Abstract
Bone morphogenetic proteins (BMPs) are morphogens with long-range signaling activities. BMP-7 is secreted as a stable complex consisting of a growth factor noncovalently associated with two propeptides. In other transforming growth factor-β-like growth factor complexes, the prodomain (pd) confers latency to the complex. However, we detected no difference in signaling capabilities between the growth factor and the BMP-7 complex in multiple in vitro bioactivity assays. Biochemical and biophysical methods elucidated the interaction between the BMP-7 complex and the extracellular domains of its type I and type II receptors. Results showed that type II receptors, such as BMP receptor II, activin receptor IIA, and activin receptor IIB, competed with the pd for binding to the growth factor and displaced the pd from the complex. In contrast, type I receptors interacted with the complex without displacing the pd. These studies suggest a new model for growth factor activation in which proteases or other extracellular molecules are not required and provide a molecular mechanism consistent with a role for BMP receptors in the establishment of early morphogen gradients.
Keywords: BMP, growth factor, receptor, signaling, propeptide
Introduction
Bone morphogenetic proteins (BMPs) perform important roles in many developmental processes, including early patterning. DPP, a Drosophila BMP, functions as a long-range morphogen during wing development.1 Vertebrate BMP-7 is expressed in many tissues during embryogenesis and is essential for normal development of the kidney, eye, and autopod.2,3 Because BMP-7 can also induce new bone formation when implanted within a suitable matrix,4 it is an attractive therapeutic agent for bone regeneration in humans. However, with the exception of BMP antagonists, such as noggin and chordin, little is known about extracellular mechanisms that regulate BMP signaling and activation.
Like transforming growth factor-β (TGF-β), BMP-7 is synthesized as a precursor protein that is processed, generating an N-terminal propeptide and a C-terminal disulfide cross-linked dimer. Like TGF-β, the secreted form of BMP-7 is a complex, consisting of the C-terminal dimer and two non-covalently associated prodomains (pds) that target the growth factor to fibrillin-1,5 the major structural component of extracellular microfibrils. TGF-β is also targeted to extracellular microfibrils through interactions between its pd and latent TGF-β binding proteins.6,7
In addition to targeting growth factors to the extracellular matrix, pds of TGF-β and GDF-8 (myostatin) are known to confer latency to the C-terminal growth factor dimer (gfd).8–10 Significant structural rearrangements have been shown to occur when the pd of TGF-β-1 (called β-1-latency-associated peptide or β-1-LAP) forms a complex with TGF-β-1.11,12 Therefore, latency may result either from β-1-LAP blocking the interaction of TGF-β with its receptors or from LAP inducing a conformational change in TGF-β such that it no longer interacts with its receptors.12 Similar structural changes were observed when BMP-7 pd forms a complex with BMP-7 gfd,5 suggesting that the pd of BMP-7 could confer latency through similar mechanisms.
Activation of TGF-β growth factor complexes can occur through various mechanisms, including thrombospondin-and integrin-mediated mechanisms.13,14 In addition, proteolytic cleavage of the pd in latent complexes of TGF-β and GDF-8 may be an important mechanism of activation.15,16 In contrast to what is known about TGF-β activation, little is known about the activation of BMPs and the role of the pd during BMP activation.
In this study, we tested whether the pd of BMP-7 confers latency to the complex and whether the pd can block receptor binding. By analogy to TGF-β and GDF-8, we expected that the BMP-7 pd would perform these functions, especially because the BMP-7 complex is very stable.5 However, we were surprised to find that bioactivity assays failed to demonstrate that the presence of the pd results in a reduction in BMP-7 activity. Therefore, additional biochemical and biophysical studies were performed in order to determine how the BMP-7 complex interacts with its receptors. These studies revealed that type II, but not type I, receptors compete with the pd for binding to the gfd and are able to displace the pd. Based on the molecular mechanisms described here, we propose a new model for BMP activation that does not require proteases or other extracellular matrix molecules.
Results
Activity of the BMP-7 pd–growth factor complex
In order to test whether the association of the BMP-7 pd with the processed gfd results in gfd latency, we measured the activity of the BMP-7 pd-gfd complex and compared it with the activity of the free gfd. C3H/10T1/2 cells, which express activin receptor (ActR) II, ActRIIB, BMP receptor (BMPR) II, and ALK2, ALK3, ALK4, and ALK5,17 were transiently transfected with the Δ3Msx2luciferase construct, containing a 1.8-kb fragment of the 5'-flanking sequence of Msx2.18,19 The cells were then incubated either with free BMP-7 gfd or with pd-gfd complex at 3.85–30.8 nM. BMP-2 gfd at the same molar concentrations was incubated as a positive control; bovine serum albumin (BSA), as a negative control. These BMP concentrations were experimentally determined to generate sufficient BMP-7 signals over basal levels [the reporter assay used is not as responsive to BMP-7 as it is to BMP-2]. After 24 h of BMP incubation, the cells were harvested and lysed, and luciferase activity was measured. The addition of both the free BMP-7 gfd and the BMP-7 complex resulted in the same induction of luciferase activity over basal levels (Fig. la). This finding was surprising, because it suggested that, unlike TGF-β-1 and GDF-8 complexes, the BMP-7 complex is not latent.
Fig. 1.
BMP-7 complex and free BMP-growth factor are equally active. (a) BMP-2, BMP-7 gfd, and BMP-7 complex were added for 1 day to C3H/10T1/2 cells that were transiently transfected with an Msx2 reporter construct. Both BMP-7 species showed the same activation of Msx2 promoter-TK-luciferase reporter gene activity over basal levels for all concentrations (3.85–30.8 nM). BMP-2 served as a positive control, whereas BSA served as a negative control. (b) BMP-7 complex (7cplx) and BMP-7 (7gfd) were added in equal molar amounts increasing from 0.32 nM (corresponding to 10 ng/ml of BMP-7 gfd and 30 ng/ml of BMP-7 complex) to 3.2 nM to ATDC5 chondroprogenitor cells and incubated for 1 h. For positive and negative controls, cells were treated with equal amounts of BMP-2 gfd (2gfd; R&D Systems) and similar or higher nanograms of BSA. Smad phosphorylation, detected by Western blotting, was induced to the same extent by both BMP-7 species. Ponceau S stain shows the total transferred proteins, (c) ATDC5 cells were treated with BMP-7 at a constant concentration of 3.2 nM (100 ng/ml of BMP-7 gfd and 300 ng/ml of BMP-7 complex). Cells were examined after 20 min or up to 6 h by Western blotting for pSmad1/5/8. BMP-7 gfd and BMP-7 complex showed equivalent kinetics and persistence of pSmads. (d) C2C12 cells were also treated with BMPs in a similar time course experiment as in (c). Total RNA was harvested, and quantitative real-time RT-PCR assays were performed in triplicate for Id3, a BMP-responsive gene. The data are representative of two independent experiments. RNA levels of the ARBP PO gene, which are constant during treatment, were used to standardize samples. Fold expression values were calculated relative to the BSA-treated sample for each time point. BMP-7 gfd and BMP-7 complex resulted in equal induction of Id3.
The protocol for this assay required an incubation period of 24 h before luciferase activity was measured. During this 24-h period of BMP treatment, BMP-7 complex may have been activated, resulting in the appearance that the BMP-7 complex was not latent. To test this possibility, we utilized assays with shorter periods for BMP treatment. Either BMP-7 complex or free BMP-7 gfd was added in equal molar amounts increasing from 0.32 nM (10 ng/ml of BMP-7 gfd; 30 ng/ml of BMP-7 complex) to 3.2 nM (100 ng/ml of BMP-7 gfd; 300 ng/ml of BMP-7 complex) to ATDC5 chondroprogenitor cells. ATDC5 cells express BMPRII, ActRIIB, ALK2, and ALK3.20 For positive and negative controls, cells were treated with equal amounts of BMP-2 gfd and similar or higher amounts of BSA. After 1 h of incubation, the cells were harvested and analyzed for Smad phosphorylation. Immunoblotting assays with an antibody recognizing the phosphorylated forms of Smadl, Smad5, and Smad821 were performed using whole cell extracts. The BMP-7 complex displayed the same bioactivity in this assay as the free BMP-7 gfd (from R&D Systems; Fig. 1b). In order to investigate the kinetics of Smad phosphorylation, we carried out a time course experiment, harvesting ATDC5 cells after 20 min to 6 h of treatment with BMP-7; no significant difference was observed between the BMP-7 complex and the free gfd (Fig. lc).
A third approach was used in order to better quantitate BMP activity. A time course experiment was conducted using C2C12 cells treated over 20 min to 6 h with BMP-2 (positive control), BSA (negative control), BMP-7 complex, or free gfd. The expression level of the Id3 element was analyzed by quantitative real-time reverse transcriptase (RT)-PCR (Fig. 1d). Significant induction of Id3, a BMP-responsive gene, was detected after 3 and 6 h of BMP treatment. For all measured time points, there was no significant difference in the induction of Id3 expression between the BMP-7 gfd and the BMP-7 complex.
The BMP-7 pd interacts with the growth factor at sites close to the type II receptor binding sites
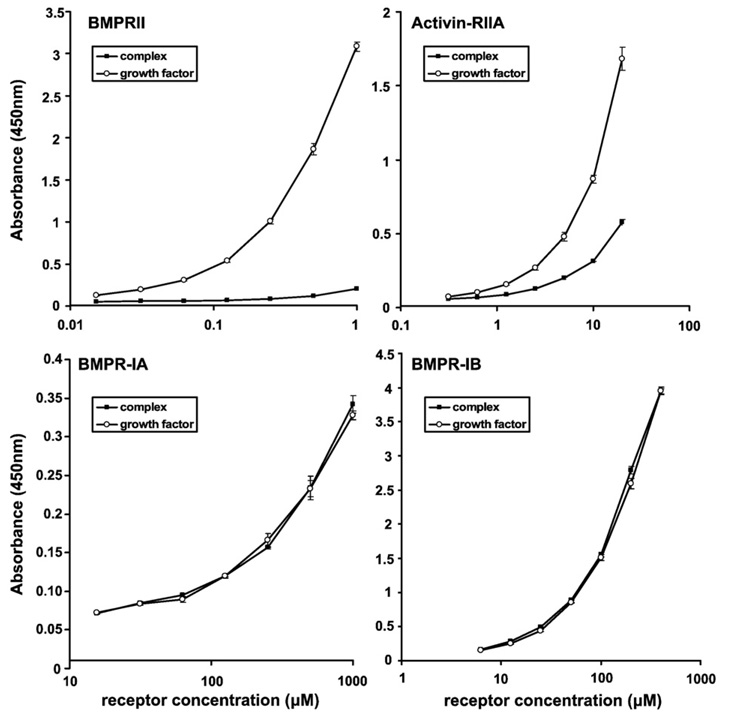
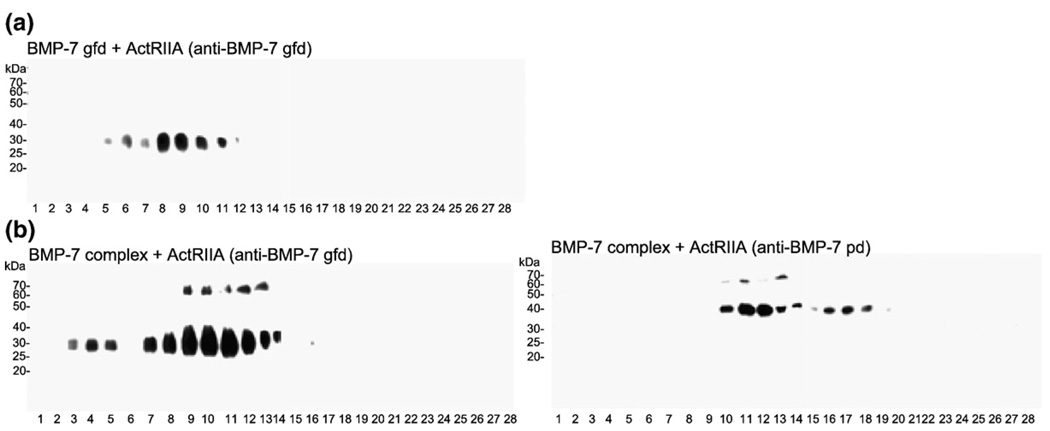
Next, receptor binding to BMP-7 was tested in the presence and absence of the pd. The ectodomains of BMPRIA (ALK3), BMPRIB (ALK6), BMPRII, and ActRIIA were tested using ELISA binding assays in which the BMP-7 gfd and the BMP-7 complex were coated onto the well (Fig. 2). ELISA and BIAcore interaction studies demonstrated no binding between the pd and the receptors (data not shown). BMPRIA and BMPRIB interacted well with both the pd-gfd complex and the separated gfd, suggesting that the presence of the pd does not affect binding of these type I receptors to the gfd. However, binding of BMPRII and ActRIIA to the complex was significantly inhibited by the presence of the pd. A similar observation was made using surface plasmon resonance (SPR) when equal molar amounts of the BMP-7 gfd and the BMP-7 complex were coupled to a biosensor chip. The response of injected ActRIIA and BMPRII onto a chip with immobilized BMP-7 complex was reduced by 90% compared with the signal obtained when the same amounts of type II receptor were injected onto a chip carrying immobilized BMP-7 gfd (Supplementary Fig. 13). These data suggested that the pd interacts with the gfd close to the type II receptor binding sites and that the pd may block binding of the type II receptor.
Fig. 2.
The BMP-7 pd interacts with the growth factor at sites close to the type II receptor binding sites. The extracellular domains of the receptors BMPRII, ActRIIA, BMPRIA, and BMPRIB (R&D Systems) were used as soluble ligands in ELISA binding assays with coated BMP-7 substrates. Binding of these ligands to the separated gfd (open circles) was compared with binding to the BMP-7 complex (filled squares). Binding of the receptor ligands was detected with monoclonal antibodies specific for each receptor (R&D Systems). Each data point represents the average value of a triplicate. Error bars are shown.
Type II receptors bind to BMP-7 and displace the pd
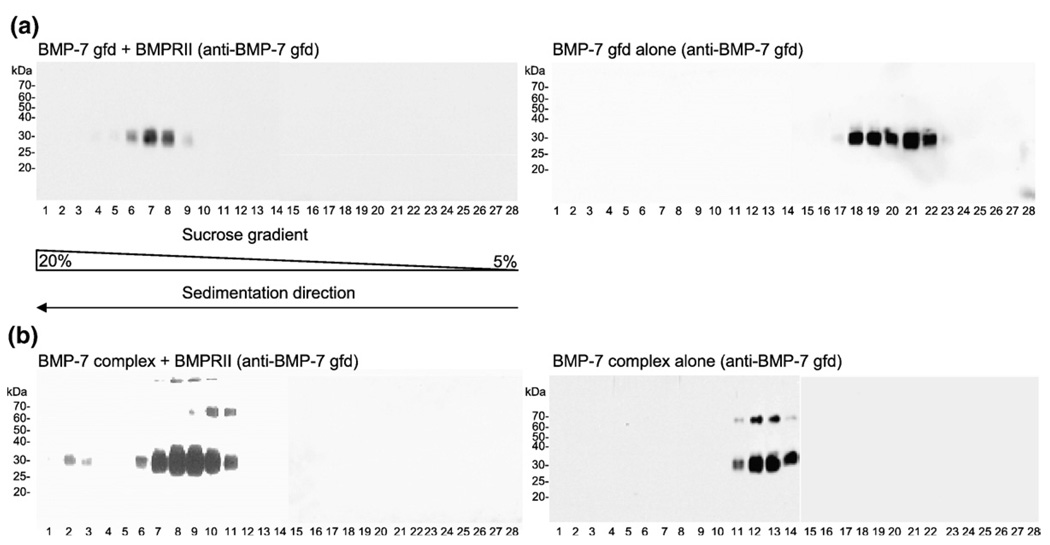
In order to further test whether the pd blocks the binding of type II receptors to the BMP-7 complex, we tested interactions in solution. Velocity sedimentation experiments were performed using 5%–20% sucrose gradients. Either BMP-7 complex (0.53 µM) or free BMP-7 gfd (0.79 µM) was dialyzed together with BMPRII at a molar ratio of 1:2.5 in TBS and then subjected to velocity sedimentation. Migration of BMP-7 throughout the gradient was monitored by immunoblotting of each fraction (Fig. 3) using monoclonal antibodies specific for the BMP-7 gfd or the BMP-7 pd. For comparison, reference gradients were established with the free BMP-7 gfd (calculated molecular mass = 31.4 kDa) alone (Fig. 3a, right panel) or with the BMP-7 complex (calculated molecular mass = 94.6 kDa) alone (Fig. 3b, right panel). Bands with slower mobility likely represent monomeric unprocessed, full-length BMP-7, which constitutes only a small percentage of the total protein in the BMP-7 complex preparation.
Fig. 3.
BMPRII binds the BMP-7 complex in solution. BMP-7 gfd and BMP-7 complex were each dialyzed with BMPRII against TBS and were then applied to a 5%–20% sucrose gradient. Results were compared with reference runs with BMP-7 gfd alone or BMP-7 complex alone. Fractions were collected starting with fraction 1 (20%) to fraction 28 (5%) and were analyzed by immunoblotting with monoclonal anti-BMP-7 gfd antibody (R&D Systems). (a) Left panel: BMP-7 gfd (0.79 µM) was dialyzed together with BMPRII at a gfd/receptor molar ratio of 1:2.5, showing a signal in fractions 6–9 (peak fraction, 7); right panel: BMP-7 gfd in TBS sediments in fractions 18–22 (peak fractions, 20 and 21). (b) Left panel: BMP-7 complex (0.53 µM; molecular mass = ∼94.6 kDa) dialyzed at a molar ratio of 1:2.5 with BMPRII in TBS shows two signals in fractions 2 and 3 and fractions 6–11 (double peak), indicating three possible peaks; right panel: BMP-7 gfd (molecular mass=31.6 kDa) signals of BMP-7 complex in TBS were detected in fractions 11–14 (peak fractions, 12 and 13). Signals ∼60–70 kDa represent small amounts of unprocessed BMP-7 full-length monomer detectable only by blotting.
As a positive control, BMPRII was incubated with free BMP-7 gfd and then subjected to velocity sedimentation. When the gradient fractions were immunoblotted with antibody to BMP-7 gfd, the resulting receptor-gfd complex appeared primarily in fractions 6–9 (Fig. 3a, left panel), 12 fractions farther down in the gradient compared with the reference gradient with free BMP-7 gfd alone (fractions 18–22, Fig. 3a, right panel). These results demonstrated, as expected, that binding of free BMP-7 gfd by BMPRII could be detected after velocity sedimentation. Equilibrium ultracentrifugation of BMPRII incubated with free BMP-7 gfd (molar ratio = 2:1) revealed that the peak in fractions 6–9 (Fig. 3a, left panel) consists of a complex of one BMPRII-Fc dimer molecule bound to two gfds, which represents a ratio of receptor binding site to gfd binding site of 1:1. Table 1 shows the molecular masses determined by equilibrium ultracentrifugation of the free BMPRII-Fc dimer and the receptor dimer bound to BMP-7 gfd.
Table 1.
Molecular masses determined by analytical ultracentrifugation of BMPRII-Fc alone and BMPRII-Fc incubated with BMP-7 gfd (molar ratio = 2:1)
| Species | Molecular mass (kDa) |
|---|---|
| BMPRII-Fc | 117±5 |
| BMPRII-Fc + BMP-7 gfd | 193±5 |
When the BMP-7 complex was tested for binding to BMPRII, the position of the immunoblotted BMP-7 gfd signal appeared predominantly in fractions 6–11 (Fig. 3b, left panel), a shift of five fractions farther down in the gradient from the peak fractions (fractions 11–14) containing the BMP-7 complex alone (Fig. 3b, right panel). In contrast to the solid-phase binding data, in which the BMP-7 complex was immobilized to the plate, these data indicated that the presence of the pd in the BMP-7 complex did not prevent BMPRII from binding to BMP-7 in solution.
Complexes of BMPRII-BMP-7 sedimented in fractions 6–9 in both experiments described above. Intriguingly, in the case of the interaction between the BMP-7 complex and BMPRII, the immunoblotted BMP-7 gfd signal showed a broader distribution, indicating the possibility of multiple peaks (fractions 2 and 3, fractions 6–11), representing the formation of distinct interaction products.
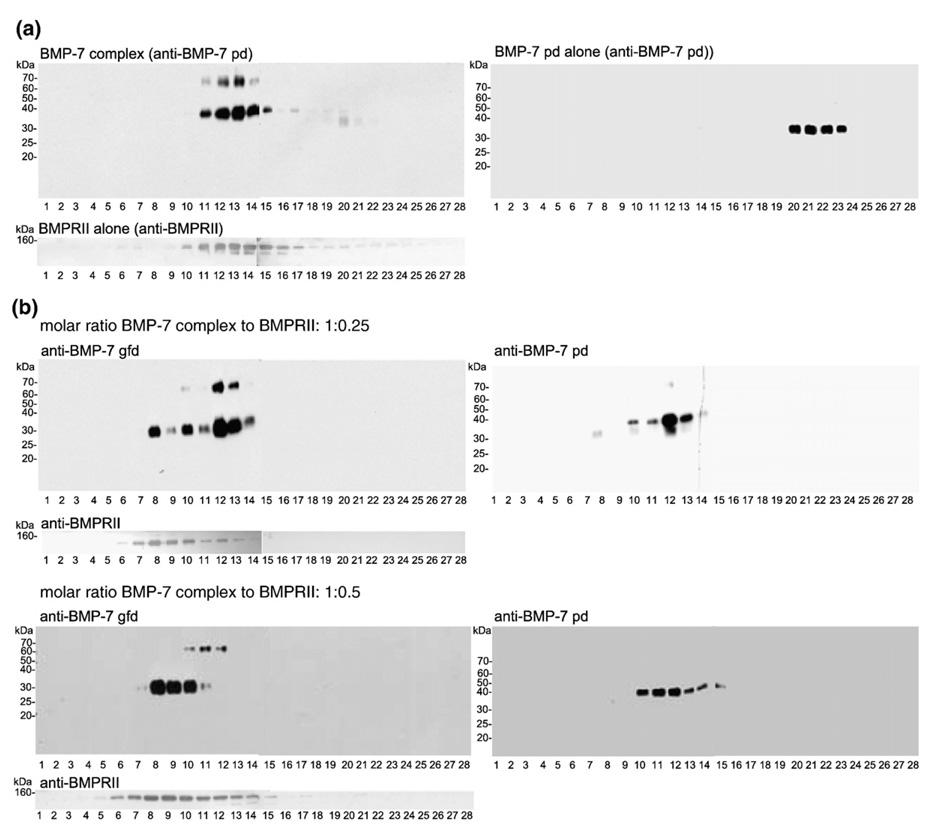
To clarify these complexes of BMPRII-BMP-7, we performed titration experiments using a constant concentration of BMP-7 complex (0.53 µM) with increasing molar ratios of BMP-7 complex to BMPRII ranging from 1:0.25 to 1:2.5 (Fig. 4 and Fig. 5). In the case of excess BMP-7 complex to BMPRII (molar ratio = 1:0.25; Fig. 4), the immunoblotted BMP-7 gfd signal was already shifted farther down in the gradient, indicated by the appearance of two additional peaks in fractions 8 and 10 (Fig. 4b, left panel) compared with the gfd signal for the BMP-7 complex reference gradient (Fig. 3b, right panel). After stripping and reincubation with anti-BMP-7 pd antibody, the blot showed signals for the BMP-7 pd only in fractions 10–14 (Fig. 4b, right panel). Therefore, fraction 8 represented freed BMP-7 gfd bound to BMPRII. Fraction 10 showed antibody signals for both BMP-7 pd and BMP-7 gfd domain, suggesting that, in this fraction, the BMP-7 complex is bound to the receptor. Incubation with anti-BMPRII supported these findings, showing that the peak signals for the receptor appeared in fractions 7–10 (Fig. 4b), four fractions farther down in the gradient compared with the reference run with BMPRII alone (Fig. 4a, fractions 11–14). At this concentration of a molar excess of BMP-7 complex to BMPRII, the main portion of BMP-7 complex remains unbound because the peak signal for both the gfd and the pd is in fraction 12 (compare Fig. 4b with the reference runs in Fig. 3b, right panel, and Fig. 4a).
Fig. 4.
Binding of BMPRII to the BMP-7 complex results in displacement of the BMP-7 pd. (a) Reference runs of BMP-7 complex in TBS (bands at 70 kDa represent unprocessed BMP-7 full-length monomer), BMP-7 pd in TBS (immunoblotted with anti-BMP-7 pd antibody), and BMPRII (immunoblotted with anti-BMPRII antibody; R&D Systems). (b) Left panel: blots with anti-BMP-7 gfd antibody; right panel: blots with anti-BMP-7 pd antibody. At a BMP-7 complex/BMPRII molar ratio of 1:0.25, the BMP-7 gfd signal is shifted farther down in the gradient, resulting in three individual peaks (fractions 8, 10, and 12). Fractions 8 and 9 showed no pd signal (right side), indicating the presence of a freed BMP-7 gfd bound to the receptor domain. Immunoblotting for BMPRII showed the presence of the receptor in the same fractions as the freed BMP-7 gfd. At a molar ratio of 1:0.5 (BMP-7 complex/BMPRII), the equilibrium is shifted more toward the state of freed gfd-receptor complex and almost no free BMP-7 complex is detectable.
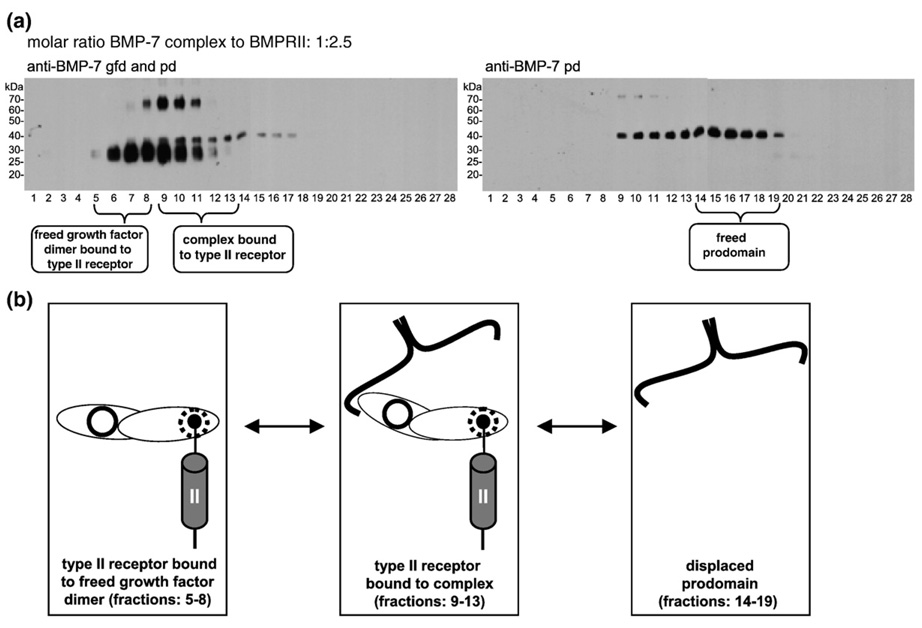
Fig. 5.
BMPRII displaces the BMP-7 pd as a dimer from the complex. (a) BMP-7 complex (0.53 µM) incubated with 1.32 µM BMPRII (1:2.5) and analyzed by immunoblotting for the BMP-7 gfd and the BMP-7 pd (left panel) and for the BMP-7 pd only (right panel) after velocity sedimentation. Three individual but overlapping peaks were recognizable, indicating the formation of three species: BMP-7 gfd bound to the BMPRII (fractions 5–8); BMP-7 complex bound to the receptor (fractions 9–13); and freed pd dimer (fractions 14–19). (b) Cartoon representation of a dynamic equilibrium of the different tentative interaction intermediates forming the three distinctive peaks in (a): BMP-7 gfd species with no attached pd bound to receptor; BMP-7 complex bound to the receptor; and freed BMP-7 pd, which is displaced from the complex by the BMPRII. Each BMP gfd has two independent interaction sites for its type I (solid circle) and type II (dashed circle) receptors.
A twofold increase of the BMPRII (1:0.5) resulted in a shift of the BMP-7 gfd to fractions 8–10 (Fig. 4b). Incubation with anti-BMPRII demonstrated that the main signals for the receptor were in the same fractions (Fig. 4b). Immunoblotting of the pd showed that peak fractions 8 and 9 contained no pd (Fig. 4b, compare the left panel with the right panel), confirming the presence of a freed BMP-7 gfd bound to its receptor in these fractions. No BMP-7 gfd was detected in fractions 12–15, demonstrating that much of the BMP-7 gfd present in the complex (found in fractions 11–14 in the reference gradient shown in Fig. 3b, right panel) was bound to BMPRII. Most interestingly, pd signals were found in fractions 12–15 without detectable gfd signals, indicating the presence of freed pd in these fractions. Compared with the reference run with separated BMP-7 pd alone (Fig. 4a, right panel, fractions 20–23), the sedimentation of the freed pd in fractions 12–15 displayed a shift of nine fractions farther down in the gradient. This finding suggests that the freed pd may be displaced as a dimer.
A 2.5-fold excess of the receptor over the complex resulted in more freed BMP-7 gfd bound to BMPRII, found in fractions 5–8 (Fig. 5a). Fractions 9–13 contained signals for both the pd and the gfd (Fig. 5a), indicating the presence of BMP-7 complex bound to BMPRII. Fractions 14–19 contained freed pd dimer (Fig. 5a). Based on these data, the cartoon in Fig. 5b depicts the possible interacting species represented in the gradient. These species are likely formed in dynamic equilibrium in the gradient, after incubation of the BMP-7 complex with BMPRII: freed BMP-7 gfd bound to the receptor; BMP-7 complex bound to the receptor; and freed pd. Sometimes a minor fraction of BMP-7 gfd shifted even farther down in the gradient (fractions 2 and 3, Fig. 3b). We interpret these results to indicate the formation of a high-molecular-weight complex, induced by the Fc receptor dimers, consisting of two BMPRII-Fc dimers and two, three, or four BMP-7 gfd molecules.
Activin type II receptors also displaced the pd from the BMP-7 complex. In sedimentation experiments using a molar ratio of BMP-7 gfd or BMP-7 complex to ActRIIA of 1:2.5 (condition of excess receptor), similar gfd and pd patterns were obtained. The reference run of free BMP-7 gfd together with ActRIIA demonstrated anti-BMP-7 gfd signals in fractions 5–11 (Fig. 6a). When the BMP-7 complex was tested with ActRIIA, distinct peaks were again detected (Fig. 6b): BMP-7 complex (fractions 11–14); BMP-7 complex bound to receptor (fractions 10–12); and freed gfd bound to receptor (fractions 7–9). Freed BMP-7 pd was also detected (fractions 15–18). Titrating ActRIIA to the BMP-7 complex (complex/receptor molar ratio = 1:0.25–10) resulted in a concentration-dependent displacement of the pd from the gfd (data not shown). An additional peak very early in the gradient (fractions 3–5) is most likely due to the binding of Fc receptor dimers to the gfd, as in the case of BMPRII. Identical results were obtained after sedimenting the BMP-7 complex bound to ActRIIB (data not shown).
Fig. 6.
ActRIIA also displaces the BMP-7 pd. (a) Sucrose gradient of ActRIIA together with the BMP-7 gfd. Immunoblotting with anti-BMP-7 gfd showed a main peak in fractions 8 and 9, indicating the formation of a receptor-gfd complex, (b) Interaction of BMP-7 complex with ActRIIA. Immunoblotting of sucrose gradient fractions revealed the appearance of four peaks: free complex (fractions 11–14); complex bound to the receptor molecule (fractions 10–12); free gfd bound to the receptor (fractions 7–9); and high-molecular-weight complexes, caused by dimerization of the Fc domains of the receptor molecules, possibly consisting of two BMPRII-Fc dimers and multiple BMP-7 gfds (fractions 3–5).
In order to exclude the possibility of artifactual pd displacement in our experiments, we tested the interaction of the GDF-8 complex with its type II receptor by velocity sedimentation. GDF-8 circulates in the blood as a latent complex, consisting of the GDF-8 gfd together with the GDF-8 pd, and requires proteolysis for activation.16,22 GDF-8 signals by binding to ActRIIB.17 Results demonstrate that ActRIIB cannot displace the GDF-8 pd (Fig. 7). To perform these experiments, we first reconstituted the GDF-8 complex in solution, using commercially available GDF-8 gfd and the GDF-8 pd. When allowed to recombine, the GDF-8 components sedimented together in fractions 10–15 (Fig. 7). Compared with the reference run of the GDF-8 pd alone (fractions 19–22; data not shown), the reconstituted GDF-8 complex sedimented eight fractions farther down in the gradient. Addition of ActRIIB to the GDF-8 complex at complex/receptor molar ratios of 1:0.5 and 1:2.5 (data not shown) resulted in no shift of the GDF-8 complex peak fractions, as shown by immunoblotting either with anti-GDF-8 pd or with anti-GDF-8 gfd (Fig. 7). Similarly, the main peak for ActRIIB remained unaffected (Fig. 7), confirming that the presence of the GDF-8 pd within the GDF-8 complex successfully blocked the interaction of the GDF-8 gfd with its receptor.
Fig. 7.
The reconstituted GDF-8 complex does not bind to its type II receptor, ActRIIB. GDF-8 complex was successfully reconstituted by dialyzing the gfd together with its pd (both components were purchased from R&D Systems). The reconstituted GDF-8 complex (0.1 µM) sample was dialyzed together with ActRIIB (0.05 µM) in TBS. The signals for the GDF-8 gfd (upper left panel) and pd (upper right panel) as well as for the receptor (lower left panel) remain unaffected in the presence of each other, compared with the reference runs (ActRIIB alone, lower right panel; reconstituted GDF-8 complex alone, fractions 10–15; GDF-8 pd alone, fractions 19–222), indicating that no higher-molecular-weight complex was formed and no interaction could be detected.
Type I receptors cannot displace the BMP-7 pd
As additional controls, we carried out titrations with the BMP-7 complex and the soluble extracellular domains of BMPRIA and BMPRIB, which were able to bind to the BMP-7 complex in solid-phase assays (Fig. 2). At a BMP-7 complex/BMPRIA molar ratio of 1:0.25, the BMP-7 gfd and the BMP-7 pd signals appeared in fractions 9–14 (Fig. 8a). Compared with reference runs of the BMP-7 complex that showed signals for both components in fractions 11–14 (Fig. 3b, right panel; Fig. 4a, left panel), these results suggested the presence of two main species: unbound complex in fractions 12–14 and BMP-7 complex bound to BMPRIA in fractions 9–10, with both species overlapping in fraction 11 (Fig. 8b). This finding of BMPRIA bound to the BMP-7 complex was confirmed by observing peak receptor signals in the same fractions (fractions 9–11, Fig. 8a), a shift of the peak receptor signals four fractions farther down in the gradient than in the reference run for BMPRIA alone (Fig. 8a). Because signals for the gfd and the pd appeared together in all fractions, the BMP-7 complex did not dissociate upon binding to BMPRIA. In contrast to experiments with type II receptor domains, increasing the concentration of BMPRIA to a ratio of 1:1 resulted only in the formation of faint signals for additional peaks farther down in the gradient (Supplementary Fig. 11). Also, negligible signals for displaced pd molecules appeared in fractions 15–17 (Supplementary Fig. 11). Titration experiments with the BMP-7 complex and BMPRIB demonstrated very similar results, although at higher receptor concentrations, no additional peak was detected (Supplementary Fig. 11).
Fig. 8.
Type I receptors, such as BMPRIA, do not displace the BMP-7 pd. (a) BMP-7 complex with BMPRIA at a molar ratio of 1:0.25. Samples were analyzed by immunoblotting for the BMP-7 gfd (left panel) or for the BMP-7 pd (right panel). Two individual overlapping peaks were recognizable: complex bound to receptor (fractions 9–11) and free complex (fractions 11–14). (b) Cartoon representation of the different species forming the two distinctive peaks in (a). At a BMP-7 complex/BMPRIA ratio of 1:0.25, BMP-7 complex species bound to the receptor were found next to free BMP-7 complex. A model of the BMP-7 complex with its type I receptor domains is shown. The pd does not obstruct the interaction site for the type I receptor ectodomains. Binding of one receptor molecule induces little to no displacement of the BMP-7 pd.
Similar velocity sedimentation experiments were performed with ActRIA (ALK2). However, after incubation of ActRIA with the BMP-7 complex, the positions of the individual components did not shift farther down in the gradient (data not shown), indicating little, if any, interaction between ActRIA and the BMP-7 complex.
BMPRII and BMP-7 pd compete for the BMP-7 growth factor
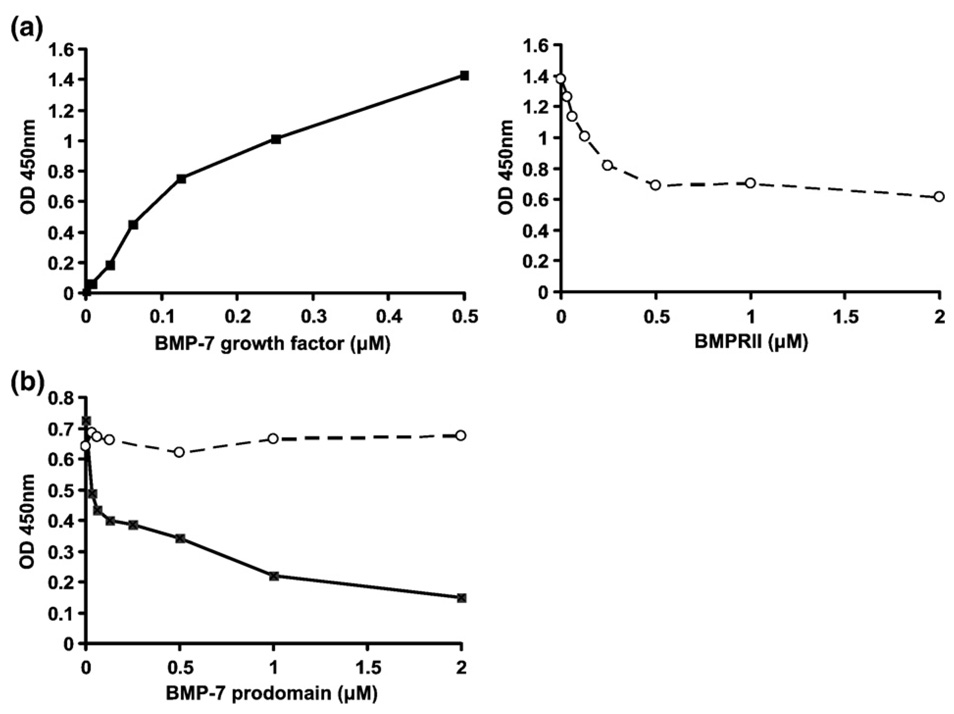
To determine whether type II receptors compete with the BMP-7 pd for binding to the gfd, we carried out competition ELISA experiments. Separated BMP-7 pd was immobilized via a BMP-7 pd-specific monoclonal capture antibody (mAb2) on an ELISA plate. Dose-dependent binding of BMP-7 gfd (filled squares) to the immobilized pd was detected (Fig. 9a, left graph). Titration of increasing amounts of BMPRII in the presence of a high constant concentration of BMP-7 gfd demonstrated competitive inhibition of gfd binding (Fig. 9a, right graph). As a second approach, BMPRII was coated on an ELISA plate and incubated with BMP-7 gfd at a constant concentration of 0.125 µM. Next, BMP-7 pd was incubated at increasing concentrations from 0 to 2.0 µM. Dose-dependent reduction of the signal for BMP-7 gfd bound to BMPRII was found (Fig. 9b), demonstrating that addition of the BMP-7 pd displaced the gfd from the preformed BMP-7 gfd-BMPRII complex. Both experiments suggested that BMPRII competes with the BMP-7 pd for the BMP-7 gfd.
Fig. 9.
BMPRII and BMP-7 pd compete for the BMP-7 growth factor. (a) Competition ELISA between BMP-7 pd and BMPRII. BMP-7 pd (separated from the BMP-7 complex) was immobilized on a microtiter plate using mAb2 (anti-BMP-7 pd) as a capture antibody. BMP-7 gfd was incubated either in increasing amounts (0–0.5 µM) without BMPRII (filled black squares connected by solid line, left) or at a constant concentration of 0.5 µM in the presence of increasing amounts of BMPRII (0–2.0 µM BMPRII; empty cycles connected by dashed line, right). The signals represent amounts of BMP-7 gfd detected by using a biotinylated polyclonal anti-BMP-7 gfd antibody. (b) Displacement of the gfd from a preformed BMPRII-BMP-7 complex by the pd. BMPRII was coated at a concentration of 0.1 µM, and BMP-7 gfd was subsequently incubated at a constant concentration of 0.125 µM. Increasing amounts of BMP-7 pd were added in a second incubation step (0–2.0 µM BMP-7 pd; black squares connected by solid line) or, as a control, no BMP-7 pd (empty circles connected by dashed line). BMP-7 gfd was detected using the same antibody as in (a).
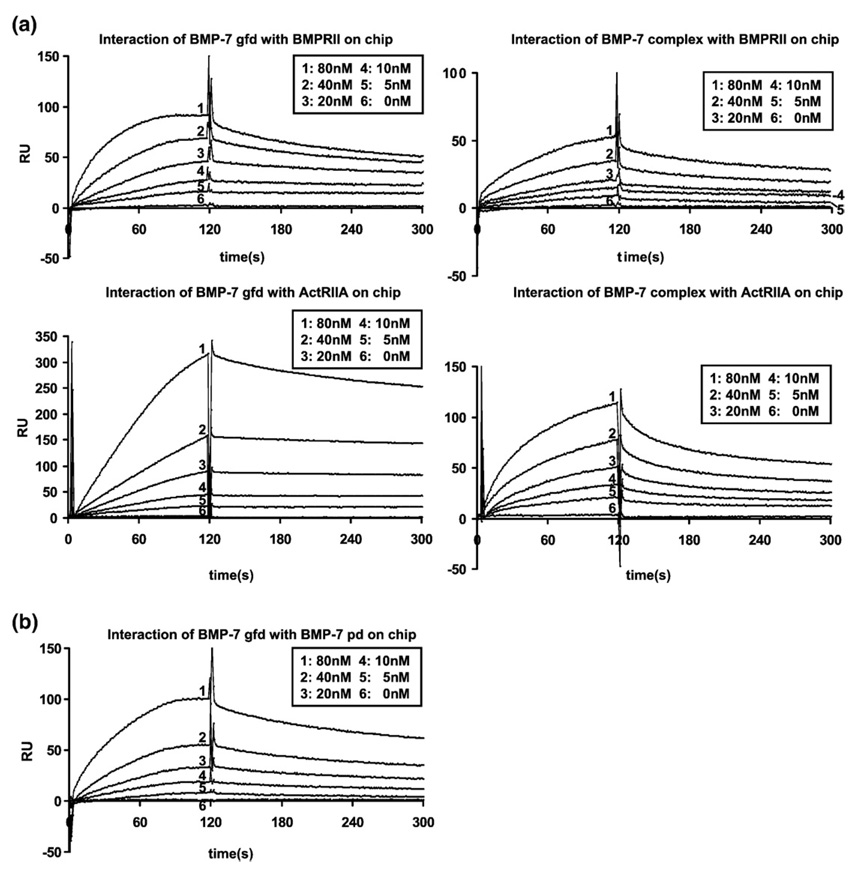
BIAcore studies (Fig. 10; summarized in Table 2) were conducted in order to obtain kinetic information to further elucidate potential mechanisms of interaction. Binding of the pd to the gfd and that of type II receptors to the gfd fit a simple 1:1 interaction model. The BMP-7 pd binds to the gfd with a dissociation constant (Kd) of 20 nM. Both the gfd and the complex bind to the BMPRII and ActRIIA with Kd values between 5 and 13 nM. These comparable binding affinities of the gfd and the complex to the type II receptors indicate that the presence of the pd in the complex does not block receptor binding of the BMP-7 gfd. Interestingly, injecting the BMP-7 complex onto immobilized receptors results in about 50% reduced response signal, when compared with curves generated by BMP-7 gfd injection, even though the molecular weight of the BMP-7 complex is three times that of the gfd. This could be due to a molecular exclusion effect of the dextran matrix, which might be in favor of the smaller gfd, or an indication that coupled type II receptors bind to the gfd and release the pd during the binding of the complex. Moreover, the binding kinetics between the BMP-7 complex and the tested type II receptors again revealed a 1:1 interaction, excluding or limiting the possibilities of more complex mechanisms.
Fig. 10.
BIAcore studies of BMP-7 growth factor and BMP-7 complex show similar binding affinity to immobilized BMPRII or ActRIIA. BMP-7 pd binds with slightly lower affinity to the BMP-7 growth factor. (a) Interaction of BMP-7 gfd or complex in concentrations of 0–80 nM diluted in HBS-EP with 500 RU of BMPRII (upper panel) or ActRIIA (lower panel) immobilized on a CM5 chip. Nonspecific binding to the control surface has been subtracted at a molar ratio of 1:1. (b) Interaction of BMP-7 gfd in concentrations of 0–80 nM diluted in HBS-EP with BMP-7 pd (500 RU) immobilized on a CM5 chip.
Table 2.
SPR affinity data for interactions
| Interaction (ligand/analyte) | kon (1/M*s)/koff (1/s) | Kd (nM) |
|---|---|---|
| BMPRII/BMP-7 gfd | 6.25 × 105/2.98 × 10−3 | 4.76 × 10−9 |
| BMPRII/BMP-7 complex | 2.16 × 105/2.13 × 10−3 | 9.86 × 10−9 |
| ActRIIA/BMP-7 gfd | 2.15 × 105/2.43 × 10−3 | 11.5 × 10−9 |
| ActRIIA/BMP-7 complex | 2.65 × 105/3.52 × 10−3 | 13.3 × 10−9 |
| BMP-7 pd/BMP-7 gfd | 9.87 × 104/2.09 × 10−3 | 21.1 × 10−9 |
Discussion
Some members of the TGF-β family are known to form latent complexes consisting of a gfd noncovalently associated with its pd, which is proteolytically processed during secretion. Recently, we demonstrated that BMP-7 is secreted as a highly stable pd-gfd complex.5 Previous characterization of soluble OP-1 (BMP-7) suggested that it was active.24 Therefore, we investigated whether the BMP-7 complex is latent and whether the BMP-7 pd can block interactions of BMP-7 gfd with its receptors. Because TGF-βs and BMPs are potent biological effectors, a better understanding of the molecular mechanisms by which they are activated and how these mechanisms may vary is required.
In vitro bioactivity assays demonstrated that the BMP-7 complex was as active as the free gfd. This was also the case even at a relatively low cytokine concentration of 0.32 nM, indicating that the BMP-7 complex is a highly potent molecule. In contrast, TGF-β-1 and GDF-8 complexes showed no in vitro activity unless they were incubated with activators, such as proteases, or were physically dissociated by certain conditions, such as low pH.16,25 Because pulse-chase experiments showed that the BMP-7 complex is stable in cell culture medium over 24 h5 and because complete dissociation of the BMP-7 complex was only accomplished using harsh denaturating conditions (8 M urea with 20 mM octylglucopyranoside),5 the BMP-7 activity observed in our assays cannot be due to spontaneous dissociation of the complex into its constituents during the incubation periods. Our results presented here with BMP-7 are similar to the in vitro bioactivity results reported for BMP-9,26 suggesting that BMP pds may not generally confer latency to their gfd domains.
Solid-phase binding studies suggested that the BMP-7 pd interacts with the BMP-7 gfd at sites close to the type II receptor binding sites. Therefore, we performed interaction studies in solution in order to determine whether the pd can block receptor binding to the gfd. Velocity sedimentation studies combined with inhibition ELISAs and BIAcore studies revealed a concentration-dependent dynamic process for the BMP-7-BMPRII interaction, in which BMPRII molecules displace the pd in a direct competitive manner and activate the signaling process. This novel activation mechanism for BMP-7 was also demonstrated for the BMP-7-ActRIIA/ActRIIB interactions.
Velocity sedimentation using sucrose gradients can be a very useful and powerful tool to investigate and monitor protein-protein interactions and protein complex formation in solution. In contrast to our solid-phase assay results (Fig. 2; Supplementary Fig. 13) in which the BMP-7 complex was immobilized to a solid surface, velocity sedimentation studies in which the BMP-7 complex and receptors were both in solution allowed the type II receptor to displace the pd. Immobilization to the solid phase likely prevented this displacement of the pd.
BMPRII and ActRII, which share the same binding sites on BMP,27 interacted equally well with the BMP-7 complex in our sedimentation experiments. These data were confirmed with the use of real-time SPR experiments, where BMPRII or ActRIIA was immobilized onto the solid phase and the gfd or complex was flowed over in solution. The dissociation constants for these interactions were all ∼10 nM, showing no inhibitory effect of the pd (Table 2), and, indeed, BIAcore data could be interpreted to suggest that displacement of the pd may occur when type II receptors bind to the complex. To exclude the possibility that the BMPRII-Fc chimeric receptor dimer might lead to higher binding affinity and displacement of the pd due to avidity effects, we performed equilibrium ultracentrifugation of BMPRII with free BMP-7 gfd and found that one BMPRII-Fc dimer bound to two gfd's, excluding artifactual avidity effects. We also produced monomeric BMPRII by enzymatic cleavage of the Fc portion using papain and found Kd values of 7–8 nM in SPR interaction studies between the immobilized monomeric BMPRII material and the BMP-7 gfd or complex, consistent with the results obtained using the intact BMPRII-Fc dimer (Supplementary Fig. 12). The observed binding values are in accord with binding affinities previously reported for immobilized ActRIIA and BMP-7 gfd in solution.28 However, these high-affinity interactions may be due to clustering of the bound receptors onto the BIAcore chip, because Kd values obtained when gfd's are coupled and receptors are in solution are sometimes in the micromolar range.28,29 Regardless of these studies, demonstrating variances in which one component is coupled and one is in solution, our experiments using velocity sedimentation, in which all components are in solution, clearly demonstrate interactions between BMP-7 and BMPRII, ActRIIA and ActRIIB, and BMPRIA and BMPRIB. We could not detect interactions between ActRIA (ALK2) and BMP-7 in velocity sedimentation experiments, indicating that this interaction may be a lower-affinity interaction.
Our velocity sedimentation studies might also suggest that the BMP-7 pd could be a dimer, because displacement of the pd from the native complex resulted in migration to a position father down in the gradient than the reference pd monomers. Due to the lack of cysteines in the pd, it has been so far unclear whether the BMP-7 pd, like LAP in the small latent TGF-β complex, is associated with the growth factor as a dimer. After separation from the BMP-7 complex using 8 M urea and 20 mM octylglucopyranoside, the pd appears to be a relatively insoluble monomer. Circular dichroism measurements of the BMP-7 pd alone compared with the BMP-7 complex revealed that the pd undergoes a conformational change when the BMP-7 complex is dissociated.5 From these observations, we suggest that folding of the BMP-7 complex may involve not only the formation of the cysteine-knot gfd but also the dimerization of the pd. When separated from its growth factor domain and denatured, the pd may lack the information required to easily dimerize. However, reassociation studies suggest that pds can renature in the presence of the gfd.23
Unlike TGF-βs, BMPs perform essential roles during very early embryogenesis and act as morphogens. Our investigations are consistent with the importance of BMPRs in the establishment of morphogen gradients during early embryogenesis. Furthermore, our investigations provide a molecular mechanism by which BMPRs can bind directly to BMP complexes, without the requirement for intervening activators that either degrade or displace the pd to release the gfd so that it can bind to its receptors. There is a growing body of evidence that BMPs are secreted as stable complexes in association with their gfds rather than as free gfds.5,23,26 The pd is suggested to target a variety of BMPs to the extracellular matrix23 and could possibly render the complex latent once it is bound to the extracellular matrix, because our studies with BMP-7 complex bound to a solid phase inhibit binding to type II receptors. However, these mechanisms might not play a predominant role during early embryogenesis, when the embryo is primarily cellular with relatively little extracellular matrix. During these early stages of development, the pd-gfd complex may facilitate diffusion and the formation of stable gradients, and it may be directly activated when it comes into contact with receptors immobilized on cells. In this case, type II receptors could displace the pd through a competitive mechanism to bind the gfd and initiate signaling. At later stages of development or during postnatal life, extracellular factors such as antagonists may then be required to control the access of BMPs to its receptors and perform critical roles in the regulation of BMPs. Finally, when the ratio of extracellular matrix to cells becomes greater than that during early stages of embryogenesis, extracellular molecules, such as fibrillin, may serve as storage scaffolds in which gfd complexes are embedded and later utilized when required.5,23 So, unlike TGF-β and GDF-8, which require activation before receptors can bind, BMPs require antagonism and sequestration from their receptors.
Materials and Methods
Cell lines
The cell lines used in this study were C3H/10/T1/2 (ATCC, CCL-226), C2C12 (ATCC, CRL-1772), and ATDC5 (Riken, RCB0565).
Recombinant proteins
Expression and purification of the BMP-7 complex were as described previously.5 Soluble extracellular receptor domains (BMPRIA/ALK3, BMPRIB/ALK6, ActRIA/ALK2, BMPRII, ActRIIA, and ActRIIB; all human and Fc chimera), gfds (human BMP-2, human BMP-7, and mouse GDF-8), and the mouse GDF-8 propeptide were purchased from R&D Systems. All purchased R&D Systems products contained 0.1% BSA as carrier protein.
Antibodies
The following antibodies were used: monoclonal anti-BMP-7 pd mAb2;5 monoclonal anti-BMPRII, anti-BMPRIB, anti-ActRIIA, anti-ActRIIA/ActRIIB, anti-BMP-7 gfd, and anti-His6 tag and polyclonal anti-BMPRIA and anti-GDF-8 from R&D Systems; polyclonal anti-phosphoSmadl/5/8 from Cell Signaling; and biotinylated polyclonal anti-BMP-7 gfd antibody from Peprotech.
Other reagents
Other reagents included an ECL chemiluminescence kit and immobilized papain (Pierce Chemical Co.), Superfect (Qiagen), a Dual-Luciferase Kit (Promega), and okadaic acid as well as calyculin A (Upstate Biotechnology).
Plasmids
The Δ3Msx2luciferase construct was a gift to Karen Lyons from Robert Maxson (USC). It contained a 1.8-kb fragment of the 5'-flanking sequence of Msx2 that was sufficient to confer BMP responsiveness by a reporter gene in cultured cells.18
Cell culture and transfection
C3H/10T1/2 cells were plated in six-well plates at 200,000 cells/well and cultured for 1 day in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). The cells were transfected with the Δ3Msx2luciferase reporter construct using Superfect (Qiagen) and 24 h later treated with BMP ligands at 3.85–30.8 nM [0.12–0.96 µg/ml for BMP-2 and BMP-7 gfd (R&D Systems) and at 0.36–2.88 µg/ml for the BMP-7 complex] for 24 h. After this 24-h treatment, the cells were harvested, and luciferase assays were performed using the dualluciferase system (Promega).
ATDC5 cells were cultured in six-well plates at 200,000 cells/well in DMEM/F12 medium supplemented with 10% FBS. After 1 day of culture, cells were treated for 20 min to 6 h with BMP ligands and were then lysed [cell lysis buffer: 50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM ethylenediaminetetraacetic acid (EDTA); 1 mM PMSF; 1 µg/ml each of aprotinin, leupeptin, pepstatin, okadaic acid, and calyculin A; 1 mM Na3VO4; and 1 mM NaF]. The contents of three wells were combined, trichloroacetic acid precipitated, and analyzed by Western blot analysis.
C2C12 cells were cultured in six-well plates at a density of 200,000 cells/well for 1 day in DMEM/10% FBS. For 20 min to 6 h, 100 ng/ml of BMP-2, 100 ng/ml of BMP-7 gfd, 300 ng/ml of BMP-7 complex, or 300 ng/ml of BSA was added. Cells were washed, and total RNA was harvested using TRIzol reagent (Invitrogen).
Real-time PCR
Total RNA preparations from treated C2C12 cells were quantified by photospectrometry. A total of 0.5 µg of RNA per sample was reverse transcribed using a BioRad iScript™ cDNA synthesis kit. Samples in triplicate were amplified using an iTaq™ SYBR Green Supermix (BioRad) in an iQ5™ Multicolor Real-Time PCR Detection System (BioRad). Analysis of data was performed using the method30 and quantitated relative to the ARBO PO gene. Gene expression was normalized to BSA-treated samples, which provided an arbitrary constant for comparative fold expression.
Velocity sedimentation
Recombinant proteins were dialyzed at concentration ratios mentioned in the figure legends against TBS. Aliquots (200 µl) were then pipetted onto the top of a 5%–20% (w/v) sucrose gradient (total volume = 3.6 ml), buffered with TBS, and formed in polyallomer tubes (11 × 3 × 60 mm; Beckman). Ultracentrifugation experiments were performed for 22 h 15 min at 42,000 rpm (ω2t: 1.55 × 1012) at 4 °C in a Beckman L8-M ultracentrifuge using a Beckman SW 60Ti rotor. After a small hole was pricked with a pin in the bottom of the tubes, eight-drop fractions were collected. Fractions were trichloroacetic acid precipitated, separated by nonreducing SDS-PAGE on 12.5% (w/v) acrylamide gels, and analyzed by Western blot analysis. Equal protein loading was checked by Ponceau stain. Nitrocellulose membranes were developed with either SuperSignal™ (Pierce) or an Opti 4-CN™ Substrate Kit (BioRad) according to the manufacturer's instructions. In some cases, membranes were redeveloped after stripping with Restore Western Blot Stripping Buffer (Pierce) and additional subsequent first and secondary antibody incubations.
ELISA binding assays
Multiwell plates were coated with purified BMP-7 complex and separated gfd (0.2 µM; 5–10 µg/ml; 100 µl/well) in 15 mM Na2CO3 and 35 mM NaHCO3, pH 9.2, at 4 °C overnight. Coated wells were blocked with 5% nonfat dry milk in TBS at room temperature for 1 h. Soluble recombinant receptor domains (100 µl/well) were serially diluted 1:2 in 2% milk in TBS and incubated for 3 h. Monoclonal antibodies against soluble ligands were diluted in 2% milk in TBS and used to detect the bound ligands, after a final incubation with enzyme-conjugated secondary antibodies. Color reaction of the enzyme immunoassay was achieved using 3,3',5,5'-tetramefhyl-benzidine (Sigma) and stopped with 0.1 N HCl. Absorbance was read at 450 nm using a Titertek Multiskan.
For inhibition ELISAs, BMPRII was coated at 0.1 µM and mAb2 was at 1 µg/ml in the same way as described above. Each blocking, ligand, or antibody incubation step was carried out in 5% FBS in 1× TBS with or without 1 M urea. For detection of BMP-7 gfd, a biotinylated polyclonal anti BMP-7 gfd antibody was used.
SPR
Binding analysis was performed using BIAcoreX (BIAcore AB, Uppsala, Sweden). BMP-7 gfd [400 or 1700 response units (RU)], BMP-7 complex (1200 or 5100 RU), BMP-7 pd, BMPRII, or ActRIIA (500 RU of each molecule) was covalently coupled to CM5 sensor chips (research grade) using the amine coupling kit following the manufacturer's instructions (BIAcore AB). Binding responses due to analyte interaction with the surface-coupled ligand were normalized by subtraction of background binding to plain control flow cells.
Binding assays were performed at 25 °C in 10 mM Hepes buffer, pH 7.4, containing 0.15 M NaCl, 3 mM EDTA, and 0.005% (v/v) P20 surfactant (HBS-EP buffer, BIAcore AB). BMP-7 gfd or BMP-7 complex was diluted in HBS-EP buffer and then injected at several concentrations and different flow rates over immobilized BMP-7 pd and BMPRII. The surface was regenerated with a pulse of 10 mM glycine, pH 1.7. Kinetic constants were calculated by nonlinear fitting (1:1 interaction model with mass transfer) to the association and dissociation curves according to the manufacturer's instructions (BIAevaluation 3.0 software). Apparent equilibrium dissociation constants (Kd) were then calculated as the ratio of kd to ka.
Analytical ultracentrifugation
Sedimentation equilibrium runs were performed in a Beckman Coulter ProteomeLab™ XL-A protein characterization system (Beckman Instruments, Fullerton, CA, USA) equipped with a scanner. Twelve-millimeter Epon double-sector cells in an An-F Ti rotor were used. The proteins were analyzed in 50 mM Tris buffer, pH 7.4, containing 150 mM NaCl. The peptide concentrations were adjusted to 0.6 mg/ml. Sedimentation equilibrium measurements were carried out at 4–6 °C with a rotor speed of 7500 rpm. Molecular masses were evaluated from In A versus r2 plots, where A represents the absorbance and r is the distance from the center of rotation. A partial specific volume of 0.72 ml/g for the BMP-7 gfd and that of 0.724 ml/g for the BMPRII-Fc receptor were used for all calculations. The data were analyzed using a least-squares method with the SCIENTIST for Windows software (MicroMath Research, St. Louis, MO, USA).
Papain cleavage
Cleavage of the BMPRII-Fc chimera by papain was performed according to the manufacturer's protocol, digesting 20 µg of BMPRII-Fc in 100 µl of reaction buffer (20 mM sodium phosphate, 10 mM EDTA, 20 mM cysteine, pH 7.0) with 100 µl of equilibrated swollen papain resin for 30 min.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2008.06.074
Acknowledgements
This work was supported by grants from the Shriners Hospitals for Children (to L.Y.S. and to H.P. B.), the National Institutes of Health (R01AR46811 and PO1AR049698 to L.Y.S.), and the Deutsche Forschungsgemeinschaft (Forschungsstipendium: SE 1115/1-1 to G.S.). We thank Noe L. Charbonneau, Bruce A. Boswell, and Sara Ota for excellent technical assistance and William Walker and Wendy Knosp for providing primers for the quantitative real-time RT-PCR experiments.
Abbreviations used
- BMP
bone morphogenetic protein
- pd
prodomain
- TGF
transforming growth factor
- gfd
growth factor dimmer
- LAP
latency-associated peptide
- ActR
activin receptor
- BMPR
BMP receptor
- BSA
bovine serum albumin
- RT
reverse transcriptase
- SPR
surface plasmon resonance
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- RU
response units
References
- 1.Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 2.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 3.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 4.Sampath TK, Coughlin JE, Whetstone RM, Banach D, Corbett C, Ridge RJ, et al. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming gfd-beta superfamily. J. Biol. Chem. 1990;265:13198–13205. [PubMed] [Google Scholar]
- 5.Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bächinger HP, Sakai LY. The pd of BMP-7 targets BMP-7 complex to the extracellular matrix. J. Biol. Chem. 2005;280:27970–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- 6.Rifkin DB. Latent transforming gfd-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J. Biol. Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 7.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, et al. Latent transforming gfd-binding protein-1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield LM, Smith DM, Broz S, Jackson M, Levinson AD, Sporn MB. Recombinant TGF-beta 1 is synthesized as a two-component latent complex that shares some structural features with the native platelet latent TGF-beta 1 complex. Growth Factors. 1989;1:203–218. doi: 10.3109/08977198908997997. [DOI] [PubMed] [Google Scholar]
- 9.Gentry LE, Nash BW. The pro domain of pre-pro-transforming growth factor beta 1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–6857. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc. Natl Acad. Sci. U. S. A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon GA, Dignam JD, Gentry LE. Structural characterization of the latent complex between transforming growth factor beta 1 and beta 1-latency-associated peptide. Biochem. J. 1996;313:343–351. doi: 10.1042/bj3130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Crescenzo G, Grothe S, Zwaagstra J, Tsang M, O'Connor-McCourt MD. Real-time monitoring of the interactions of transforming growth factor-β (TGF-β.) isoforms with latency-associated protein and the ectodomains of the TGF-β type II and III receptors reveals different kinetic models and stoichiometries of binding. J. Biol. Chem. 2001;276:29632–29643. doi: 10.1074/jbc.M009765200. [DOI] [PubMed] [Google Scholar]
- 13.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-betal in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 14.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 15.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J. Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, et al. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc. Natl Acad. Sci. U. S. A. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, et al. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–5165. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- 19.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, Shukunami C, Nakamura T, Hiraki Y. Differential expression of BMP family genes during chondrogenic differentiation of mouse ATDC5 cells. Cell Struct. Funct. 2000;25:195–204. doi: 10.1247/csf.25.195. [DOI] [PubMed] [Google Scholar]
- 21.Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127:2917–2931. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- 22.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 23.Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, et al. Targeting of BMP growth factor complexes to fibrillin. J. Biol. Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones WK, Richmond EA, White K, Sasak H, Kusmik W, Smart J, et al. Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth Factors. 1994;11:215–225. doi: 10.3109/08977199409046919. [DOI] [PubMed] [Google Scholar]
- 25.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 26.Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J. Biol. Chem. 2005;280:25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 27.Kirsch T, Nickel J, Sebald W. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J. 2000;19:3314–3324. doi: 10.1093/emboj/19.13.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald J, Groppe J, Gray P, Wiater E, Kwiatkowski W, Vale W, Choe S. The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol. Cell. 2003;11:605–617. doi: 10.1016/s1097-2765(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 29.Saremba S, Nickel J, Seher A, Kotzsch A, Sebald W, Mueller TD. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 2008;275:172–183. doi: 10.1111/j.1742-4658.2007.06187.x. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2008.06.074