Abstract
Objective
Automated and disease-specific classification of textual clinical discharge summaries is of great importance in human life science, as it helps physicians to make medical studies by providing statistically relevant data for analysis. This can be further facilitated if, at the labeling of discharge summaries, semantic labels are also extracted from text, such as whether a given disease is present, absent, questionable in a patient, or is unmentioned in the document. The authors present a classification technique that successfully solves the semantic classification task.
Design
The authors introduce a context-aware rule-based semantic classification technique for use on clinical discharge summaries. The classification is performed in subsequent steps. First, some misleading parts are removed from the text; then the text is partitioned into positive, negative, and uncertain context segments, then a sequence of binary classifiers is applied to assign the appropriate semantic labels.
Measurement
For evaluation the authors used the documents of the i2b2 Obesity Challenge and adopted its evaluation measures: F1-macro and F1-micro for measurements.
Results
On the two subtasks of the Obesity Challenge (textual and intuitive classification) the system performed very well, and achieved a F1-macro = 0.80 for the textual and F1-macro = 0.67 for the intuitive tasks, and obtained second place at the textual and first place at the intuitive subtasks of the challenge.
Conclusions
The authors show in the paper that a simple rule-based classifier can tackle the semantic classification task more successfully than machine learning techniques, if the training data are limited and some semantic labels are very sparse.
Introduction
Biomedical text mining has become a thriving field because it proved its efficiency in a wide scope of application areas, such as the identification of biological entities in text, 1 assigning insurance codes to clinical records, 2 facilitating querying in biomedical databases, 3 etc. For a survey see Cohen and Hersh, 2005. 4 Discharge summaries offer a rich source of information for information extraction (IE) tasks, including classification. Several open challenges have been announced in this field: automated assignments of insurance codes to radiology reports 5 and smoking status identification task. 6
The processing of textual medical records like discharge summaries facilitates medical studies by providing statistically relevant data for analysis. Analysis of a particular disease and its comorbidities on sets of patients is an example of this. The findings drawn from connections observed between elements of a set of diseases are of key importance in treatment and prevention issues.
In this paper we present results on the i2b2 Obesity Challenge shared task, which is a multiclass multilabel classification task focused on obesity and its 15 most common comorbidities (termed diseases). For each document, the task was to assign for each disease one of the following semantic labels: present, absent, questionable, or unmentioned (full description in Uzuner 7 ).
The problem of the Obesity Challenge is an atypical, two-dimensional classification problem with disease and semantic dimensions.
The top 10 solutions are dominated by rule-based systems, while, interestingly, no machine learning based approach can be found among them (see the survey of Uzuner 7 and the online only version available at www.jamia.org).
Rule-based text classifiers (aka expert systems) were widespread (see, e.g., Hayes et al 8 ) before the steady growth of computational capacity made machine learning approaches more popular. The rule-based approach is often criticized due to the knowledge acquisition bottleneck (Sebastiani 9 ). That is, each rule must be manually created, and the portability and flexibility of such systems are often very limited. These concerns are valid at categorization problems, where the rules are domain-dependent and the semantics of categories may shift. However, if the expert knowledge is available in knowledge bases (such as ontologies, typical for the biomedical domain), and the rules can be generated automatically, the overhead of manual processing can be minimized to error analysis. Consequently, expert and rule-based systems are often applied for different problems on medical domain (see, e.g., Zeng et al 10 and Chi et al 11 ).
In the medical field, there is a growing need for interactive systems; however, the challenge did not address this aspect. Health experts usually do not trust a system that acts like a black-box, but instead they want to verify the evidences that support the decision made. Our system is transparent for humans, while a system that is using sophisticated and, hence, not easy-to-understand machine learning techniques may require additional efforts to achieve this goal.
Next we describe our context-aware rule-based classifier, present its performance on the i2b2 Obesity Challenge, and briefly discuss the results and lessons learnt from our study. For the community, we provide an online appendix to this paper (available as an online data supplement at www.jamia.org) and on-line demo (available at categorizer.tmit.bme.hu/∼illes/i2b2/obesity_demo).
Methods
Problem Definition
In general, the problem is a multiclass multilabel classification task, but the applied semantic labeling and the selected evaluation criteria make the problem setting unusual. For each disease the annotators labeled documents with Y, N, Q, and U. Here Y means that the disease is present, N means that it is absent, Q means that it is questionable in the patient, and U means that the disease is unmentioned in the document. The labeling was performed in two different aspects: textual and intuitive. Textual contains only judgments strictly based on text; intuitive is based also on implicit information found in narrative text (and here only Y, N, and Q labels are assigned).
Systems were evaluated primarily based on their averaged F1-macro value and secondarily based on their averaged F1-micro value calculated over the 16 disease classes.
The problem can be considered as a two-dimensional task, where documents should be assigned to 16 disease classes and 4 (resp. 3) semantic classes. The two sets of classes are not independent.
• The semantic classes are distributed very unevenly: there exist classes even without training examples (6 textual N, 7 textual Q, and 5 intuitive Q classes out of 16).
• For the textual task, the annotators had to assign labels based on text fragments of documents, however, these fragments are not marked in the training set. The existence of such mark up could be extremely helpful when developing an automatic classifier.
Preprocessing of Text
To address the special characteristics of the tasks and assist manual exploration of the data, we first applied the following preprocessing steps:
Abbreviation Resolution
Resolving abbreviations aids nonprofessionals in understanding medical texts, by contrast decoding and disambiguating abbreviations in medical texts can improve the accuracy of information extraction. 12 It can be observed that periods, spaces and letter capitalization are used almost freely. In addition to dictionary lookups and web searches, we developed a regular expression driven string replacement dictionary to decode all occurrences of relevant abbreviations (see the online only version).
The style of abbreviations in a record may be characteristic of the health care institution where it originates. The deidentification of medical records usually covers only the obfuscation of named entities, however, we found that documents could be clustered by their abbreviation style. Consequently, we suspect that an adversary could bind documents to the note taking person observing the clusters and corresponding patterns (with better than uniform probability), however, we cannot verify this claim due to the lack of data. Nevertheless, we suggest that concealment of abbreviation styles should be the part of the deidentification process.
Identify Discharge Summary Elements (Zones)
The exploration of documents is facilitated by a visually enhanced version with text being chunked into zones. Zones were recognized by the surface features of the headings (e.g., Clinical Course, Diagnosis, etc).
Classifiers
Next we describe our context-aware rule-based classifier for both the textual and intuitive subtasks. The classifiers only differ in the set of rules. When creating rules, we intended to mimic the work of the annotators. We found most documents to be labeled with Y/N/Q (textual) if at least one mention of the disease occurred explicitly in the text, and with U otherwise. A mention can be various forms of the disease name or a directly associated term/phrase. Annotators had to decide on the label based on the context of the mentions.
The Baseline Y-Classifier
We built a dictionary that included for each disease a set of clue terms. The sets contain the disease's name and its alternatives:
• Abbreviations (e.g., HTN for hypertension),
• Synonyms (e.g., cardiac failure for heart failure),
• Plain English equivalents (e.g., high cholesterol),
• Spelling variants (e.g., gall stone instead of gallstones),
• Frequent typos (e.g., dislipidemia instead of dyslipidemia),
• Suffixed forms (e.g., gouty),
• Related terms (e.g., dyslipidemia for Hypercholesterolemia).
Based on the set of clue terms, a rule-based baseline classifier was created for Y label, which assigned the label if any of the clue terms was present in the document.
Family History
Obviously, the baseline classifier fails to distinguish between mentions occurring in different zones. However, the zone information can be crucial. A typical case is the group of family history zones, in which the text is not directly related to the patient's health. Therefore, we removed full zones and other fragments containing family history. During development, the removed fragments were systematically checked for false-positive matches. We ignored text fragments with family history and allergies (see details in the online only version).
Context-aware Partitioning, N- and Q-Classifiers
The baseline classifier was a binary classifier that only assigns Y labels. We found documents labeled with N (or Q) if the disease was mentioned in a negative (or uncertain) context. Since the primary evaluation function is F1-macro, correctly assigning the rare N and Q labels to documents was a key issue.
First, we addressed the semantic dimension of the task. All documents were partitioned into family history, negative, uncertain, and positive contextual segments by a sequence of splitters, which reduced the 4-way partitioning problem to the binary case.
Inspired by NegEx, 13 a framework to identify negative statements in medical text, we used trigger phrases to recognize negative and uncertain contexts. The differences between our solution and NegEx are the following. We use a different set of triggers and scope-terminating words. We built our solution for the Obesity Challenge using solely the given training records, surprisingly, without resulting in overfitting to the medical domain (see Section 4.). As an improvement over NegEx, we also applied midscope and word prefix triggers. We provide some illustrative fragments with triggers (bold) not included in the latest version of NegEx:
-
1 Pre- and postscope triggers
Example: “[?history of diabetes.]uncertain” yields Q for Diabetes, “[outpatient OSAscreen.]uncertain” yields Q for OSA
-
2 Scope limiting triggers
Example: “[No other significant past medical history]negativebesideshypertension.” yields Y for Hypertension
-
3 Mid-scope triggers
Syntax: [words] trigger [words]
Example: “[Right lower lobe pneumonia versusCHF.]uncertain” yields Q for CHF
-
4 Word-prefix triggers
Syntax: trigger (non-space characters) Example: “Abdomen: soft, [non-obese]negative, and [non-tender]negative” yields N for Obesity
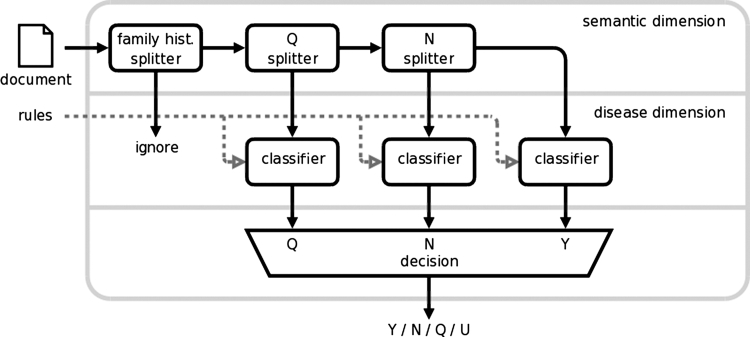
Semantic classification is followed by tackling the disease dimension. Once the documents were partitioned based on the context, they were fed to the appropriate binary baseline classifiers to obtain Q, N, and Y judgments. Thus, more than one label could be assigned to a document. The conflicts were resolved as follows. The Q-classifier is the least certain one: if a disease appears in both an uncertain and positive/negative context, then the latter rules out the former. Similarly, any positive mention takes precedence over negative mentions. Therefore, we applied the 3 classifiers in a cascade like pipeline: first Q labels were assigned, then N labels, finally Y labels (labels are overwritten), and unlabeled documents received U labels. The document processing and classification workflow is depicted in ▶.
Figure 1.
Document processing and classification workflow.
Our solution separates semantic and disease dimensions: the Q-, N- and Y-classifiers are based on the same set of rules that makes the classification simple, but more importantly, it allows us to use one common training set (per disease) for all classifiers, which is crucial due to the very limited number of Q and N labels.
Intuitive Judgments
At this point we have textual judgments. The text- and rule-based approach of the baseline classifier was less powerful on intuitive judgments, while the corpus had virtually no textual indicators recognizable by human experts or rule-based learners. Still, we found some simple rules that, in addition to those created for textual judgments, improved performance on the intuitive set. These rules include:
• Disease-specific, non-preventive medications and their brand names (e.g., SSRI or Zoloft for Depression)
• Related procedures (e.g., panniculectomy for Obesity)
• Symptoms with very high correlation (e.g., leg ulcers for Venous Insufficiency)
Example
Let us illustrate the complete work of the text splitter and classifier method, on a paragraph taken from the test document #1,058:
“CARDIAC RISK FACTORS: Hypertension, smoking [family history of coronary artery disease]family and CVA. [No diabetes.]negative she is [not postmenopausal]negative [no history of elevated cholesterol.]negative [No previous myocardial infarction history.]negative”
For this paragraph the overall classifier system would assign Y for hypertension, N for Diabetes and Hypercholesterolemia, and U for all others (note: including CAD).
Results
▶ includes the main evaluation measures of our best submissions for both subtasks as calculated by the organizers. We also present the confusion matrices in ▶. ▶
Table 1.
Table 1 F1-Macro and F1-Micro Results of Our Best Submissions
| Textual |
Intuitive |
|||
|---|---|---|---|---|
| F1-Macro | F1-Micro | F1-Macro | F1-Micro | |
| Disease | ||||
| Asthma | 0.9434 | 0.9921 | 0.9784 | 0.9894 |
| CAD | 0.8561 | 0.9256 | 0.6122 | 0.9192 |
| CHF | 0.7939 | 0.9355 | 0.6236 | 0.9315 |
| Depression | 0.9716 | 0.9842 | 0.9346 | 0.9539 |
| Diabetes | 0.9032 | 0.9761 | 0.9682 | 0.9729 |
| Gallstones | 0.8141 | 0.9822 | 0.9729 | 0.9857 |
| GERD | 0.4880 | 0.9881 | 0.5768 | 0.9131 |
| Gout | 0.9733 | 0.9881 | 0.9771 | 0.9900 |
| Hypercholesterolemia | 0.7922 | 0.9721 | 0.9053 | 0.9072 |
| Hypertension | 0.8378 | 0.9621 | 0.8851 | 0.9283 |
| Hypertriglyceridemia | 0.9732 | 0.9980 | 0.7981 | 0.9712 |
| OA | 0.9594 | 0.9761 | 0.6286 | 0.9589 |
| Obesity | 0.4879 | 0.9675 | 0.9724 | 0.9732 |
| OSA | 0.8781 | 0.9920 | 0.8805 | 0.9939 |
| PVD | 0.9682 | 0.9862 | 0.6348 | 0.9763 |
| Venous insufficiency | 0.8403 | 0.9822 | 0.8083 | 0.9625 |
| Overall | 0.8000 | 0.9756 | 0.6745 | 0.9590 |
CAD = coronary artery disease; CHF = congestive heart failure; GERD = gastroesophageal reflux disease; OA = osteo arthritis; OSA = obstructive sleep apnea; PVD = peripheral vascular disease.
Table 2a.
Table 2a Textual Confusion Matrix on the Test Set
| Y | N | U | Q | |
|---|---|---|---|---|
| Y | 2117 | 8 | 66 | 1 |
| N | 10 | 41 | 14 | 0 |
| U | 71 | 16 | 5681 | 2 |
| Q | 5 | 0 | 3 | 9 |
Rows represent the gold standard, while columns indicate the label assigned by our system.
Table 2b.
Table 2b Intuitive Confusion Matrix on the Test Set
| Y | N | Q | |
|---|---|---|---|
| Y | 2106 | 178 | 1 |
| N | 110 | 4989 | 1 |
| Q | 6 | 7 | 1 |
Rows represent the gold standard, while columns indicate the label assigned by our system.
The choice of F1-macro for the main evaluation metric motivated participants to focus on sparse classes. Applying F1 also implies that misclassification from one label to another is penalized in a degree dependent on the distribution of labels, and not according to the degree of the mistake's seriousness. For example, in the textual case, misclassifying a document as N instead of its gold standard Y, is penalized less than misclassifying it as Q, though the semantics of the labels suggest the opposite. Not concentrating on the sparsest Q labels—the approach followed by many other participants—would give an upper bound on F1-macro of three-fourth (textual), and two-thirds (intuitive). Our system was able to break both upper bounds.
We achieved a second position in textual and a first position in the intuitive subtask with our system that was built from scratch: it does not incorporate any third party software, or other external resources. In comparison with the other top teams' submissions, the main advantage of our system over other participants' was the low number of misclassified Q labels, in other aspects its performance is on par with those ones.
Our system is scalable: the number of rules grows linearly with the number of classes (health conditions), while the context identification components (text splitters) can be entirely reused without modification for the medical domain, thus reducing development time. Classification rules do not have to be created manually, they can also be bootstrapped from domain-specific ontologies (like UMLS 14 ) or obtained from machine learners.
Though only the most common comorbidities of obesity were selected for this challenge, the training examples were still very limited in number, forecasting even sparser data when scaling up to other conditions. Our system performs relatively stable across all classes: F1-macro is on average higher on classes with more data, but drops only slightly for sparser classes (see Fig 2a in online only version).
We also investigated the portability of our system to other domains. For comparison, we evaluated NegEx and our system on the biomedical corpus provided by the BioNLP2009 Shared Task 15 that featured negation and speculation annotations. The results summarized in ▶ show that our context-aware system slightly outperforms NegEx also on the biomedical domain, without any corpus-dependent adaptation.
Table 3.
Table 3 Performance of Assertion Classification on the BioNLP2009 Corpus
| Semantic Class | System | Precision | Recall | F1-Measure |
|---|---|---|---|---|
| Negation | NegEx | 0.4070 | 0.5152 | 0.4548 |
| Negation | Our original system | 0.5015 | 0.4626 | 0.4813 |
| Negation | Our adapted system | 0.7662 | 0.7460 | 0.7561 |
| Speculation | NegEx | 0.0000 | 0.0000 | 0.0000 |
| Speculation | Our original system | 0.1340 | 0.0236 | 0.0402 |
| Speculation | Our adapted system | 0.6049 | 0.5472 | 0.5746 |
We compared NegEx (v1.01 of Imre Solti's implementation, code.google.com/p/negex/), our system developed for the i2b2 Obesity Challenge, and the adaptation of our system towards the BioNLP2009 Shared Task (for comparison only).
Conclusions
In this paper we reported on our approach for the i2b2 Obesity Challenge. We developed a context-aware rule-based classification model that was able to cope successfully with this unusual multiclass multilabel classification task by a two-dimensional approach that handles disease and semantic classes separately. Thus, we could exploit efficiently the very limited training data.
Using manually fine-tuned regular expressions for identifying contexts and diseases, the performance could be slightly increased compared to those systems using preexisting domain specific tools and resources. By keeping the system's structure simple, its output can be presented in a way that makes human verification quick and easy; our system is thus suitable for both fully automated black-box operation and incorporation into interactive systems. Our system scales up well with the number of classes and is portable to other not necessarily clinical corpora. As an extension of this paper, we also provide a detailed online appendix that contains the resources we applied and an on-line demo with most of the functionalities of our system.
Acknowledgments
The authors thank György Biró for his machine learning related tips, and Ferenc P. Szidarovszky for a part of text preprocessing, and finally Katalin Tóth, MD, for giving some domain-specific hints for rule creation. The authors thank the challenge organizers for providing them with this invaluable dataset and research experience; special thanks to Özlem Uzuner, for her role as organizer and correspondent.
Footnotes
Domonkos Tikk was supported by the Alexander von Humboldt Foundation.
References
- 1.Krallinger M, Erhardt RA, Valencia A. Text-mining approaches in molecular biology and biomedicine Drug Discov Today 2005;10(6):439-445. [DOI] [PubMed] [Google Scholar]
- 2.Farkas R, Szarvas G. Automatic construction of rule-based ICD-9-CM coding systems BMC Bioinform 2008;9(S3):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plake C, Schiemann T, Pankalla M, et al. PubMed as a graph Bioinformatics 2006;22(19):2444-2445. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AM, Hersh WR. A survey of current work in biomedical text mining Brief Bioinform 2005;6(1):57-71. [DOI] [PubMed] [Google Scholar]
- 5. International Challenge: Classifying Clinical Free Text Using Natural Language Processinghttp://www.computationalmedicine.org/challenge/index.php 2005. Accessed May 2009.
- 6.Uzuner Ö, Szolovits R, Kohane I. i2b2 workshop on natural language processing challenges for clinical records 2006. Proc. of the Fall Symp. of the Amer. Med. Info. Assoc. (AMIA 2006), Washington, DC.
- 7.Uzuner Ö. Recognizing obesity and Co-morbidities in sparse data J Am Med Inform Assoc 2009. [DOI] [PMC free article] [PubMed]
- 8.Hayes PJ, Andersen PM, Nirenburg IB, Schmandt LM. Tcs: A shell for content-based text categorization 1990. In: 6th Conference on Artificial Intelligence Applications.
- 9.Sebastiani F. Machine learning in automated text categorization ACM Comput Surv 2002;34(1):1-47. [Google Scholar]
- 10.Zeng Q, Goryachev S, Weiss S, et al. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: Evaluation of a natural language processing system BMC Med Inform Decis Mak 2006;6(1):30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi CL, Street WN, Ward MM. Building a hospital referral expert system with a prediction and optimization-based decision support system algorithm J Biomed Inform 2008;41(2):371-386. [DOI] [PubMed] [Google Scholar]
- 12.Gaudan S, Kirsch H, Rebholz-Schuhmann D. Resolving abbreviations to their senses in MEDLINE Bioinformatics 2005;21(18):3658-3664. [DOI] [PubMed] [Google Scholar]
- 13.Chapman WW, Bridewell W, Hanbury P, Cooper GF, Buchanan BG. A simple algorithm for identifying negated findings and diseases in discharge summaries J Biomed Inform 2001;34:301-310. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg DA, Humphreys BL, McCray AT. The Unified medical language system Methods Inf Med 1993;32:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsujii Laboratory [homepage on the Internet]Tokyo, Japan: BioNLP'09 Shared Task on Event Extraction: in conjunction with BioNLP, a NAACL-HLT 2009 workshop, June 4-5 2009, Boulder, Coloradohttp://www-tsujii.is.s.u-tokyo.ac.jp/GENIA/SharedTask/ 1993. Accessed May 2009.