Abstract
To distinguish the extract of notoginseng root from that of other species in the genus Panax, we used reverse phase high performance liquid chromatography (HPLC) coupled with a principal component analysis (PCA) method. The content of 12 saponins in notoginseng root extracts from different sources was evaluated. Herbal extracts from different plant parts of notoginseng, Asian ginseng, and American ginseng were also evaluated. With an HPLC assay, however, it is difficult to determine whether notoginseng root extract has been adulterated with other plant parts or other Panax species before extraction. Therefore, we introduced PCA to identify adulteration in notoginseng root extract. PCA was performed on the data set obtained from the HPLC chromatogram. The HPLC-PCA assay not only distinguished notoginseng root extract from the extract of other plant parts of notoginseng, but also from the extract of Asian or American ginseng plant parts.
Keywords: Panax notoginseng, extract authentication, adulteration, genus Panax, dammarane saponins, HPLC, PCA
INTRODUCTION
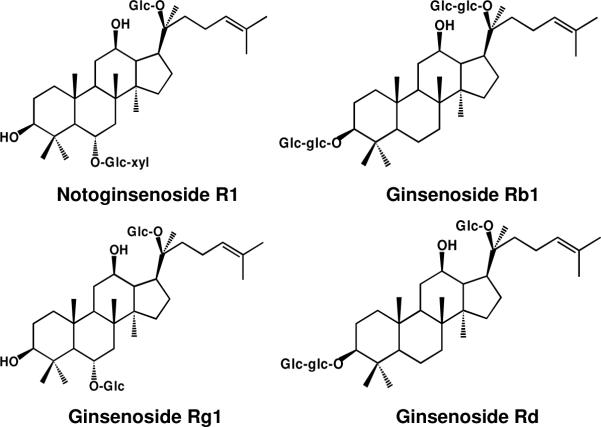
The root of Panax notoginseng (Burk.) F. H. Chen (Araliaceae) is a remedy that has a long history of use in China, Japan and other Asian countries (1, 2). Notoginseng is cultivated commercially in the southwest regions of China, especially in the Yunnan province. The main bioactive compounds in notoginseng are dammarane saponins, commonly referred to as notoginseng saponins (Figure 1) (2, 3). Notoginseng root extract exerts various effects on the cardiovascular system, the central nervous system, the endocrine system, and the body's inflammatory response (1, 4). Although the saponins from notoginseng are similar to those in other ginsengs such as Panax ginseng (Asian ginseng) and Panax quinquefolius (American ginseng), these herbs differ in their effects on symptoms (2, 5). To reduce the expense of ginseng product in the marketplace, however, some notoginseng leaf and flower extracts or extracts of other Asian ginseng are marked and sold as notoginseng extract in China (6). Since different ginseng saponins may possess opposing pharmacological activities (7), knowing the real content of saponins and their proportions is vital for the safe use of notoginseng extract. If notoginseng extract is adulterated, its therapeutic effects are not achieved. Moreover, adulteration may cause an adverse or opposite effect (8, 9). New regulations by the FDA in the United States for alternative complementary supplements require botanical extracts to be standardized (10). Therefore, accurate identification of notoginseng root extract is important.
Figure 1.
Chemical structures of four major saponins in notoginseng roots. Abbreviations for carbohydrates are as follows: Glc, β-D-glucose; Xyl, β-D-xylose.
Currently, the most popular herbal products are in extract forms (10, 11). The identification of the correct species used to make the extract is an essential step in the production of an herbal product. Several methods have been used to identify the source of an extract, including chemical, spectroscopic, and chromatographic techniques (12, 13). Among these methods, high performance liquid chromatography (HPLC) has been preferred (14). HPLC studies of notoginseng root have been performed by different groups (15-19). In those reports, two HPLC methods to identify the root of three Panax species were developed; however, only the constituents in the roots were determined; aerial plant parts were not assayed (18, 19). Since notoginsenoside R1 is found in notoginseng, R1 was used as the marker compound for the identification of notoginseng (18, 19). In the process of preparation and before extraction, notoginseng root can be adulterated with its other aerial parts or with other Panax species. Previous studies have not considered adulteration (18, 19) since notoginsenoside R1 can be detected from the adulterated extracts.
In this study, we determined the saponin content of extracts from different Panax species and different plant parts. We established a quantitative method with HPLC for the simultaneous determination of 12 saponins in notoginseng. If notoginseng root is adulterated with other ginsengs before extraction, the chromatograms of adulterated product may be similar to those of notoginseng root extract. We introduced principal component analysis (PCA), therefore, to find patterns in data and express their statistical similarities and differences (20, 21). Data obtained from HPLC were subjected to PCA to identify adulteration of notoginseng root extract.
MATERIALS AND METHODS
Chemicals
Standards for ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rg1 were obtained from Indofine Chemical Company (Somerville, NJ); ginsenosides Rb3, Rg2, 20R-Rg2, Rg3, Rh1, and notoginsenoside R1 were obtained from the Delta Information Center for Natural Organic Compounds (Xuancheng, Anhui, China). All standards were of biochemical-reagent grade and at least 95% pure as confirmed by HPLC.
Herbal Materials
Different plant parts of Panax notoginseng, the root, rootlet, corm, leaf, flower and berry were obtained from Wenshan, Yunnan, China. Fourteen lots of notoginseng root were obtained from various pharmacies in Beijing and Nanjing, China. The root, rootlet, leaf and berry of Asian ginseng (Panax ginseng C.A. Meyer) were obtained from Fusong, Jilin, China. Standard Asian ginseng root and leaf extracts were obtained from the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. The roots of notoginseng and Asian ginseng were identified according to the Chinese Pharmacopoeia (2005 edition). American ginseng (Panax quinquefolius L.) root, rootlet, leaf, berry were obtained from Roland Ginseng, LLC (Wausau, WI) and American ginseng roots were identified according to the United States Pharmacopoeia NF 21. The voucher samples were deposited at the Tang Center for Herbal Medicine Research at the University of Chicago (Table 1).
Table 1.
Panax Extracts from Different Plant Sources
| Group | Classification | Part | Sample numbers |
|---|---|---|---|
| 1 |
P. notoginseng underground |
Root | 16 |
| Rootlet | 3 | ||
| Corm |
2 |
||
| 2 |
P. notoginseng aerial |
Leaf | 2 |
| Flower | 1 | ||
| Berry |
1 |
||
| 3 |
P. ginseng underground |
Root | 3 |
| Rootlet |
1 |
||
| 4 |
P. ginseng aerial |
Leaf | 2 |
| Berry |
1 |
||
| 5 |
P. quinquefolius underground |
Root | 4 |
| Rootlet |
1 |
||
| 6 |
P. quinquefolius aerial |
Leaf | 3 |
| Berry |
4 |
||
| 7 |
P. notoginseng |
Root, leaf |
1 |
| 8 |
P. notoginseng | Root | 1 |
|
P. ginseng |
Root |
||
| 9 | P. notoginseng | Root | 1 |
| P. ginseng | Leaf |
Sample Preparation
Dried herbal samples from different plant parts from notoginseng, Asian ginseng and American ginseng, were pulverized into powder and passed through a 40-mesh screen. The sample powder was extracted with 70% ethanol. The extract solution was condensed under vacuum and extracted with water-saturated n-butanol. The saponins were extracted into the n-butanol phase. The n-butanol phase was evaporated under vacuum and then lyophilized. The herbal extract was dissolved in methanol (1 mg/mL and 10 mg/mL) and filtered through a Millex 0.2-μm nylon membrane syringe filter (Millipore Co., Bedford, MA) before use.
HPLC Analysis
The HPLC system was a Waters 2960 instrument (Milford, MA) with a quaternary pump, an automatic injector, a model 996 photodiode array detector, and Waters Millennium 32 software for peak identification and integration. The separation was carried out on an 250 mm × 3.2 mm i.d., 5μ, Alltech Ultrasphere C18 column (Deerfield, IL) with a 7.5 mm × 3.2 mm i.d. guard column. For HPLC analysis, a 20-μL sample was injected into the column and eluted at room temperature with a constant flow rate of 1.0 mL/min. For the mobile phase, acetonitrile (solvent A) and water (solvent B) were used. Gradient elution started with 17.5% solvent A and 82.5% solvent B, followed by 20 min linear gradient from 17.5% to 21% A, 3 min linear gradient from 21% to 26% A, 19 min isocratic elution with 26% A, 13 min linear gradient from 26% to 36% A, 9 min linear gradient from 36% to 50% A, 2 min linear gradient from 50% to 95% A, 3 min isocratic elution with 95% A, 3 min linear gradient from 95% to 17.5% A, and 8 min isocratic elution with 17.5% A. The detection wavelength was set to 202 nm.
Principal Component Analysis (PCA)
A similar evaluation system for the chromatographic fingerprint of the different plant parts of notoginseng, Asian ginseng and American ginseng was used to process a two-dimensional fingerprint. The HPLC fingerprint data were processed on a Pentium IV computer. PCA and other involved programs were coded in MATLAB (Mathworks Inc.).
RESULTS AND DISCUSSION
Optimal Separation of Notoginseng Saponins
The separation of 12 notoginseng saponins was evaluated by changing the gradient eluting programs. In the chromatogram, 12 saponins were separated into three groups: 1) notoginsenoside R1, ginsenosides Rg1 and Re; 2) ginsenosides Rh1, Rg2 and 20R-Rg2; and, 3) ginsenosides Rb1, Rc, Rb2, Rb3, Rd and Rg3. Saponins in groups 1) and 3) were relatively easy to separate. Separation of the three ginsenosides in group 2) was difficult using the gradient eluting system of acetonitrile and water. The modification of HPLC conditions in this study was focused on the separation of ginsenosides Rh1, Rg2 and 20R-Rg2.
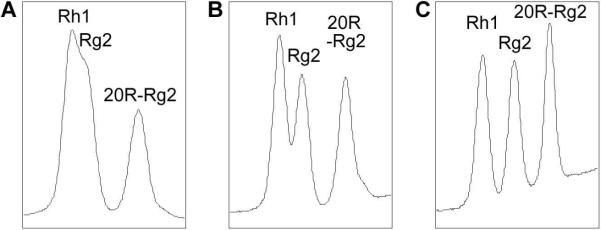
The first 20 min of elution, beginning with 17.5% acetonitrile and 82.5% water and then changing to 21% acetonitrile, was for the separation of notoginsenoside R1 and ginsenosides Re and Rg1. The percentage of acetonitrile was then increased to separate ginsenosides Rh1, Rg2 and 20R-Rg2. As shown in Figure 2, when the composition of the eluting solvents was kept at 28% acetonitrile and 72% water beginning at 23 min, ginsenosides Rh1 and Rg2 could not be separated. At a composition of 27% acetonitrile and 73% water, ginsenosides Rh1, Rg2 began to separate, but with low separation efficiency. When the composition of acetonitrile was changed to 26%, baseline separation for Rh1 and Rg2 was achieved. Small changes in the composition of eluting solvents influenced the separation of ginsenosides Rh1 and Rg2 noticeably.
Figure 2.
Representative HPLC chromatograms of the separation of ginsenosides Rh1, Rg2 and 20RRg2. After 23 min, eluting solvents were held for 19 min in (A) 28% acetonitrile and 72% water, (B) in 27% acetonitrile and 73% water, or (C) in 26% acetonitrile and 74% water.
Validation of Analytical Method
The calibration curves for all 12 saponins showed good linearity (R2>0.9990) in the concentration ranges of 1−200 μg/mL for ginsenosides Rb2, Rc, Rd, Rg2, 20 R-Rg2, Rh1, Rg3 and notoginsenoside R1; 2−400 μg/mL for ginsenosides Rb1, Rb3, Re and Rg1.
The precision of the HPLC method was determined for intra- and inter-day variations. A notoginseng root extract was weighed and dissolved in methanol. The intra-day variability was performed five times on the same extract prepared on a single day. The inter-day reproducibility was determined by analyzing the samples on three separate days. The validation studies showed overall intra- and inter-day variations (RSD) of less than 9.2% and 11.0%, respectively.
The percentage difference between amounts determined and spiked was considered a measure of accuracy. Various amounts of 12 saponins (2 mg and 1 mg) were added to 10 mg of notoginseng extract and then prepared as a test solution. The determination was performed in triplicate and the average recoveries and relative standard deviation (RSD) was calculated. An accuracy of higher than 87.1% was obtained for each of the analytes tested, and RSD was less than 11.5%.
Constituent Analysis of Samples
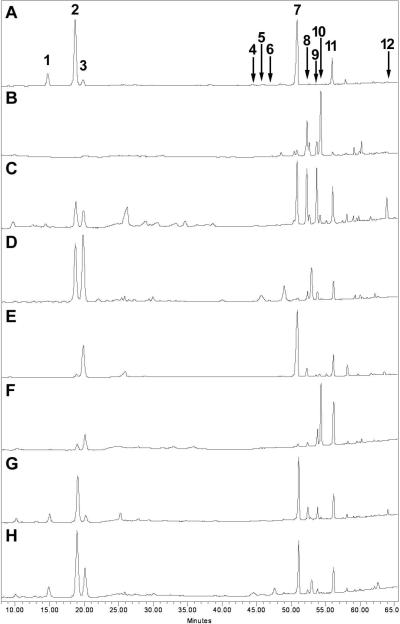
The HPLC method was subsequently applied to determine the twelve saponins in the botanical extracts simultaneously (Figure 3). Table 2 shows typical analytical results of extracts from root, rootlet, corm, leaf, flower and berry of notoginseng, and root, leaf and berry of Asian ginseng and American ginseng. Although the HPLC method has been used to identify ginseng saponins (18, 19, 22), the results of the comparison studies on the different plant parts of the Panax species are not sufficient. Our HPLC data supplied comprehensive information. The quantitative HPLC assay, however, could not identify notoginseng root extract from that adulterated with its aerial parts or other Panax plant parts (Figure 3G, H). Therefore, it was necessary to introduce an additional method to identify notoginseng root extract.
Figure 3.
Typical chromatograms of extracts from (A) the root and (B) leaf of Panax notoginseng; (C) the root and (D) leaf of Panax ginseng; and (E) the root and (F) leaf of Panax quinquefolius; a mixture of Panax notoginseng root with (G) Panax ginseng root and (H) leaf. Saponin peaks: notoginsenoside R1 (1), and ginsenosides Rg1 (2), Re (3), Rh1 (4), Rg2 (5), 20R-Rg2 (6), Rb1 (7), Rc (8), Rb2 (9), Rb3 (10), Rd (11), and Rg3 (12).
Table 2.
Typical Saponin Content in Panax Extracts (mg/g)
| Extracts | R1 | Rg1 | Re | Rh1 | Rg2 | 20R-Rg2 | Rb1 | Rc | Rb2 | Rb3 | Rd | Rg3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Panax notoginseng |
root | 60.2a | 299.8 | 33.9 | 6.7 | 9.2 | b | 269.9 | 2.7 | 1.3 | 0.6 | 70.3 | 0.5 |
| rootlet | 65.7 | 250.1 | 30.8 | 9.0 | 8.3 | 175.2 | 1.6 | 0.4 | 0.7 | 34.3 | 0.6 | ||
| corm | 71.4 | 270.7 | 33.6 | 3.9 | 6.7 | 243.5 | 0.8 | 7.5 | 0.7 | 66.8 | 0.6 | ||
| leaf | 0.4 | 0.5 | 10.4 | 1.1 | 15.6 | 119.8 | 35.8 | 182.1 | 7.6 | 0.4 | |||
| flower | 0.7 | 0.5 | 6.7 | 1.6 | 54.5 | 112.9 | 46.4 | 152.4 | 18.0 | 5.2 | |||
| berry |
|
0.1 |
1.9 |
|
0.0 |
|
8.7 |
69.6 |
27.8 |
111.5 |
5.1 |
0.2 |
|
|
Panax ginseng |
root | 33.6 | 27.8 | 3.8 | 64.5 | 62.0 | 52.4 | 7.9 | 30.1 | 0.5 | |||
| leaf | 146.0 | 204.2 | 2.0 | 46.4 | 3.2 | 16.8 | 14.6 | 1.7 | 26.0 | ||||
| berry |
|
16.7 |
212.6 |
4.1 |
76.6 |
0.4 |
5.2 |
32.9 |
42.2 |
8.0 |
100.5 |
0.6 |
|
| Panax quinquefolius | root | 14.0 | 197.9 | 341.8 | 34.2 | 4.6 | 6.8 | 65.0 | 0.6 | ||||
| leaf | 32.8 | 97.5 | 0.4 | 3.1 | 0.6 | 8.3 | 16.2 | 54.5 | 204.8 | 101.6 | 0.7 | ||
| berry | 1.4 | 68.7 | 0.3 | 2.2 | 5.5 | 30.5 | 70.0 | 245.7 | 47.6 | 1.5 | |||
The data are the average of duplicates from one extract of each plant part.
Blank cell, trace content (<0.1 mg/g) or not detected.
PCA assay
To evaluate the similarity/diversity of notoginseng root extract and adulterated extract, we used PCA assay. PCA assay was performed on the data set obtained from the HPLC chromatogram. Twelve characteristic saponins, notoginsenoside R1, ginsenosides Rg1, Re, Rh1, Rg2, 20 R-Rg2, Rb1, Rc, Rb2, Rb3, Rd and Rg3, were chosen to build up a 12-dimensional data set, which represents 12 vectors; the contents are the value of the vectors. The data was normalized to reduce the effect of signal strength, which corresponded to a different concentration in each sample. As shown in Figure 4, all samples were classified into one of six groups: (1) notoginseng underground parts, (2) notoginseng aerial parts, (3) Asian ginseng underground parts, (4) Asian ginseng aerial parts, (5) American ginseng underground parts, and (6) American ginseng aerial parts. The points of the notoginseng rootlet and corm extracts were in the area of notoginseng root extracts, suggesting that because of high similarity of chemical constituents, either the rootlet or corm of notoginseng can be used as root.
Figure 4.
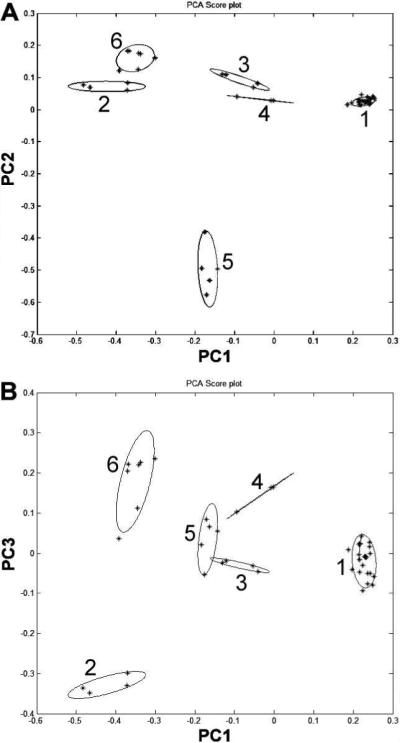
Principal component analysis (PCA) plots with (A) principal components 1 and 2 and (B) principal components 1 and 3, using contents of 12 saponins as input data. The 12 saponins are notoginsenoside R1, ginsenosides Rg1, Re, Rh1, Rg2, 20R-Rg2, Rb1, Rc, Rb2, Rb3, Rd and Rg3. The group numbers of Panax extracts are listed in Table 1.
To simplify data management, array optimizations were performed. In this evaluation, some characteristic saponins were “removed” from the array to make a smaller one. Fewer vectors simplified data collection requirements for model building. Since the content of ginsenosides Rh1, Rg2, 20R-Rg2 and Rg3 in the different extracts were relatively low, they may be removed from the array. A subset of eight saponins, notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3 and Rd, was chosen to build the PCA model. This array performed separation as good as that with the 12-peak array and simplified the mode (Figure 5).
Figure 5.
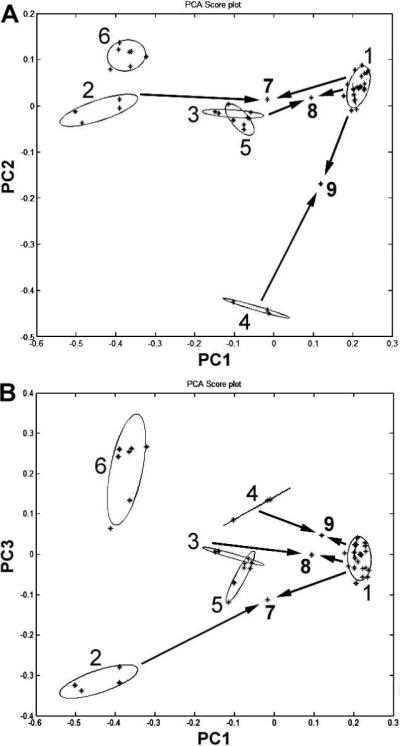
Principal component analysis (PCA) plots with (A) principal components 1 and 2 and (B) principal components 1 and 3, using contents of 8 saponins as input data. The 8 saponins are notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3 and Rd. The group numbers of Panax extracts are listed in Table 1. Arrows indicate groups 7, 8 and 9 were extracted from mixed plant parts.
We also selected as few as any four saponins, with a subset of notoginsenoside R1, and ginsenosides Rg1, Re, Rc for PCA assay, which improved separation among different groups. This set separated the groups with more scatter of the notoginseng underground group. The first two components (PC1+PC2) kept variance information at 95%. The third component contributed little to the separation. Data obtained from this study suggest that the set of eight saponins described above is the better choice for the simplified mode.
The by-products of notoginseng, such as leaf and flower, and other species in the genus Panax, such as Asian ginseng root and leaf, are cheaper than notoginseng root; hence commercially marked “notoginseng extract” may have been extracted from these other plant resources. Moreover, the pesticide concentration in the leaf extract could be high. Former identification methods were unsatisfactory because of the similarities of chemical constituents between notoginseng extracts and its adulterants. This HPLC-PCA method may identify adulterations in notoginseng such as notoginseng leaf or other ginsengs mixed with notoginseng root before extraction. Using the PCA method, we tried to identify the adulterations. As shown in Figure 5, when notoginseng root is mixed with notoginseng leaf (1:1), it can be separated from notoginseng root extract (point 7 in Figure 5). Separation was also good when notoginseng root was mixed with the root or leaf of Asian ginseng (1:1) (points 8 and 9 in Figure 5). Separation was similar when notoginseng root was mixed with American ginseng root or leaf before extraction, although the possibility of adulteration with American ginseng is low, given that American ginseng is a product of North America. Data obtained from this study suggest that using our HPLC-PCA method, adulterants added to notoginseng root such as notoginseng leaf; Asian ginseng root or leaf; or American ginseng root or leaf can be accurately identified. This method ensures the safe use of notoginseng root extract.
The HPLC fingerprint is a powerful method for the identification of constituents in botanical extracts and has been used with many herbal medicines (23), avoiding the time-consuming isolation of compounds to be identified (14, 24). Because Asian and American ginseng are commonly used herbs that are taxonomically related to notoginseng, the differentiation of extracts of notoginseng from Asian or American ginseng is difficult. Our study supplies a new method for distinguishing notoginseng root extract from its adulterants.
In summary, the present study proposes a quantitative HPLC method for the analysis of 12 saponins in extracts from the root, and other plant parts of Panax notoginseng, P. ginseng and P. quinquefolius. Our data showed comprehensive results for various plant parts of the commonly used species in the genus Panax. PCA was applied to obtain information about the different extracts in a data set of chromatographic fingerprints. A subset of eight saponins, notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3 and Rd, was distinguished. Notoginseng root extract can be separated from its aerial plant parts and from Asian or American ginseng. With this method, adulteration of the extract from notoginseng root by other ginseng plant parts or species can be revealed. The HPLC-PCA method supplies both a quantitative assay and qualitative identification. It could be a critical reference for the future evaluation of notoginseng products.
ACKNOWLEDGEMENT
This work was supported in part by the NIH/NCCAM grants AT003255 and AT004418.
LITERATURE CITED
- 1.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J. Pharm. Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 2.Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) F.H. Chen. J. Nat. Med. 2006;60:97–106. [Google Scholar]
- 3.Wang CZ, Xie JT, Zhang B, Ni M, Fishbein A, Aung HH, Mehendale SR, Du W, He TC, Yuan CS. Chemopreventive effects of Panax notoginseng and its major constituents on SW480 human colorectal cancer cells. Int. J. Oncol. 2007;31:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- 4.Chan P, Thomas GN, Tomlinson B. Protective effects of trilinolein extracted from Panax notoginseng against cardiovascular disease. Acta Pharmacol. Sin. 2002;23:1157–1162. [PubMed] [Google Scholar]
- 5.Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am. J. Chin. Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian Y. Identification of adulteration of Panax notoginseng. Shizhen Guoyi Guoyao. 1997;8(4):61. [Google Scholar]
- 7.Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- 8.Steele T, Rogers CJ, Jacob SE. Herbal remedies for psoriasis: what are our patients taking? Dermatol. Nurs. 2007;19:448–450. 457–463. [PubMed] [Google Scholar]
- 9.Poon WT, Ng SW, Lai CK, Chan YW, Mak WL. Factitious thyrotoxicosis and herbal dietary supplement for weight reduction. Clin. Toxicol. (Phila) 2008;46:290–292. doi: 10.1080/15563650701381179. [DOI] [PubMed] [Google Scholar]
- 10.Rapaka RS, Coates PM. Dietary supplements and related products: a brief summary. Life Sci. 2006;78:2026–2032. doi: 10.1016/j.lfs.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Yao P, Song F, Li K, Zhou S, Liu S, Sun X, Nussler AK, Liu L. Ginkgo biloba extract prevents ethanol induced dyslipidemia. Am. J. Chin. Med. 2007;35:643–652. doi: 10.1142/S0192415X07005132. [DOI] [PubMed] [Google Scholar]
- 12.Hon CC, Chow YC, Zeng FY, Leung FC. Genetic authentication of ginseng and other traditional Chinese medicine. Acta Pharmacol. Sin. 2003;24:841–846. [PubMed] [Google Scholar]
- 13.Ji S, Huo K, Wang J, Pan S. Molecular identification of twelve medicinal plants of Huperziaceae. Am. J. Chin. Med. 2007;35:1061–1071. doi: 10.1142/S0192415X0700551X. [DOI] [PubMed] [Google Scholar]
- 14.He XG. On-line identification of phytochemical constituents in botanical extracts by combined high-performance liquid chromatographic-diode array detection-mass spectrometric techniques. J. Chromatogr. A. 2000;880:203–232. doi: 10.1016/s0021-9673(00)00059-5. [DOI] [PubMed] [Google Scholar]
- 15.Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 16.Lau AJ, Seo BH, Woo SO, Koh HL. High-performance liquid chromatographic method with quantitative comparisons of whole chromatograms of raw and steamed Panax notoginseng. J. Chromatogr. A. 2004;1057:141–149. doi: 10.1016/j.chroma.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 17.Guan J, Lai CM, Li SP. A rapid method for the simultaneous determination of 11 saponins in Panax notoginseng using ultra performance liquid chromatography. J. Pharm. Biomed. Anal. 2007;44:996–1000. doi: 10.1016/j.jpba.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Leung KS, Chan K, Bensoussan A, Munroe MJ. Application of atmospheric pressure chemical ionisation mass spectrometry in the identification and differentiation of Panax species. Phytochem. Anal. 2007;18:146–150. doi: 10.1002/pca.962. [DOI] [PubMed] [Google Scholar]
- 19.Wan JB, Li SP, Chen JM, Wang YT. Chemical characteristics of three medicinal plants of the Panax genus determined by HPLC-ELSD. J. Sep. Sci. 2007;30:825–832. doi: 10.1002/jssc.200600359. [DOI] [PubMed] [Google Scholar]
- 20.Jurs PC, Bakken GA, McClelland HE. Computational methods for the analysis of chemical sensor array data from volatile analytes. Chem. Rev. 2000;100:2649–2678. doi: 10.1021/cr9800964. [DOI] [PubMed] [Google Scholar]
- 21.Norden B, Broberg P, Lindberg C, Plymoth A. Analysis and understanding of high-dimensionality data by means of multivariate data analysis. Chem. Biodivers. 2005;2:1487–1494. doi: 10.1002/cbdv.200590120. [DOI] [PubMed] [Google Scholar]
- 22.Fuzzati N. Analysis methods of ginsenosides. J. Chromatogr. B. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Xie P, Chen S, Liang YZ, Wang X, Tian R, Upton R. Chromatographic fingerprint analysis--a rational approach for quality assessment of traditional Chinese herbal medicine. J. Chromatogr. A. 2006;1112:171–180. doi: 10.1016/j.chroma.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 24.Liang YZ, Xie P, Chan K. Quality control of herbal medicines. J. Chromatogr. B. 2004;812:53–70. doi: 10.1016/j.jchromb.2004.08.041. [DOI] [PubMed] [Google Scholar]