Abstract
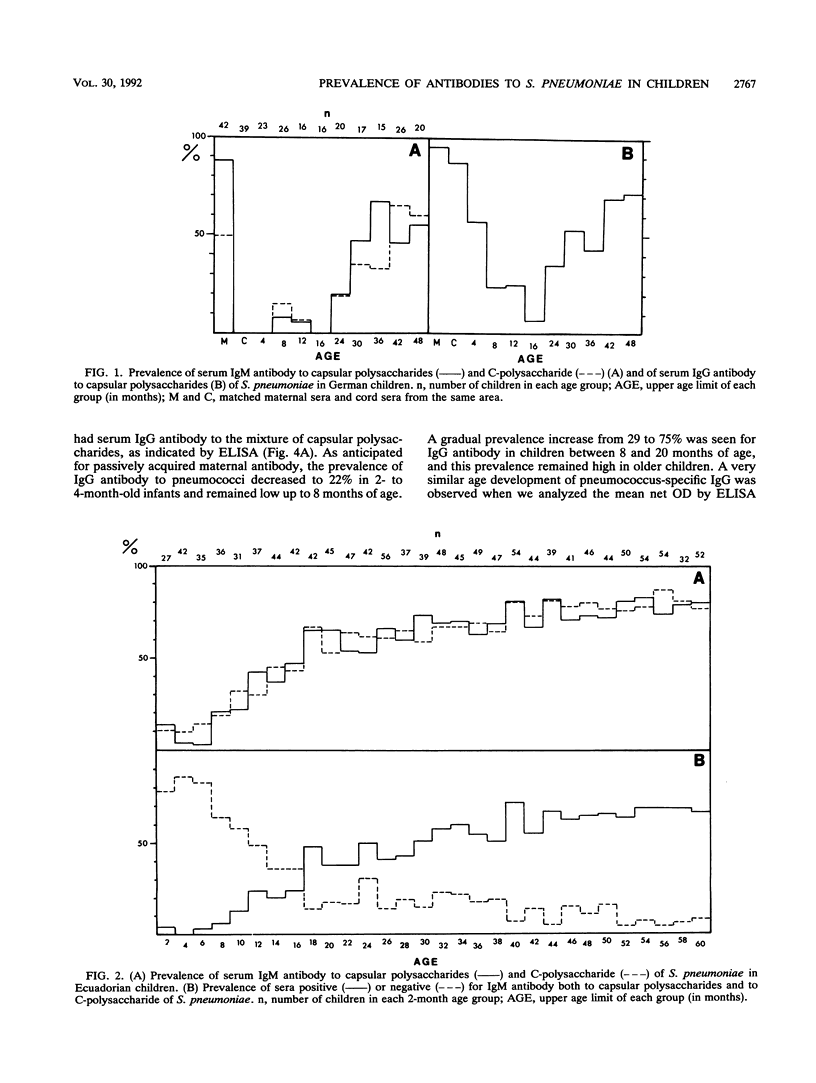
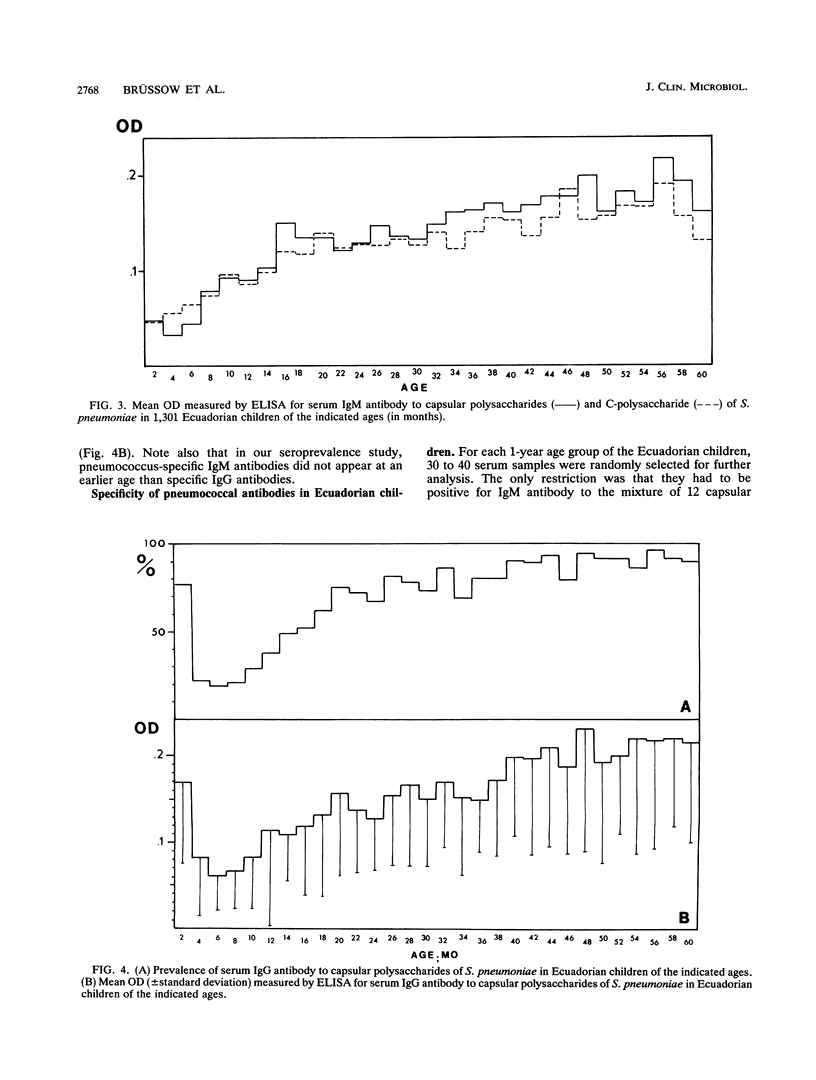
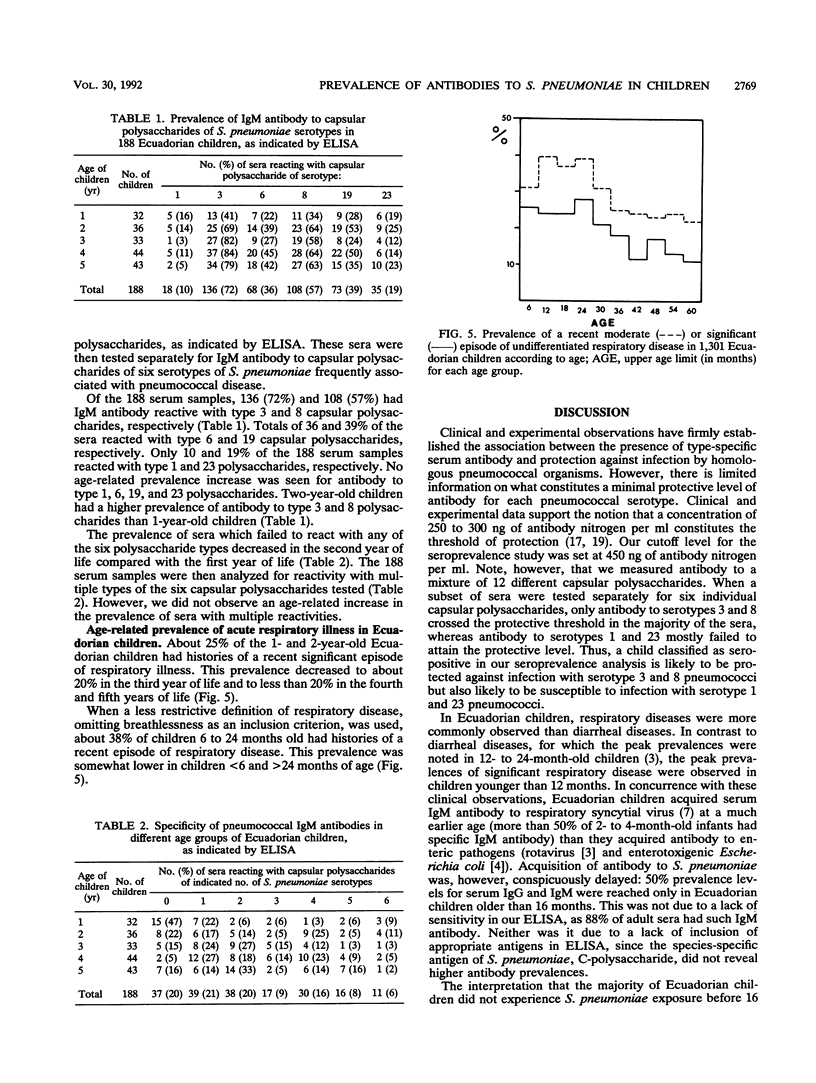
The age-specific prevalence of serum immunoglobulin M (IgM) antibody to capsular polysaccharides of Streptococcus pneumoniae, as detected by enzyme-linked immunosorbent assay, was studied in 1,301 Ecuadorian children enrolled in a national nutrition and health survey. This prevalence was 6% in infants < 6 months old and increased to 28% in children 6 to 11 months old, 49% in those 12 to 17 months old, and 58% in those 18 to 23 months old. About 80% of the 5-year-old children had this antibody. When tested separately against six different capsular polysaccharides, serum IgM antibody reacted with decreasing frequency with serotype 3, 8, 19, 6, 23, and 1 capsular polysaccharides. We did not observe a broadening of the antibody response with increasing age in the sense that more and more serotypes were recognized. A similar age-related prevalence was found for IgM antibody to the species-specific C-polysaccharide of S. pneumoniae and for IgG antibody to capsular polysaccharides of S. pneumoniae. A smaller German serum collection showed a comparable age-related prevalence of pneumococcus-specific serum IgG and IgM antibodies. The highest incidence of respiratory diseases was observed in 1- and 2-year-old Ecuadorian children. It thus seems that acquisition of serum antibody to S. pneumoniae reflects more the developmental maturation of an immune response than an actual exposure to different pneumococcal serotypes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonkhai V. I., Landesman S. H., Fikrig S. M., Schmalzer E. A., Brown A. K., Cherubin C. E., Schiffman G. Failure of pneumococcal vaccine in children with sickle-cell disease. N Engl J Med. 1979 Jul 5;301(1):26–27. doi: 10.1056/NEJM197907053010106. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Sidoti J., Barclay D., Sotek J., Dirren H., Freire W. B. Prevalence and serotype specificity of rotavirus antibodies in different age groups of Ecuadorian infants. J Infect Dis. 1990 Sep;162(3):615–620. doi: 10.1093/infdis/162.3.615. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Sidoti J., Link H., Hoang Y. K., Barclay D., Dirren H., Freire W. B. Age-specific prevalence of antibody to enterotoxigenic Escherichia coli in Ecuadorian and German children. J Infect Dis. 1990 Oct;162(4):974–977. doi: 10.1093/infdis/162.4.974. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Sidoti J., Rahim H., Dirren H., Freire W. Infectious gastroenteritis does not act as a triggering mechanism for the synthesis of serum IgG antibody to beta-lactoglobulin. J Pediatr Gastroenterol Nutr. 1991 Nov;13(4):402–408. doi: 10.1097/00005176-199111000-00011. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Sidoti J. Reactivity of human serum antibody with lipopolysaccharide O 78 antigen from enterotoxigenic Escherichia coli. Epidemiol Infect. 1992 Apr;108(2):315–322. doi: 10.1017/s0950268800049785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Werchau H., Liedtke W., Lerner L., Mietens C., Sidoti J., Sotek J. Prevalence of antibodies to rotavirus in different age-groups of infants in Bochum, West Germany. J Infect Dis. 1988 May;157(5):1014–1022. doi: 10.1093/infdis/157.5.1014. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Werchau H., Sidoti J., Ballo S., Rahim H., Dirren H., Freire W. B. Age-related prevalence of serum antibody to respiratory syncytial virus in Ecuadorian and German children. J Infect Dis. 1991 Mar;163(3):679–680. doi: 10.1093/infdis/163.3.679a. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Paton J. C., Duncan S. J., Hansman D. J. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983 Jul;148(1):131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- Gratten M., Gratten H., Poli A., Carrad E., Raymer M., Koki G. Colonisation of Haemophilus influenzae and Streptococcus pneumoniae in the upper respiratory tract of neonates in Papua New Guinea: primary acquisition, duration of carriage, and relationship to carriage in mothers. Biol Neonate. 1986;50(2):114–120. doi: 10.1159/000242576. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980 Dec;142(6):923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Huhta N., Johnston R. B., Jr, Pichichero M. E., Schiffman G., Dillon H. C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: antibody response to nasopharyngeal carriage of types 3, 19, and 23. J Infect Dis. 1981 Oct;144(4):312–318. doi: 10.1093/infdis/144.4.312. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr Epidemiological studies of Streptococcus pneumoniae in infants: antibody to types 3, 6, 14, and 23 in the first two years of life. J Infect Dis. 1988 Nov;158(5):948–955. doi: 10.1093/infdis/158.5.948. [DOI] [PubMed] [Google Scholar]
- Gwatkin D. R. How many die? A set of demographic estimates of the annual number of infant and child deaths in the world. Am J Public Health. 1980 Dec;70(12):1286–1289. doi: 10.2105/ajph.70.12.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger M., Jann K., Jann B. Crossreactions of Escherichia coli K and O polysaccharides in antipneumococcal and anti-Salmonella sera. J Exp Med. 1985 Oct 1;162(4):1350–1358. doi: 10.1084/jem.162.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg H., Krook A., Sjögren A. M. Determination of antibodies to pneumococcal C polysaccharide in patients with community-acquired pneumonia. J Clin Microbiol. 1985 Nov;22(5):808–814. doi: 10.1128/jcm.22.5.808-814.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. A., Landesman S. H., Schiffman G. A comparison of antibody concentration measured by mouse protection assay and radioimmunoassay in sera from patients at high risk of developing pneumococcal disease. Mol Immunol. 1984 Nov;21(11):1061–1065. doi: 10.1016/0161-5890(84)90116-0. [DOI] [PubMed] [Google Scholar]
- Koskela M., Leinonen M., Häivä V. M., Timonen M., Mäkelä P. H. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr Infect Dis. 1986 Jan-Feb;5(1):45–50. doi: 10.1097/00006454-198601000-00009. [DOI] [PubMed] [Google Scholar]
- Landesman S. H., Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S184–S197. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Banks S. D., Li J. P. Virulence, immunity, and vaccine related to Streptococcus pneumoniae. Crit Rev Microbiol. 1991;18(2):89–114. doi: 10.3109/10408419109113510. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Sibakov M., Herva E., Henrichsen J., Luotonen J., Timonen M., Leinonen M., Koskela M., Pukander J., Pöntynen S. Pneumococcal vaccine and otitis media. Lancet. 1980 Sep 13;2(8194):547–551. doi: 10.1016/s0140-6736(80)91989-3. [DOI] [PubMed] [Google Scholar]
- Riley I. D., Everingham F. A., Smith D. E., Douglas R. M. Immunisation with a polyvalent pneumococcal vaccine. Effect of respiratory mortality in children living in the New Guinea highlands. Arch Dis Child. 1981 May;56(5):354–357. doi: 10.1136/adc.56.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley I. D., Lehmann D., Alpers M. P. Pneumococcal vaccine trials in Papua New Guinea: relationships between epidemiology of pneumococcal infection and efficacy of vaccine. Rev Infect Dis. 1991 May-Jun;13 (Suppl 6):S535–S541. doi: 10.1093/clinids/13.supplement_6.s535. [DOI] [PubMed] [Google Scholar]
- Shann F., Gratten M., Germer S., Linnemann V., Hazlett D., Payne R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet. 1984 Sep 8;2(8402):537–541. doi: 10.1016/s0140-6736(84)90764-5. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Priehs C., Madore D. V. Standardization of antibody assays for measuring the response to pneumococcal infection and immunization. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S84–S91. [PubMed] [Google Scholar]
- Sloyer J. L., Jr, Howie V. M., Ploussard J. H., Ammann A. J., Austrian R., Johnston R. B., Jr Immune response to acute otitis media in children. I. Serotypes isolated and serum and middle ear fluid antibody in pneumococcal otitis media. Infect Immun. 1974 Jun;9(6):1028–1032. doi: 10.1128/iai.9.6.1028-1032.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. A., Warren K. S. Selective primary health care: an interim strategy for disease control in developing countries. N Engl J Med. 1979 Nov 1;301(18):967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Kim H. W., Kapikian A. Z., Chanock R. M. Immunological response to infection with human reovirus-like agent: measurement of anti-human reovirus-like agent immunoglobulin G and M levels by the method of enzyme-linked immunosorbent assay. Infect Immun. 1978 Feb;19(2):540–546. doi: 10.1128/iai.19.2.540-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


