Abstract
The condensation between dienophiles and α,β-unsaturated hydrazone azadienes was previously reported to afford piperidines. During an attempt to adapt this reaction to the preparation of piperidine-based conformationally-restricted analogs of glutamate, it was discovered that the electrophile, dimethyl oxoglutaconate (DOG) led to highly substituted dihydropyrans in 20–50% yield. The unexpected pyran product likely results from an initial 1,4-addition of the hydrazone to the oxoglutaconate followed by intramolecular cyclization of the resultant enolate oxygen to the α,β-unsaturated iminium ion. Further manipulations afford substituted tetrahydropyran 6-methamino-2,4-dicarboxylic acids.
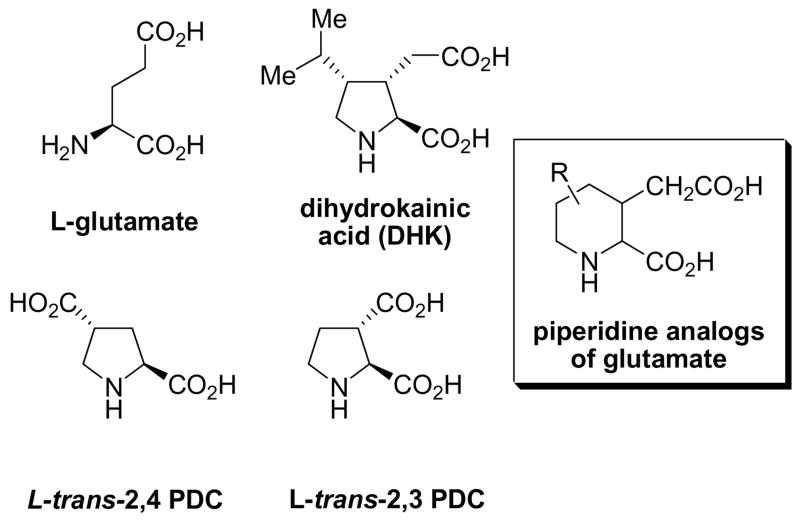
The use of conformationally-restricted amino acid analogues have greatly advanced our understanding of glutamatergic receptor and transporter pharmacology.1 By appropriately positioning or locking key functional groups via ring systems, conformational bias can yield useful information in the design of new pharmacologic agents.1ab For example, conformationally-restricted analogs of glutamate including dihydrokainate (DHK), 2,4-pyrrolidine dicarboxylate (L- trans-2,4-PDC)2 and L-trans-2,3-pyrrolidine dicarboxylate (L-trans-2,3-PDC)3 (Fig. 1) all show a high degree of selectivity at excitatory amino acid transporters (EAAT).1a,4 In each of these structures, the glutamate C2–C3 bond is restricted within the pyrrolidine ring. Given the subtle binding differences between the EAAT subtypes and other glutamate transporters, the preparation of the corresponding piperidine analogues would be of value (Fig. 1).
Fig 1.
Structures of glutamate and conformationally restricted piperidine analog of glutamate.
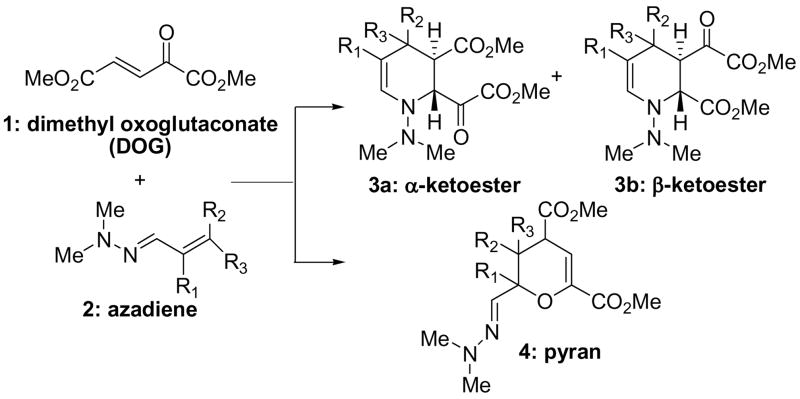
One possible synthetic approach to make piperidine dicarboxylic acid analogs would employ the azadiene Diels Alder reaction pioneered by Ghosez5 adapted for use with the dienophile dimethyl oxoglutaconate (1; DOG). We showed previously that DOG reacts with dienes in a Diels-Alder reaction or with anilines in an addition-cyclization to form quinolines in route to conformationally-restricted glutamate analogs.6 The reaction between DOG and the azadienes of α,β-unsaturated hydrazones 2 is then expected to afford either α-ketoester piperidine 3a or β-ketoester piperidine dicarboxylates 3b that could be elaborated to the desired targets (Scheme 1).
Scheme 1.
Proposed synthesis of piperidines via an azadiene-DOG reaction.
Therefore, an azadiene Diels-Alder sequence was envisioned as a rapid approach to making a new panel of glutamate analogues (Scheme 1): formation/separation of the substituted tetrahydropiperidine regioisomers, reduction of the ketone, scission of the N-N(Me)2 bond, and ester deprotection.
Nine unsymmetrical dimethyl hydrazones were prepared in 60–95% yield by the reaction of α,β-unsaturated aldehydes with N,N-dimethyl hydrazine in diethyl ether. The azadiene-based hydrazones of acyloin, methacrolein and cinnamaldehyde were reacted with dimethyl oxoglutaconate (DOG) in 1.1:1.0 ratio in CH3CN for 24–48 h to afford the presumed piperidine-[trans]-2, 3-diesters as oils in 20–50% yield after chromatography.7 Interestingly, a single product was isolated and assigned as the piperidine α-oxoester 3a (Scheme 1) based on 1H NMR assignments with the corresponding piperidine formed from dimethyl fumarate.5 The modest yield for this reaction was perplexing because the starting material was consumed in each instance resulting only in product and baseline material by tlc. Combustion analysis, high resolution mass analysis and the 1H NMR spectra were largely consistent with piperidine 3a although the 13C NMR spectra showed only two carbonyl signals between 165–170 ppm curiously lacking the ketone. We preliminarily ascribed this anomaly as possible formation of an enol owing to a singlet at 6.4 ppm and the susceptibility of α-amino ketoesters to racemize. However, deuterium exchange experiments (acid or base promoted) indicated no evidence of keto-enol equilibrium. Attempts to isolate the enol as silyl, methyl or acetyl enol ether also failed. Mild reduction (NaBH4, etc.) to convert the ketone to alcohol in the presence of the esters was unsuccessful and strong reduction (LAH) typically led to a mixture of over-reduced molecules. Use of Zn/HCl reported for the bis-reduction of both enamine and N-N moiety in the reported tetrahydropiperidine adducts5 failed to provide the saturated piperidine. The inconsistent and failed outcomes in these experiments, formation of a single regioisomers, and lack of a 13C NMR absorbance for a ketone suggested that the reaction between DOG and an azadiene does not afford the desired piperidines and a more thorough spectral examination was undertaken.
A 2D NMR study revealed a structure more consistent with a dihydropyran (Scheme 1) although the azomethine N=CH appears well upfield (6.4–6.5 ppm) of the usual position (7.0–7.5 ppm) due to the electron donating -N(CH3)2 group.8 In order to identify and confirm pyrans as the products, a DEPT experiment was conducted on the putative 6-methyl pyran product 4c isolated from the reaction between DOG and methacrolein azadiene. The DEPT shows three methine CH carbons and two quaternary carbons. The corresponding piperidine product (3a or 3b) would also show three methines but only one quaternary carbon. Dihydropyran 4c also shows a CH carbon at 135.5 ppm consistent with the exocyclic hydrazino –CH carbon and a –CH at 108.5 ppm consistent with an alkene –CH carbon in the pyran ring. Carbon 4 at 36.0 ppm matches well with a CH adjacent to a methyl ester. The two quaternary carbons (carbon 2 and 6) show shifts of 143.0 and 78.5 ppm and are consistent with a cyclic enol ether and quaternary ether, respectively. Like analysis of the other dihydropyrans 4 showed complete agreement of the carbon connectivity.
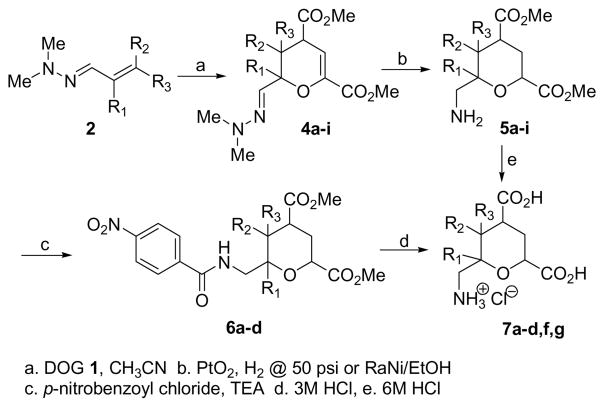
Based on this evidence, we have identified a new reaction between DOG and azadienes to afford tri- or tetrasubstituted pyrans (Scheme 2). Thus, the panel of azadienes originally planned for piperidine synthesis clearly affords the corresponding pyrans 4 (Scheme 2). This conclusion is also consistent with our failure to reduce the ketone, to functionalize the mistaken enol and the upfield shift in the azomethine. The formation of pyrans also explains the single regioisomer question. Six additional azadiene hydrazones were found to result in the corresponding pyrans 4 all of which are summarized in Table 1. The stereochemical arrangement of substituents could not be ascertained from the product mixture.
Scheme 2.
Formation and functionalization of pyrans formed via DOG-azadiene condensation
Table 1.
| Entry | R1 | R2 | R3 | 4 (%) | 5 (%) | 6 (%) | 7 (%) |
|---|---|---|---|---|---|---|---|
| a | H | H | H | 32 | 70 | 42 | 96 |
| b | H | nPr | H | 26 | 92 | 40 | 72 |
| c | Me | H | H | 40 | 82 | 52 | 62 |
| d | iPr | H | H | 54 | 92 | 51 | 97 |
| e | H | Et | H | 20 | 61b | - | n/a |
| f | H | Ph | H | 40 | 35b | - | 52c |
| g | H | Me | H | 28 | 20b | - | 50c |
| h | H | Me | Me | 25 | 48b | - | n/a |
| i | Me | Ph | H | 41 | 50b | - | n/a |
all structures showed satisfactory combustion analysis and/or HRMS in addition to spectral identification.
RaNi, MeOH,
6N HCl from 5 directly. n/a = not obtained
To further confirm this reaction sequence, the structure of the novel pyran products, and the potential for this addition-cyclization reaction deprotection of the pyrans were undertaken. The first objective was to remove the exocyclic hydrazone group from pyrans 4a–i. We discovered that H2/PtO2 (10% by weight) and CF3CO2H (4 eq.) reduces the C=N bond and cleaves the N-N bond and also reduces the enol ether unsaturation to afford primary amines 5. Likewise, Ra-Ni (6%) in methanol at room temperature converted the pyrans to primary amine 5.9 Although the yields for the RaNi process (entries 4e–i) were lower, isolation and workup were superior to PtO2.
Pyrans 5fg were amenable to direct conversion to the fully deprotected pyran dicarboxylic acids 7fg using 6M HCl (~50% yield). However, pyrans 5a–ehi were challenging to isolate and separate from impurities by this reaction and therefore, were not amenable to direct deprotection. As a result, the primary amines were reacted with 1.5 equiv p-nitrobenzoyl chloride (1.5 eq) in CH2Cl2 with TEA (2.0 eq) to afford amides 6a–d as tan foams in 40–45% yield. We were unable to convert pyrans 5ehi to the amides 6 or directly to product 7 presumably due to their sensitivity to acid (decomposition noted by tlc). High resolution mass spectrometry, 1H NMR, 13C NMR and IR supported the structures of amides 6a–d. The analysis was consistent with assignment as tetrahydopyrans.
Although the stepwise deprotection reactions of amide and esters were examined, we preferred to identify a onestep process. Dual hydrolysis of the methyl esters and amide bond under basic conditions failed leading to a complex mixture of products. However, reacting 6a–d in 3 M HCl for 24 h at reflux cleanly hydrolyzed both ester and amide protecting groups with the p-nitrobenzoic acid byproduct precipitating during the course of the reaction. Standard workup afforded the 6-methanaminopyran 2,4-diacids 7a–d in 50–60% yield without further purification.
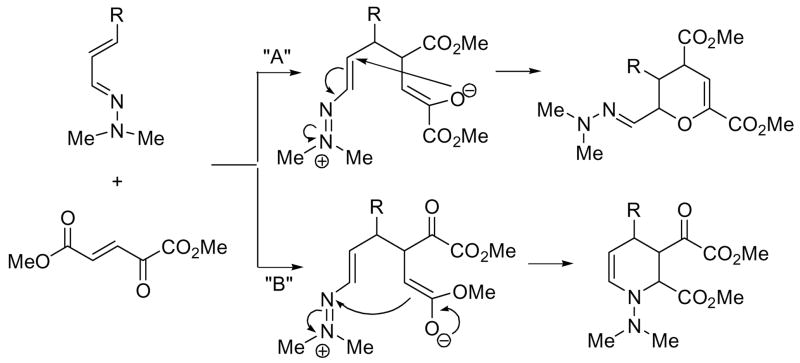
The mechanism of the reaction is deserving of note. The pyran products 4 likely result from initial addition of the azadiene onto the DOG backbone followed by an intramolecular conjugate addition by the enolate oxygen. (Scheme 3, path “A”). The planned azadiene-DOG synthesis of piperidines could occur either by a Diels-Alder or via the same initial addition but cyclization via the enolate carbon (Scheme 3, path “B”). In an effort to alter the reaction outcome and favor C-alkylation, solvents other than CH3CN were explored including polar, protic solvents (water, isopropanol, etc.) and non-polar solvents (ether, pentane) but the reaction led to the same pyran product (lower yields) regardless of the azadiene employed. Because certain transannular amino acid analogs show activity as inhibitors of glutamate receptors, the pyrans 7a–dfg prepared in this study were tested at the AMPA,10 NMDA11 and kainate12 glutamate receptors using radioligand binding assays (MDS Pharma Services; Taipei, Taiwan). The results indicated that the pyrans were inactive except the 6-methyl analog 7c that showed 17% inhibition of the AMPA receptor at 100 nM. We are currently exploring variants of this structure as selective inhibitors.
Scheme 3.
Pathway leading to pyran and piperidine from azadiene and DOG.
Acknowledgments
The authors acknowledge financial support of the NIH to CMT (RO1 NS38248). The authors are also grateful for the support of the Core Laboratory for Neuromolecular Production (NIH P30-NS055022), the Center for Structural and Functional Neuroscience (NIH P20-RR015583) and scientific advice from Prof. C. Sean Esslinger and Dr. Travis T. Denton.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Bridges RJ, Esslinger CS. Pharmacol Ther. 2005;107:271–85. doi: 10.1016/j.pharmthera.2005.01.002.Bridges RL. The Ins and Outs of Glutamate Transporter Pharmacology. Tocris Reviews. 2001;(17)Acher FC. Metabotropic Glutamate Receptors Tocris Bioscience Scientific Review Series. 2006.
- 2.Bridges RJ, Stanley MS, Anderson MW, Cotman CW, Chamberlin AR. J Med Chem. 1991;34:717–25. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- 3.Willis CL, Humphrey JM, Koch HP, Hart JA, Blakely T, Ralston L, Baker CA, Shim S, Kadri M, Chamberlin AR, Bridges RJ. Neuropharmacology. 1996;35:531–539. doi: 10.1016/0028-3908(96)84623-5. [DOI] [PubMed] [Google Scholar]
- 4.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. J Neurosci. 1994;14:5559–69. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serckx-Poncin B, Hesbain-Frisque AM, Ghosez L. Tetrahedron Lett. 1982;23:3261–4. [Google Scholar]
- 6.Carrigan CN, Bartlett RD, Esslinger CS, Cybulski KA, Tongcharoensirikul P, Bridges RJ, Thompson CM. J Med Chem. 2002;45:2260–2276. doi: 10.1021/jm010261z.Denton TT, Seib T, Bridges RJ, Thompson CM. Bioorg Med Chem Lett. 2002;12:3209–13. doi: 10.1016/s0960-894x(02)00520-6.
- 7.Representative synthesis. Dimethyl 2-[(E)-(dimethylhydrazino)methyl]-2-methyl-3,4-dihydro-2H-pyran-4,6-dicarboxylate (4c). To a solution of methacrolein azadiene (0.36 g, 3.2 mmol) in CH3CN (5 mL) was added 1 (0.5 g, 2.9 mmol) and stirred for 24 h. The CH3CN was removed and the red oil chromatographed (SiO2,1:4 EtOAc:hex) to afford 4c as a light yellow oil (40%). 1H NMR (CDCl3): δ6.39 (br s, 1H), 6.18 (br s, 1H), 3.80 (s, 3H), 3.73 (s, 3H), 3.55 (m, 1H), 2.74 (s, 6H), 2.55 (dd, J = 6.7, 13.5 Hz, 1H), 1.91 (br t, J = 12.3 Hz, 1H), 1.50 (s, 3H); 13C NMR (CDCl3): δ172.8, 163.2, 143.1, 135.5, 108.4, 78.4, 52.2, 52.1, 42.6, 36.3, 31.5, 27.3; Anal. Calcd for C13H20N2O5 : C, 54.92; H, 7.09; N, 9.85. Found C, 55.04; H, 7.02; N, 9.69. Dimethyl 6-methyl-6-{[(4-nitrobenzoyl)amino]-methyl}tetrahydro-2H-pyran-2,4-dicarboxylate (6c). To 4c (0.29 g, 1.0 mmol) in MeOH (10 mL) was added 10% PtO2 (0.29 g) and TFA (0.46 g, 4.0 mmol) in a Parr flask. The reaction with H2 (50 psi) was conducted for 18 h, filtered through Celite and concentrated. The resulting clear oil was extracted from CH2Cl2 to afford the primary amine 5c (0.20 g, 82 %) as a pale yellow oil. Amine 5c (0.2 g, 0.83 mmol) was dissolved in CH3OH (10 mL) and reacted with TEA (0.13 g, 1.25 mmol) and p-nitrobenzoyl chloride (0.15 g, 0.83 mmol) at rt for 24 h. Workup and purification (40 % EtOAc:hex) afforded the amide 6c (0.17 g, 52%) as a tan foam. 1H NMR (CDCl3): δ 8.28 (d, J = 8.4 Hz, 2H), 7.94 (d, J = 8.4 Hz, 2H), 6.52 (br s, 1H), 4.20 (dd, J = 2.2, 12.1 Hz 1H) 3.75 (s, 3H), 3.72 (m, 1H), 3.71 (s, 3H), 3.52 (dd, J = 7.0, 14.3 Hz 1H), 2.86 (m, 1H), 2.27 (m, 1H), 1.95 (m, 1H), 1.68 (m, 2H), 1.35 (s, 3H); 13C NMR (CDCl3): δ 173.7, 170.7, 165.9, 149.6, 139.7, 128.2, 123.8, 74.0, 69.8, 52.4, 52.2, 41.85, 36.5, 35.2, 29.7, 27.0; Anal. Calcd for C18H22N2O8 : C, 54.82; H, 5.62; N, 7.1. Found C, 54.68; H, 5.63; N, 6.85. 6-(Aminomethyl)-6-methyltetrahydro-2H-pyran-2,4-dicarboxylic acid (7c). Compound 6c (0.05 g, 0.13 mmol) was dissolved in 3M HCl (5 mL) and brought to reflux for 24 h, cooled, filtered, and extracted with EtOAc (3 × 25 mL). The resulting H2O layer was concentrated to a solid in vacuo, and triturated with water to afford the diacid hydrochloride product 7c (0.017 g, 62%) as a tan solid. 1H NMR (D2O): δ 4.12 (d, J = 12.1 Hz, 1H), 3.41 (d, J = 13.9 Hz, 1H), 2.72 (m, 2H), 2.12 (d, J = 13.9 Hz, 1H), 1.83 (d J = 14.2 Hz, 1H), 1.49–1.39 (br m, 2H) 1.16 (s, 3H); 13C NMR (D2O): δ177.8, 174.8, 72.9, 69.4, 40.9, 35.9, 34.8, 29.7, 25.3. HRMS Calcd for C9H16NO5: 218.1036. Found: 218.1028. (M+H)+.
- 8.Remuzon P, Dussy C, Jacquet JP, Roty P, Bouzard D. Tetrahedron: Asymmetry. 1996;7:1181–1188.Solladie-Cavallo A, Bonne F. Tetrahedron: Asymmetry. 1996;7:171–80.
- 9.Alexakis A, Lensen N, Mangeney P. Tetrahedron Lett. 1991;32:1171–1173. [Google Scholar]
- 10.Honore T, Lauridsen J, Krogsgaard-Larsen P. J Neurochem. 1982;38:173–8. doi: 10.1111/j.1471-4159.1982.tb10868.x. [DOI] [PubMed] [Google Scholar]
- 11.Siegel BW, Sreekrishna K, Baron BM. Eur J Pharmacol. 1996;312:357–65. doi: 10.1016/0014-2999(96)00477-3. [DOI] [PubMed] [Google Scholar]
- 12.London ED, Coyle JT. Mol Pharmacol. 1979;15:492–505. [PubMed] [Google Scholar]