMed] [Google Scholar]
- 11.Rughetti A, Biffoni M, Pierelli L, et al. Regulated expression of MUC1 epithelial antigen in erythropoiesis. Br J Haematol. 2003;120:344–52. doi: 10.1046/j.1365-2141.2003.04038.x. [DOI] [PubMed] [Google Scholar]
- 12.Fatrai S, Schepers H, Tadema H, Vellenga E, Daenen SM, Schuringa JJ. Mucin1 expression is enriched in the human stem cell fraction of cord blood and is upregulated in majority of the AML cases. Exp Hematol. 2008 doi: 10.1016/j.exphem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Singh PK, Wen Y, Swanson BJ, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67:5201–10. doi: 10.1158/0008-5472.CAN-06-4647. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–32. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 15.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–22. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362:740–6. doi: 10.1016/j.bbrc.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem. 2003;278:38029–39. doi: 10.1074/jbc.M304333200. [DOI] [PubMed] [Google Scholar]
- 18.Scheller M, Huelsken J, Rosenbauer F, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–47. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 19.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–56. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 20.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 21.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 23.Novak EK, Reddington M, Zhen L, et al. Inherited thrombocytopenia caused by reduced platelet production in mice with the gunmetal pigment gene mutation. Blood. 1995;85:1781–9. [PubMed] [Google Scholar]
- 24.Michalke M, Cariers A, Schliess F, Haussinger D. Hypoosmolarity influences the activity of transcription factor NF-kappaB in rat H4IIE hepatoma cells. FEBS Lett. 2000;465:64–8. doi: 10.1016/s0014-5793(99)01719-6. [DOI] [PubMed] [Google Scholar]
- 25.Spicer AP, Parry G, Patton S, Gendler SJ. Molecular cloning and analysis of the mouse homologue of the tumor- associated mucin, MUC1, reveals conservation of potential O- glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. J Biol Chem. 1991;266:15099–109. [PubMed] [Google Scholar]
- 26.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with EGFR and correlates with MAP kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–64. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 27.Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Targeted disruption of the murine mucin gene 1 decreases susceptibility to cholesterol gallstone formation. J Lipid Res. 2004;45:438–47. doi: 10.1194/jlr.M300468-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Al Masri A, Gendler SJ. Muc1 affects c-Src signaling in PyV MT-induced mammary tumorigenesis. Oncogene. 2005;24:5799–808. doi: 10.1038/sj.onc.1208738. [DOI] [PubMed] [Google Scholar]
- 29.McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–24. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu W, Hisatsune A, Koga T, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006;176:3890–4. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 31.Smith DG, Buffet M, Fenwick AE, et al. 3-Anilino-4-arylmaleimides: potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3) Bioorg Med Chem Lett. 2001;11:635–9. doi: 10.1016/s0960-894x(00)00721-6. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3beta and beta-catenin. J Biol Chem. 2001;276:6061–4. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Ren J, Yu Wh W, et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001;276:35239–42. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 34.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–8. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paraiso KH, Ghansah T, Costello A, Engelman RW, Kerr WG. Induced SHIP deficiency expands myeloid regulatory cells and abrogates graft-versus-host disease. J Immunol. 2007;178:2893–900. doi: 10.4049/jimmunol.178.5.2893. [DOI] [PubMed] [Google Scholar]
- 36.Nefedova Y, Huang M, Kusmartsev S, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–74. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 37.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory s100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 40.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–31. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larrivee B, Pollet I, Karsan A. Activation of vascular endothelial growth factor receptor-2 in bone marrow leads to accumulation of myeloid cells: role of granulocyte-macrophage colony-stimulating factor. J Immunol. 2005;175:3015–24. doi: 10.4049/jimmunol.175.5.3015. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki-Arai J, Iwasaki H, Miyamoto T, Watanabe S, Akashi K. Enforced granulocyte/macrophage colony-stimulating factor signals do not support lymphopoiesis, but instruct lymphoid to myelomonocytic lineage conversion. J Exp Med. 2003;197:1311–22. doi: 10.1084/jem.20021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder JA, Masri AA, Adriance MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–47. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder JA, Adriance MC, McConnell EJ, Thompson MC, Pockaj B, Gendler SJ. ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. J Biol Chem. 2002;277:22692–8. doi: 10.1074/jbc.M201975200. [DOI] [PubMed] [Google Scholar]
- 47.Tinder TL, Subramani DB, Basu GD, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181:3116–25. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pochampalli MR, Bitler BG, Schroeder JA. Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res. 2007;67:6591–8. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.