Abstract
Tau aggregates into neurofibrillary tangles in Alzheimer’s disease and tauopathies. There is ongoing debate about whether tau aggregation is toxic and which form of tau is toxic. Based on recent studies showing that mature tau tangles can be dissociated from neuronal loss and cognitive deficits, it can be hypothesized that the intermediate pre-fibrillar tau aggregate is the predominant neurotoxic tau species. The toxicity of tau aggregation includes loss of physiological functions of native tau and gain of pathological functions of pre-fibrillar tau species. Mature tau tangles per se might be relatively inert or even represent failed cytoprotective efforts of protein quality control machineries in response to accumulating toxic tau species. Further studies on the mechanisms of tau aggregation, the structure of intermediate tau forms and their toxicity are needed to settle this debate.
Keywords: Alzheimer’s disease, intermediate tau aggregates, neurotoxicity, tangles, tauopathy
INTRODUCTION
In 1986, the microtubule-associated protein tau was demonstrated to be the major component of neurofibrillary tangles (NFTs), a neuropathological hallmark of Alzheimer’s disease (AD) [21,23]. Since this time, researchers have made significant progress in unmasking the possible mechanisms of tau aggregation, and the relationship between the pathogenic processing of tau and the aberrant effects of amyloid-β peptide (Aβ), which is the major component of senile plaques, the other neuropathological hallmark of AD. However, the question about whether tau aggregation is cytotoxic or cytoprotective is still under debate. Tau aggregation in AD and tauopathies, similar to aggregation of many other proteins such as Aβ in AD, α-synuclein in Parkinson’s disease and huntingtin in Huntington’s disease, is a complex and yet to be fully elucidated process in which tau monomers likely form intermediate pre-fibrillar structures ranging from dimers, oligomers to higher ordered multimers, which eventually form the fully organized mature fibrils or NFTs [24]. Therefore, the question about the toxicity of tau aggregation indeed comes down to whether the mature fibrillar tangles, which occur in the final stages, or the earlier staged intermediate forms or “pre-aggregates” are the predominant toxic tau species involved in the pathogenesis of AD and tauopathies.
HYPOTHESIS: INTERMEDIATE PRE-FIBRILLAR TAU FORMS ARE THE MAJOR TOXIC TAU SPECIES
As we seek to answer the question of which form(s) of tau is toxic, it would help to take a closer look at a similar question regarding the neurotoxic species of Aβ. Early studies provided evidence that Aβ aggregation was likely essential for neurotoxicity [10,38], and given that the highly organized insoluble Aβ fibrils were readily detected in both human brain and transgenic mice, it was initially assumed that the fibrillar aggregates mediated the observed toxicity of Aβ. However, this assumption has been challenged at several levels. First, it was shown that the load of amyloid plaques does not correlate positively with the severity of cognitive deficits [48]. Furthermore, over the past ten years, studies have actually shown a correlation between soluble non-fibrillar Aβ and the extent of neurodegeneration [31,50], supporting the hypothesis that the pre-fibrillar Aβ assemblies are potent neurotoxins whereas the organized fibrils or plaques are relatively inert, although the exact characteristics of the toxic forms of soluble Aβ in brain are yet to be defined [49]. Similar studies on pre-fibrillar protein aggregates and their inherent cytotoxicity have been extended to polyglutamine proteins, α-synuclein and even a range of proteins not associated with amyloid diseases, supporting the concept that aggregation-prone proteins with distinct primary sequences and functions might share a common pathway of fibrillization, common structure of pre-fibrillar aggregates and even common mechanisms of toxicity [32,44].
Due to the intrinsic high solubility of tau and the technical difficulties in studying the assembly of paired helical filaments (PHFs), the major basic element of NFTs, it has been difficult to determine whether or not PHFs are actually amyloid fibrils [30]. However, studies have demonstrated that PHFs isolated from AD brain, and filaments assembled in vitro from recombinant tau, contain a β-structure core that typically defines amyloid fibrils [5,7]. Thus, based on the amyloid nature of tau fibrils and the generic property of amyloid formation of different proteins, it can be hypothesized that in the case of tau, the primary pathogenic species might well be the pre-fibrillar aggregates and not the highly organized mature NFTs.
NFTs CAN BE DISSOCIATED FROM NEUROTOXICITY
Several layers of evidence support the hypothesis that NFTs can be separated from neurotoxicity. First, the cytotoxicity of pro-aggregation prone mutant tau preceded the appearance of thioflavin S positive tau aggregates in an inducible cell model of tauopathy [22]. Similarly, in a triple transgenic AD mouse model memory dysfunction was detected before the appearance of NFTs [35]. Additionally, in a mutant tau transgenic Drosophila model progressive neurodegeneration occurred without NFT formation [52]. This indicates that certain forms of tau lacking the fibrillar feature are probably toxic and the NFTs are not required to confer toxicity, at least in the early stages of disease progression. Second, in a repressible tau transgenic mouse model for tauopathy, turning off tau expression attenuated the memory impairment and neuronal loss, but NFTs continued to accumulate [43]. Furthermore, inhibition of tau hyperphosphorylationin another tauopathy mouse model prevented severe motor deficits and reduced the amount of soluble tau aggregates without affecting NFT counts [26]. These two studies suggest that NFTs are not sufficient to cause neuronal death and cognitive decline and more importantly, by reducing the expression or pathogenic modification of mutant tau, the neurotoxicity can be at least partially reversed. Third, reduction of endogenous wild type tau prevented behavioral abnormalities in an amyloid-β protein precursor transgenic mouse model, in which substantial neurofibrillary pathology is absent [41]. Therefore, NFTs alone can be dissociated from and are likely not the major cause of neuronal toxicity and cognitive deficits. This conclusion seems contradictory to the observation that the amount of NFTs correlates positively with the extent of cognitive impairment [20]. It is also well established that the NFT load can be used as a marker to clinically classify the stages of disease progression [8]. However, the correlation between NFTs and cognitive deficits is not sufficient to demonstrate a cause-effect relationship. Nonetheless, before we can conclude that pre-fibrillar tau forms cause toxicity, in addition to the indirect evidence cited above, there needs to be direct evidence showing that the early stage aggregated tau species do exist and they do exert neurotoxicity.
EXISTENCE OF INTERMEDIATE TAU AGGREGATES
Based on studies of the formation of tau filaments in vitro and the structure of isolated PHFs from AD brain, a model for tau fibrillization has been proposed. In this model [25], tau monomers experience critical conformational changes that make tau competent to assemble. The increased concentration and stabilization of these assembly-competent conformations initiate the process of nucleation and elongation to form fibrils. This dynamic process is controlled by the equilibrium between non-fibrillar intermediate tau species and tau fibrils. Though technically challenging due to the very hydrophilic nature of tau, intermediate tau forms during the formation of tau filaments have been detected in in vitro assays using recombinant tau [11,29].
The existence of intermediate tau aggregates in AD brain has been demonstrated by immunohistochemistry showing non-fibrillar deposits that display a punctate staining pattern in the cytoplasm and are not reactive with fluorescent dyes recognizing β-sheet structure [16]. Furthermore, granular tau oligomers, as detected by atomic force microscopy, precede PHF formation and are elevated in the prefrontal cortex in Braak stage I AD brain compared with stage 0 [28]. Recently, using biochemical approaches including fractionation by centrifugation and size exclusion chromatography, Berger et al. detected two forms of tau multimers with molecular weight of 140 and 170 KDa in mouse models of tauopathy. These tau multimers, either soluble or sarkosyl-insoluble, accumulated early in the development of tau pathology but only the levels of insoluble tau species (sarkosyl-insoluble and possibly other unknown insoluble tau forms) correlated with memory loss [6]. In contrast, in other studies, reducing soluble tau assemblies contributed to improved cognitive function [26,36]. The discrepancy with regard to toxic tau species in these studies (insoluble vs. soluble) could be due to different mouse models and different techniques and criteria used to define solubility of tau fraction. But in a way, this discrepancy points to the necessity of characterizing the exact composition and biophysical nature of these tau species. And more importantly, although these studies are some of the first efforts to relate behavioral impairments to specific potentially pathogenic tau species, the mechanisms of how they exert their toxicity are not known yet.
POTENTIAL TOXICITY OF TAU AGGREGATION: LOSS OF FUNCTION AND GAIN OF FUNCTION
Studies have shown that the pathogenic events induced by amyloid oligomers include membrane permeabilization, Ca2+ disturbances, elevated levels of reactive oxygen species and mitochondrial dysfunction [18, 44]. However, because of the lack of knowledge of the structures of intermediate tau aggregates, and difficulties in isolating these aggregates for in vitro studies, research aimed at pinpointing the mechanisms of toxicity induced by these tau species has largely been hampered. Considering that the primary toxicity of protein aggregates could be generic, it would not be unwise to hypothesize that tau oligomers might share similar mechanisms of pathogenesis. First, tau has the ability to interact with the plasma membrane through its N-terminal region, which is believed to be part of the mechanism of the involvement of tau in cell signaling pathways such as those involving Src-related kinases [9]. It is tantalizing to speculate that the conformational changes accompanying the formation of tau oligomers might enhance the interaction between tau and the membrane and as a consequence, the membrane structure could be disturbed and thus affect cellular homeostasis. In addition, the cell signaling pathways that tau is involved in might also be disrupted. Second, our lab has observed decreased Ca2+ buffering capability and a loss of mitochondrial membrane potential and integrity in thapsigargin challenged immortalized cortical cells that stably express tau truncated at D421, but not in cells expressing full-length tau [13]. Tau cleavage at D421 by caspase occurs in AD brain and enhances tau polymerization in vitro [17,40]. The cleavage of tau by caspase may be an important link between Aβ and tau pathology as Aβ treatment of cortical neurons results in the cleavage of tau at D421, and there is evidence that tau cleavage at this site may occur in the early stage of NFT formation [17,40]. The inter-molecular association of tau and tau cleaved at D421 has been shown by FRET analysis in situ [12] and furthermore, we detected the formation of tau oligomers (at least tetramers) using a β-galactosidase complementation assay [13]. This raises the possibility that the compromised Ca2+ regulation and mitochondrial dysfunction could be associated with oligomeric truncated tau.
The toxicity of tau aggregation may originate not only from the gain of pathological functions of the intermediate non-fibrillar tau species described above, but also from the loss of physiological functions of the native tau. Although the driving force of tau aggregation in vivo is yet to be elucidated, in vitro studies show that hyperphosphorylation of tau, at least at certain sites, promotes its aggregation [1,3]. Although it is still controversial as to whether hyperphosphorylation of tau is required for the initiation of tau aggregation, it is widely accepted that one of the consequences of hyperphosphorylation is a decrease in the binding of tau to microtubules, which may contribute to tau aggregation and fibrillization by increasing the concentration of free cytosolic tau. Furthermore, the decrease in the affinity of tau for microtubules may result in a decrease in the stability and function of microtubules [2]. In turn, the dynamics of microtubule network cannot be maintained and axonal transport that relies on microtubules is impaired. Axonal abnormalities and transport deficits have been proposed to be one of the earliest pathogenic events in AD pathogenesis [45]. Therefore, the loss of tau function resulting from early stage tau aggregation is closely associated with neurotoxicity. Interestingly, microtubule-stabilizing drugs can reverse axonal transport deficits and ameliorate motor impairments in a tauopathy model, presumably by offsetting loss of tau function [54].
MATURE TANGLES: A CYTOPROTECTIVE EFFORT BY PROTEIN QUALITY CONTROL MACHINERIES?
It is often found that heat shock proteins (Hsps), ubiquitin ligases and proteasome components co-exist with microscopically detectable large tau aggregates in cell culture models, transgenic mouse models and AD brain [14,15,19,37]. The mechanism of how these critical proteins in charge of protein quality control are related to the accumulation of tau aggregates is still unknown. One possible explanation is that these proteins co-aggregate with tau. But this is unlikely in light of the observation that protein aggregation exhibits exquisite specificity even among extremely hydrophobic substrates expressed at very high levels [39]. Another more intriguing and likely explanation is that these cellular machineries are mobilized as part of the cell defense mechanism to either solubilize or refold “misfolded” tau (by Hsps), or clear intermediate irreversible tau aggregates (by proteasomal degradation and autophagy). This idea has gained support from the finding that Hsp90 and Hsp70 promoted tau solubility and facilitated tau partitioning to microtubules [14], and the pivotal roles of chaperones in influencing the conformation and aggregation of other proteins such as α-synuclein, huntingtin and superoxide dismutase 1 [34]. Thus, the presence of chaperones in tau aggregates itself might already suggest the inherent toxicity of intermediate tau species. However, due to the continuous accumulation of potentially harmful tau species, the chaperone system might be overwhelmed and cannot keep up with the need for refolding and disaggregation. At the same time, prolonged occupation of chaperones by tau might deplete them from performing their functions in other important cellular events, which has been proposed to partially account for the secondary cytotoxicity induced by tau aggregation.
In addition to refolding “misfolded” proteins through the activity of chaperones, cells are also endowed with the ability to eliminate large protein aggregates. In a conditional transgenic mouse model of Huntington’s disease, suppression of mutant huntingtin led to the disappearance of inclusion bodies [53]. Silencing expression of pro-aggregation prone mutant tau in both conditional cell model and mouse model can also lead to attenuation of sarkosyl-insoluble tau aggregates and even tangles, although the tangles composed of endogenous mouse tau seemed irreversible, possibly due to the continuous synthesis of mouse tau [22,33]. The clearance of late stage protein aggregates may involve proteasomes and autophagy [42,51]. Given the potential toxicity of scattered intermediate aggregating species and the reversibility of concentrated late stage aggregates, it has been hypothesized that the formation of large aggregates such as tangles could be cytoprotective by sequestering diffuse small aggregates to minimize their toxicity and eventually clearing them by proteasomal activity and autophagy [4,42]. This is supported by data collected from cell models of polyglutamine diseases and Parkinson’s disease, in which increased toxicity was observed when the formation of large aggregates was disrupted [46,47]. However, it is yet to be directly tested in animal models, and whether the same phenomenon happens with tau pathology also needs to be tested. Further elucidation of the mechanisms involved in sequestration and clearance of aggregates should help develop new approaches to clarify this issue.
CONCLUSION
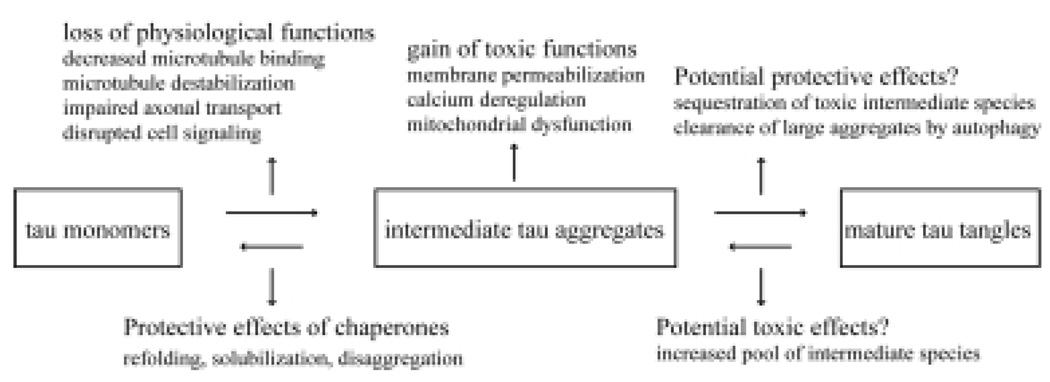
Taking everything together, the answer to the question as to whether tau aggregation is toxic or protective may not be simply one way or the other. On the one hand, tau aggregation causes loss of physiological functions of tau and produces intermediate tau aggregates that may gain pathological functions. On the other hand, the mature tangles may be relatively inert or even may represent a cytoprotective effort by protein quality control machineries when facing the challenge of accumulating toxic tau species. Despite the evidence suggesting that “pre-tangle” forms of tau are toxic, the possible toxic effects of tau tangles cannot be ruled out. Considering the intrinsic lability of PHFs shown in vitro [27], it is possible that tau tangles are dynamic in such a way that individual tau molecules or aggregated intermediate tau comes on and off tangles depending on the equilibrium. Thus, tau tangles could serve as a reservoir for toxic tau species at certain stages of disease progression. Nonetheless, there is still a long way to go before a conclusion to this question can be drawn. Further intensive studies on the structures of different intermediate tau species and mechanisms involved in the formation of tau fibrils in vivo will provide a solid basis to study the toxicity of tau aggregation and justify therapeutic strategies targeting toxic tau species.
Fig. 1.
Diagram of potentially toxic and protective processes in the formation of tau tangles.
ACKNOWLEDGMENTS
The research from the authors’ laboratory was supported by NIH grant NS051279 and a grant from the Alzheimer’s Association. The authors would also like to thank Dr. Shirley Shelton for suggesting the title.
References
- 1.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 4.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 5.Barghorn S, Davies P, Mandelkow E. Tau paired helical filaments from Alzheimer’s disease brain and assembled in vitro are based on beta-structure in the core domain. Biochemistry. 2004;43:1694–1703. doi: 10.1021/bi0357006. [DOI] [PubMed] [Google Scholar]
- 6.Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berriman J, Serpell LC, Oberg KA, Fink AL, Goedert M, Crowther RA. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc Natl Acad Sci USA. 2003;100:9034–9038. doi: 10.1073/pnas.1530287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busciglio J, Lorenzo A, Yankner BA. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol Aging. 1992;13:609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- 11.Chirita CN, Kuret J. Evidence for an intermediate in tau filament formation. Biochemistry. 2004;43:1704–1714. doi: 10.1021/bi036034b. [DOI] [PubMed] [Google Scholar]
- 12.Chun W, Johnson GV. Activation of glycogen synthase kinase 3beta promotes the intermolecular association of tau. The use of fluorescence resonance energy transfer microscopy. J Biol Chem. 2007;282:23410–23417. doi: 10.1074/jbc.M703706200. [DOI] [PubMed] [Google Scholar]
- 13.Ding H, Johnson GV. New application of β-galactosidase complementation to monitor tau self-association. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05496.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci USA. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fergusson J, Landon M, Lowe J, Dawson SP, Lay-field R, Hanger DP, Mayer RJ. Pathological lesions of Alzheimer’s disease and dementia with Lewy bodies brains exhibit immunoreactivity to an ATPase that is a regulatory subunit of the 26S proteasome. Neurosci Lett. 1996;219:167–170. doi: 10.1016/s0304-3940(96)13192-x. [DOI] [PubMed] [Google Scholar]
- 16.Galvan M, David JP, Delacourte A, Luna J, Mena R. Sequence of neurofibrillary changes in aging and Alzheimer’s disease: A confocal study with phospho-tau antibody, AD2. J Alzheimers Dis. 2001;3:417–425. doi: 10.3233/jad-2001-3409. [DOI] [PubMed] [Google Scholar]
- 17.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Goldbaum O, Richter-Landsberg C. Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the recruitment of ubiquitin to tau-positive aggregates in oligo-dendrocytes in culture. J Neurosci. 2004;24:5748–5757. doi: 10.1523/JNEUROSCI.1307-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB. Memory and mental status correlates of modified Braak staging. Neurobiol Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 21.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khlistunova I, Biernat J, Wang Y, Pickhardt M, von Bergen M, Gazova Z, Mandelkow E, Mandelkow EM. Inducible expression of Tau repeat domain in cell models of tauopathy: aggregation is toxic to cells but can be reversed by inhibitor drugs. J Biol Chem. 2006;281:1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 23.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuret J, Chirita CN, Congdon EE, Kannanayakal T, Li G, Necula M, Yin H, Zhong Q. Pathways of tau fibrillization. Biochim Biophys Acta. 2005;1739:167–178. doi: 10.1016/j.bbadis.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Kuret J, Congdon EE, Li G, Yin H, Yu X, Zhong Q. Evaluating triggers and enhancers of tau fibrillization. Microsc Res Tech. 2005;67:141–155. doi: 10.1002/jemt.20187. [DOI] [PubMed] [Google Scholar]
- 26.Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci USA. 2006;103:9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, von Bergen M, Mandelkow EM, Mandelkow E. Structure, stability, and aggregation of paired helical filaments from tau protein and FTDP-17 mutants probed by tryptophan scanning mutagenesis. J Biol Chem. 2002;277:41390–41400. doi: 10.1074/jbc.M206334200. [DOI] [PubMed] [Google Scholar]
- 28.Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res. 2006;54:197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Maeda S, Sahara N, Saito Y, Murayama M, Yoshiike Y, Kim H, Miyasaka T, Murayama S, Ikai A, Takashima A. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46:3856–3861. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- 30.Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Pathol. 2007;17:83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Meredith SC. Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins. Ann N Y Acad Sci. 2005;1066:181–221. doi: 10.1196/annals.1363.030. [DOI] [PubMed] [Google Scholar]
- 33.Mocanu MM, Nissen A, Eckermann K, Khlistunova I, Biernat J, Drexler D, Petrova O, Schonig K, Bujard H, Mandelkow E, Zhou L, Rune G, Mandelkow EM. The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J Neurosci. 2008;28:737–748. doi: 10.1523/JNEUROSCI.2824-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 35.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 36.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 37.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 38.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 39.Rajan RS, Illing ME, Bence NF, Kopito RR. Specificity in intracellular protein aggregation and inclusion body formation. Proc Natl Acad Sci USA. 2001;98:13060–13065. doi: 10.1073/pnas.181479798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 42.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 43.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81:678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 45.Stokin GB, Goldstein LS. Axonal transport and Alzheimer’s disease. Annu Rev Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem. 2004;279:4625–4631. doi: 10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- 47.Taylor JP, Tanaka F, Robitschek J, Sandoval CM, Taye A, Markovic-Plese S, Fischbeck KH. Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet. 2003;12:749–757. doi: 10.1093/hmg/ddg074. [DOI] [PubMed] [Google Scholar]
- 48.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 49.Walsh DM, Selkoe DJ. A beta oligomers – a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 51.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, Rubinsztein DC. Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- 52.Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VM, Trojanowski JQ. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci USA. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]