Abstract
Metastasis-associated tumor antigen 1 (MTA1), a component of the nucleosome remodeling and deacetylating (NuRD) complex, is routinely up regulated in several cancers. In the present study, we investigated the potential role of MTA1 in BRCA1 transcriptional repression and subsequent chromosomal instability. MTA1-NuRD complex was found to negatively regulate BRCA1 transcription by physically associating with an atypical estrogen-responsive element (ERE) on the BRCA1 promoter. Moreover, MTA1 and HDAC complex recruited to the ERE of BRCA1 promoter in an ER alpha dependent manner. Accordingly, BRCA1 protein levels were enhanced by silencing of either MTA1 expression or by treatment with the specific histone deacetylase inhibitor trichostatin A. MTA1’s strong repressive effects on BRCA1 expression was supported by our observation that cells stably over expressing MTA1 showed centrosome amplification which has been long implicated as a phenotype for BRCA1 repression. Accordingly, over expression of BRCA1 in cells stably over expressing MTA1 resulted in restoration of normal centrosome numbers. Together, these findings strongly implicate MTA1 in the transcriptional repression of BRCA1 leading to abnormal centrosome number and chromosomal instability.
INTRODUCTION
Metastasis tumor antigen 1 (MTA1), a candidate member of the metastasis-associated gene family, is a component of the nucleosome remodeling and deacetylating (NuRD) complex. The NuRD complex has been implicated in adenosine triphosphate-dependent chromatin remodeling and transcriptional regulation. As a part of the NuRD complex, MTA1 is thought to modulate transcription by influencing chromatin remodeling (Toh et al. 1994), (Kumar et al. 2003). Indeed, MTA1 is over expressed in a variety of human cancers including cancers of the breast, ovary, lung, gastrointestinal system, and colorectum (Balasenthil et al. 2006; Hamatsu et al. 2003; Jang et al. 2006; Martin et al. 2006; Toh et al. 1994; Toh et al. 1999). It also plays an important role in metastasis and tumor aggressiveness (Jang et al. 2006; Toh et al. 1994; Toh et al. 1999).
Another equally important gene that has long been implicated in metastasis is BRCA1. A large body of evidence shows a strong correlation between BRCA1 down regulation or dysfunction and increased metastasis. BRCA1-the first breast and ovarian cancer susceptibility gene was identified in 1994 and since then, significant progress has been made towards understanding its functions in cells. BRCA1 is an important regulatory protein. It is involved in regulation of the cell cycle, DNA damage repair, transcription, and chromatin remodeling (Deng 2006; Ko et al. 2006; Mullan et al. 2006; Rosen et al. 2006; Starita and Parvin 2003; Yarden and Papa 2006). Recent evidence revealed a role of BRCA1 in the regulation of numeral homeostasis of centrosomes (Indraccolo et al. 2006; Ouchi 2006). Breast cancer occurs in both familial and sporadic forms. Mutations in BRCA1 account for about 10-15% of familial breast cancers however; the role of BRCA1 in sporadic breast cancer has been ambiguous. Although hyper-methylation and loss of heterozygosity on chromosome 17 has been associated with sporadic breast cancer, very little is known about BRCA1’s transcriptional repression (Turner et al. 2007). Recent data suggest that the Ets-2 transcription factor may play a role in repression of the BRCA1 promoter (Baker et al. 2003). The notion that MTA1 might be a co-repressor was merely speculative until the recent discovery that MTA1 associates with histone deacetylases (HDACs) and thereby helps to repress estrogen receptor-α (ERα) transactivation in breast cancer cells (Mazumdar et al. 2001; Talukder et al. 2003). Since the BRCA1 promoter contains an atypical estrogen-responsive element (ERE) (Xu et al. 1997), we hypothesized that MTA1 might play a role in BRCA1 repression. To test this, we addressed the mechanism for BRCA1 repression by MTA1 and further, evaluated the mechanistic link, if any, between MTA1-mediated BRCA1 repression and centrosome number amplification in breast cancer cells. We identified centrosome amplification in MTA1-overexpressing cells and used centrosome number amplification as a functional marker of MTA1-mediated BRCA1 repression.
RESULTS
MTA1 over expression represses BRCA1 gene transcription
We used MTA1 over expressing MCF7 cells as a model system to evaluate effect of MTA1 on the status ofBRCA1 and its downstream target poly -ADP-ribose-polymerase 1 (PARP1) (Kanai et al. 2003; Simbulan-Rosenthal et al. 2001). By RT-PCR analysis we found that both BRCA1 and PARP1 were reduced at the mRNA level in MTA1-overexpressing cells when compared with control vector-transfected cells (Figs. 1A). To confirm this, northern blot analysis was conducted in MCF7/pcDNA and MTA1 clones. BRCA1 mRNA levels were found to be drastically down regulated in the MCF7/MTA1 cells (Fig. 1B). A concomitant reduction of the protein levels of BRCA1 and PARP1 in MCF7/MTA1 clones was seen by western blotting analysis (Fig. 1C). Together, these results suggested that MTA1 over expression repressed BRCA1 transcription. This notion was confirmed by knockdown of endogenous MTA1 by MTA1-specific RNA interference (RNAi) in MCF-7 cells, resulting in increased levels of BRCA1 protein (Fig. 1D). Together, these findings established an inverse correlation between MTA1 and BRCA1 expression in cancer cells.
Figure 1. Inverse relationship between expression of MTA1 and BRCA1.
A, RT-PCR analysis showing decreased BRCA1 and PARP1 expression in MTA1-overexpressing MCF7 clones (first and second panels, right lane) when compared with control cells. Actin expression was used as an internal control for both RNA samples (third panel). B, Northern blot analysis showing a drastic decrease in BRCA1 mRNA levels in MCF7/MTA1 cells when compared with MCF7/pcDNA (control) cells (first panel, second lane), thus implicating MTA1 over expression in the repression of BRCA1 gene transcription. C, Significant decreases in the protein levels of BRCA1 (left) and PARP1 (right) in the presence of MTA1 over expression (first panel, second lane). Actin or Vinculin expression was used as an internal control. D, Increase in BRCA1 levels as a result of MTA1 knockdown with small interfering RNA (siRNA) (first panel, right lane). A decreased level of MTA1 expression (second panel, right lane) shows effective knockdown of MTA1 by MTA1 siRNA. Actin expression was used as an internal control (third panel). Expression levels were quantified using the ImageQuant version 5.1 program.
MTA1 represses BRCA1 transcription
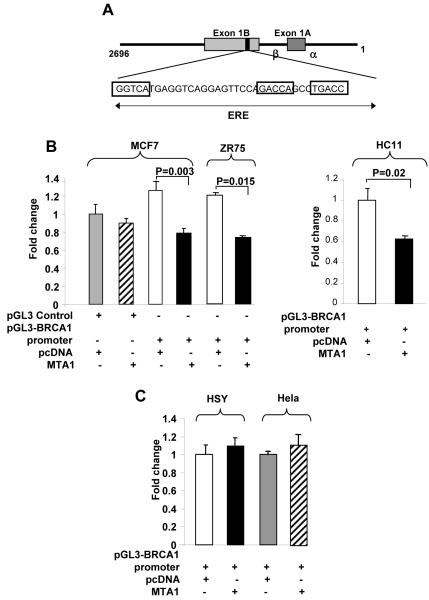
Using a pGL3-BRCA1 promoter (1-2696) reporter system that includes one atypical ERE site (Fig. 2A), we showed that transient co-transfection of MTA1 significantly reduced the amount of transcription driven by a BRCA1-luc reporter. In MCF7, ZR75 and HC11 cells (ERα- and ERβ-positive, Supplementary Fig. 1). BRCA1 transcription was repressed by approximately 40% upon MTA1 over expression (Fig. 2B and 2C). However, no inhibitory effect of MTA1 on BRCA1-reporter activity was noted in either an ERβ-positive cell line (i.e., human salivary gland carcinoma HSY cells) or an ERα- and ERβ-negative line (HeLa cells) (Fig. 2D). Together, these findings suggested that MTA1 over expression repressed BRCA1 transcription via ERα. Using a pGL3-BRCA1 promoter we showed that both MCF7/pcDNA and MCF7/MTA1 cells was able to induce BRCA1 expression on estrogen (E2) treatment. However, under conditions of E2 presence or absence MTA1 over expression in comparison to the MCF7/pcDNA control cells, repressed BRCA1 expression (Supplementary Fig. 2). Thus suggesting, MTA1 mediated repression of BRCA1 is E2 independent.
Figure 2. MTA1-mediated repression of BRCA1 promoter activity.
A, An atypical ERE located on the human BRCA1 gene promoter and through which estrogen regulates transcription of the BRCA1 gene. B, Luciferase assay in cells transfected with a pGL3-BRCA1 promoter (1-2696) reporter construct containing one atypical ERE. Luciferase activity was assayed 48 h after transfection. Values were normalized to β-galactosidase activity (n=3). BRCA1 promoter activity was reduced approximately 50% in MCF7, ZR75 and HC11 cells over expressing MTA1. C, Luciferase assay of HSY (ERα-negative) or HeLa cells (ERα-, ERβ-negative) showing no change in BRCA1 promoter activity in the presence of MTA1 over expression, thus implicating ERα in MTA1-mediated repression of BRCA1.
MTA1-NuRD complex associates with an ERE in the BRCA1 promoter
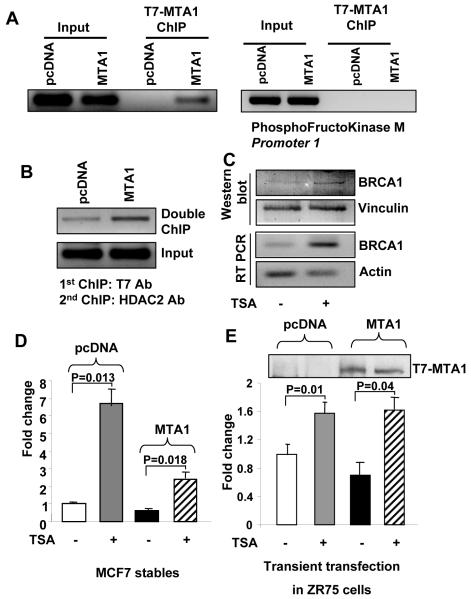
To study the mechanism of BRCA1 repression in presence of MTA1, we hypothesized that ERα may be recruiting MTA1 to the promoter via the E2-responsive element. To test this hypothesis, we conducted ChIP assays in MCF7/pcDNA and MCF7/MTA1 clones using a T7-tag specific antibody. MTA1 clearly associated with the 200-bp region of the promoter containing the ERE. An aliquot of the same ChIP elute was used to PCR-amplify the phosphofructokinase-muscle-type isoform (PFK-M) gene (Fig. 3A). Band for PFK-M gene in the input samples and no band in T7-Chip samples demonstrated specific association of MTA1 to the BRCA1 promoter which in-turn may explain the noted repression of BRCA1 in MTA1 over expressing cells. This association of the MTA1-containing NuRD complex with the promoter and the involvement of ERα in its recruitment were subsequently confirmed by sequential double ChIP assays in MCF7/pcDNA and MCF7/MTA1 clones, first with an anti-T7-Mab to immunoprecipitate T7-MTA1 and then with an HDAC2 antibody. We found that indeed, MTA1-HDAC complex was recruited to the BRCA1 promoter in MCF-7/MTA1 cells (Fig. 3B). Moreover, treatment of MTA1-overexpressing MCF7 cells with the HDAC inhibitor TSA increased BRCA1 expression at both the mRNA and protein level, as demonstrated by RT-PCR and Western blot analysis, respectively (Fig. 3C). Using BRCA1-luc reporter assays, the repression of BRCA1 transcription in MTA1-overexpressing MCF7 cells relative to pcDNA-transfected control cells was partially relieved by TSA treatment (Fig. 3D). In addition, ZR75 cells transiently transfected with pcDNA or MTA showed complete relief of repression of BRCA1 transcription on TSA treatment (Fig. 3E). Together, these studies provided strong support to the association of the MTA1-NuRD complex to the BRCA1 promoter.
Figure 3. MTA1-mediated repression of the BRCA1 promoter via its atypical ERE site.
A, ChIP assay in MCF7/pcDNA and MCF7/MTA1 clones with a T7-tag specific antibody. Results demonstrate the physical association of MTA1 with BRCA1 promoter chromatin and not with the PhosphoFructokinase M promoter chromatin. B, Double or sequential ChIP in MCF7/pcDNA and MCF7/MTA1 clones. Results demonstrate the recruitment of both MTA1 and HDAC2 to the BRCA1 promoter, thus implying recruitment of the MTA1-containing NuRD complex to that chromatin region. C, Decreased MTA1-induced repression of BRCA1 at both the protein (first panel, right lane) and mRNA levels (third panel, right lane) in MCF7 cells treated with the specific histone deacetylase (HDAC) inhibitor trichostatin A (TSA; 300 nmol/L per milliliter of culture medium). Actin expression was used as an internal control. Functional assay of pGL3 BRCA1-luciferase activity demonstrating the effect of TSA treatment on MTA1-mediated repression of BRCA1 expression in MCF7 cells. In brief, MCF7 pcDNA and MCF7 MTA1 over expressing cells were treated with TSA (D) or ZR75 cells were transiently transfected with a pcDNA or T7-tagged MTA1 expression construct (1μg) and then treated with TSA (E). Western blot for T7 in TCA precipitated luciferase samples shows efficient transfection (above the bar chart). The TSA treatment relieved MTA1-induced repression of BRCA1, thus implying that HDACs were involved in such repression.
Role of ERα in association of MTA1-NuRD complex with the ERE in BRCA1 promoter
ChIP assays demonstrated MTA1’s recruitment to the BRCA1 promoter was dependent on ERα. As shown in double ChIP assays of MCF7/pcDNA and MCF7/MTA1 clones, first with an anti-ER α antibody and then with an anti-T7-mAb to precipitate T7-MTA1 bound to ERα, association of the ERα/MTA1 complex with the BRCA1 promoter ERE was distinctly demonstrated (Fig. 4A). Comparison of the association of MTA1 to the BRCA1 promoter in ER-positive and negative cell lines provided further proof of the dependence of MTA1 on ERα to be recruited to the promoter. Simultaneous ChIP assays conducted in MCF7 and HeLa cells with MTA1-specific antibody showed association of MTA1 to the BRCA1 promoter only in the ER positive MCF7 cells but not in ER-negative (HeLa) cells (Fig. 4B). To further validate the role of ERα in recruitment of MTA1-NuRD complex on BRCA1 promoter, we treated MCF7 cells with ICI, an ERα antagonist. The ChIP assay analysis demonstrated loss of MTA1 recruitment to the ERE element on the BRCA1 promoter on treatment with ICI. Similarly, the repression of BRCA1 transcription was relieved on ICI treatment using BRCA1-luc reporter assay (Fig. 4D). Together, these results demonstrated ERα-mediated recruitment of the MTA1-NuRD complex to the ERE on the BRCA1 promoter led to BRCA1 repression.
Figure 4. ERα-mediated recruitment of the MTA1-NuRD complex to the BRCA1 promoter.
A, Single Chip with ERα antibody and IgG control shows recruitment of ERα on BRCA1 promoter. Double ChIP assays with ERα antibody and anti-T7-tag antibody proved that ERα was involved in recruiting MTA1 to the BRCA1 promoter. B, ChIP assay with MTA1-specific antibody showed that MTA1 was recruited to the BRCA1 promoter only in ER-positive (MCF7) cells and not in ER-negative (HeLa) cells, thus implicating ERα in such recruitment. Western blot showing endogenous MTA1 levels in MCF7 and Hela cells. Actin was used as an internal control. C, ChIP assay with MTA1-specific antibody of MCF7 cells treated with anti-estrogen ICI-182780 for 1h before E2 treatment. Results demonstrated that MTA1 was not recruited to the BRCA1 promoter in the treated cells. D, functional assay of pGL3 BRCA1-luciferase activity demonstrating relief of MTA1-mediated repression of BRCA1 expression in MCF7 cells over expressing MTA1 on ICI treatment, further implicating ERα as a mediator of MTA1’s recruitment to the BRCA1 promoter.
Co-operation between MTA1 and ERα in regulation of BRCA1 repression
To study effect of ERα on BRCA1 gene expression, we silenced ERα expression in both MCF7 and HC11 cells using ERα -specific RNAi and examined expression of BRCA1 using BRCA1-luc reporter assay. BRCA1 levels increased when ERα expression was knocked-down (Fig. 5A and 5B). Western blot of the TCA precipitated luciferase cell lysate with ERα antibody confirmed the successful knocking down of ERα by the RNAi used. The same blot when probed for MTA1 showed almost equal levels of MTA1 in both the control and ERα knockdown samples thus confirming the role of ERα in MTA1-HDAC mediated BRCA1 repression. We next silenced MTA1 in ERα transfected Hela cells using MTA1 -specific RNAi and examined BRCA1 expression using BRCA1-luc reporter assay. Knocking down of MTA1 resulted in increased BRCA1 expression compared to that in the control cells (Fig. 5C). Western blot of the TCA precipitated luciferase cell lysate with MTA1 antibody confirmed the successful knocking down of MTA1 by the RNAi used. To validate the role of ERE in MTA1 mediated BRCA1 repression, we mutated the ERE on BRCA1 promoter sequentially using site directed mutagenesis. Using BRCA1-luc reporter and BRCA1 with mutated ERE-luc reporter assays, BRCA1 expression in MTA1-overexpressing MCF7 cells relative to pcDNA-transfected control cells was studied. The atypical ERE on BRCA1 promoter is stretched across 35 amino acids. Mutation of the ERE-1 region continued to show MTA1 mediated repression of BRCA1, probably because of the remaining ERE-2 and ERE-3 regions capable of recruiting MTA1 on BRCA1 promoter. However, simultaneous mutation of ERE-1 + ERE-2 was able to partially block MTA1 mediated BRCA1 repression and mutation of ERE-1 + ERE-2 + ERE-3 regions completely blocked MTA1 mediated BRCA1 repression (Fig. 5D). This indicated that MTA1 mediated repression of BRCA1 is via the sites marked as ERE-2 and ERE-3 and not by ERE-1 in the BRCA1 promoter. To further confirm the involvement of ERα in recruiting MTA1 to the promoter of BRCA1, we checked the association of MTA1 to the BRCA1 promoter chromatin under conditions of depleted ERα levels. In MCF7/MTA1 stable cells, the expression of ERα was silenced by transfecting ERα-specific RNAi. Association of MTA1 to the BRCA1 promoter was studied by ChIP assay. Results showed that on depletion of ERα, the recruitment of MTA1 to BRCA1 promoter was decreased drastically. This was evident by a loss of association of MTA1 to the promoter (Fig 5E, Lane 4) under ERα knockdown conditions in comparison to the clearly evident recruitment of MTA1 in MCF7/MTA1 cells transfected with control or scrambled RNAi (Fig 5E, lane 3). These results, in addition to the ChIP experiments done on treatment with anti-estrogen ICI clearly showed that ERα was mediating the recruitment of MTA1 to the ERE elements of the BRCA1 promoter and was indispensable for the MTA1-mediated repression of BRCA1 gene transcription. In all, these studies indicated co-operation between ERα and MTA1 in regulating BRCA1 repression via the ERE on BRCA1 promoter (Fig. 5F).
Figure 5. Co-operation between MTA1 and ERα in regulation of BRCA1 repression.
pGL3 BRCA1-luciferase activity demonstrating relief of MTA1-mediated repression of BRCA1 expression in MTA1 over expressing MCF7 cells A, and in HC11 cells B, on ERα knockdown with small interfering RNA (siRNA). A decreased level of ERα expression (Top panel, right lane) shows effective knockdown of ERα by ERα siRNA. Equal MTA1 levels in both contol siRNA and ERα knockdown samples (second panel) indicate specificity of ERα siRNA. Actin expression was used as an internal control (third panel). C, Functional assay of pGL3 BRCA1-luciferase activity demonstrating relief of BRCA1 repression in ERα transfected Hela cells on MTA1 knockdown with siRNA. A decreased level of MTA1 expression (Top panel, right lane) shows effective knockdown of MTA1. Actin expression was used as an internal control (second panel). D, Luciferase activity demonstrating that mutated ERE on BRCA1 promoter blocks MTA1-mediated repression of BRCA1 (SM, mutated ERE-1; DM, mutated ERE-1 + ERE-2; TM, mutated ERE-1 + ERE-2 + ERE-3). E, ChIP assay with MTA1-specific antibody of MCF7 cells treated with control siRNA and ERα specific siRNA. Results demonstrated a loss of MTA1 recruitment to the BRCA1 promoter under ERα knockdown conditions in comparison to the cells transfected with control RNAi. F, Schematic diagram of regulation of BRCA1 expression by the MTA1/NuRD complex.
MTA1 over expression leads to generation of multiple spindle poles
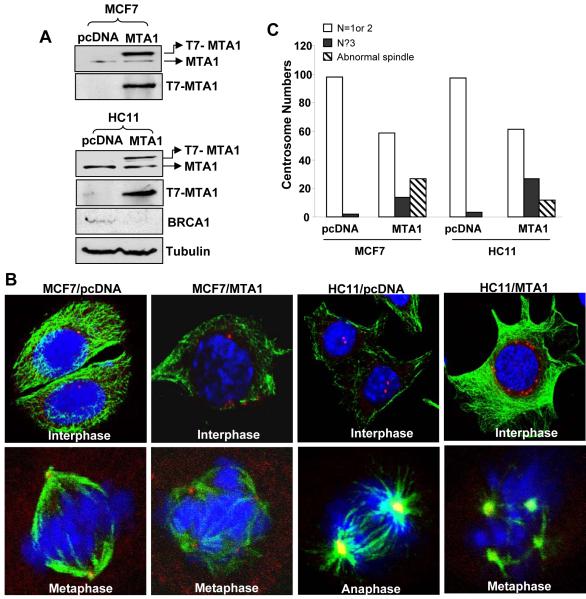
Large body of evidence shows that cells on BRCA1 suppression exhibit increased number of centrosomes and eventually develop genomic instability. To explore if MTA over expression had any effect on centrosome amplification, we used stable cell lines over expressing MTA i.e., MCF7 and HC11 cells (Fig. 6A). Similar to MCF7/MTA1 stable cells, over expression of MTA1 in HC11 cells led to BRCA1 repression (Fig. 6A). Both MCF7 and HCll cells were synchronized using double thymidine block and immunostained with antibody to α-tubulin and pericentrin (Fig. 6B). Pericentrin localization was used as an indicator of centrosomes. A total of 100 cells were counted in 5 different fields for each cell line and classified by centrosome number (i.e., 1or 2, ≥3 or abnormal spindle) (Fig. 6C). Careful analysis of these findings revealed a correlation between having high levels of MTA1 and having ≥3 centrosomes or abnormal spindles. This finding implicated a mechanistic link between MTA1-mediated BRCA1 repression and centrosome number amplification in MTA1 over expressing cells.
Figure 6. MTA1 over expression and resulting increases in centrosome number.
A, Western blot analysis showing endogenous MTA1, T7-pcDNA, T7-MTA1 plasmid expression in transfected MCF7 and HC11 cells. Significant decreases in the protein BRCA1 level in the presence of MTA1 over expression in HC11 cells. Tubulin was used as an internal control. B, Representative immunofluorescent images for α-tubulin (green), pericentrin (red) and DNA (blue) as seen under a confocal laser microscope are shown here. Cells were synchronized with double thymidine block. A total of 100 cells were counted. C, Bar chart showing quantified results. MTA1 over expression led to an increase in number of cells with multiple spindle poles.
Reintroduction of BRCA1 into MTA1-over expressing cells compromises abnormal centrosome number phenotype
In light of our findings, we hypothesized that if centrosome accumulation in MTA1-over expressing cells were due to repression of BRCA1, then reintroduction of BRCA1 would compromise the abnormal phenotype, at least partially. To test this hypothesis, MCF7/MTA1 stable cells were transfected with either GFP vector or GFP-BRCA1 (Fig. 7A). Cells were immunostained with antibody to α-tubulin and pericentrin and with DAPI to visualize DNA. A total of 100 cells were counted in 5 different fields for each cell line and classified by centrosome number (i.e., 1, 2, or ≥3 centrosomes). MTA1 over expressing cells transfected with GFP control vector continued to exhibit increase in number of cells with abnormal centrosome amplification. However, just as we hypothesized, reintroducing BRCA1 into MTA1 over expressing cells compromised the abnormal phenotype, thus implicating BRCA1 in MTA1-mediated centrosome amplification (Fig. 7B).
Figure 7. Role of BRCA1 in the multiple spindle phenotype observed in MTA1-over expressing cells.
A, MCF7/MTA1 cells transfected with GFP vector or GFP-BRCA1 was assessed by immunofluorescence staining for GFP (green), α-tubulin (Magenta), pericentrin (red) and DAPI stained DNA (blue). MTA1 over expression led to an increase in the number of centrosomes; this phenotype was compromised, however, by the reintroduction of BRCA1, thus implicating BRCA1 repression in the centrosome amplification observed in MTA1-overexpressing cells. B, Bar chart showing quantified results.
DISCUSSION
The transformation of a normal cell into a malignant cancerous cell involves profound alterations in the cellular physiology leading to the appearance of distinct characteristics including anchorage independent growth, immortalization, and chromosomal instability. A major focus of research in cancer biology is directed at deciphering molecular mechanisms responsible for these malignant transformations. Numerous tissue- and cancer-type-specific genes have been identified whose mutation or abnormal transcription (or both) results in drastic changes in cellular function that ultimately promote cancerous transformations. One such gene, the tumor suppressor gene BRCA1, has been implicated in breast cancer. This is evident by the fact that half of all familial breast cancers, which represents 10% of the total breast cancers, BRCA1 protein function is impaired as a result of germline mutations in the gene (Deng 2006; Fukasawa 2005). On the other hand, the remaining 90% of breast cancers occur sporadically in the absence of any apparent genetic predisposition. In these non-familial cases, tumorigenesis is likely not due to mutation but rather to a drastic decrease of BRCA1 mRNA or protein levels (Magdinier et al. 1998; Rio et al. 1999). Multiple studies have shown a correlation between loss of BRCA1 expression and increased metastatic disease. In light of these findings and because MTA1 is widely over expressed in human cancers including breast cancer (Jang et al. 2006; Nawa et al. 2000; Toh et al. 1994), and its over expression has been associated with metastasis, it appears that the MTA1-containing NuRD complex exerts a repressive effect on BRCA1 expression. Consistent with this notion was the relatively significant decrease in the mRNA and protein levels of BRCA1 and its target PARP1 that we observed in MCF7 cells stably over expressing MTA1 when compared with control MCF7/pcDNA cells (Fig. 1).
Previously, MTA1 has been shown to inhibit the expression of estrogen-responsive genes by repressing the transactivating functions of ERα (Mazumdar et al. 2001). In addition, BRCA1 is itself an estrogen-responsive gene whose promoter contains an atypical ERE (Fig. 2A) (Xu et al. 1997). These observations point to the possibility that MTA1 might be recruited to this element via ERα to bring about transcriptional repression of BRCA1. Luciferase assay with the full length BRCA1 promoter cloned into pGL3 vector showed repressing effect of MTA1 in MCF7 and ZR75 (ER positive cells), while there was no repression in HSY cells (ERα negative but ERβ positive) and Hela cells (ERα and ERβ negative) cells (Fig. 2B and 2C). This provided additional proof of the repressive effect of MTA1 on BRCA1 transcription and demonstrated the dependency of this repression on ERα. ChIP analysis provided further proof of the physical association of MTA1-NuRD complex with the BRCA1 promoter in the region of the estrogen responsive element, and thus, validating MTA1 as an effective co-repressor of BRCA1 transcription (Fig. 3A and 3B). An enhancement of the levels of BRCA1 by a HDAC inhibitor TSA (Fig. 3C, 3D and 3E) and by MTA1 silencing (Fig. 1D and 5B) resulted in BRCA1 expression, and further validating an inverse mechanistic link between the levels of MTA1 and BRCA1. Consistent with the above findings, ChIP analysis showed a simultaneous association of ERα and MTA1 on the BRCA1 promoter (Fig. 4A and 5E) demonstrating association of MTA1 to the ERE via ERα. The observation that the recruitment of MTA1 to BRCA1 promoter could be only seen in ERα-positive MCF7 cells and not in ERα-negative Hela cells in spite of comparative higher levels of MTA1 in Hela (Fig. 4B) and the loss of this recruitment in MCF7 cells on treatment with ICI, a pure anti-estrogen or under conditions of ERα depletion using siRNA to ERα, demonstrated the absolute dependency of this recruitment on ERα (Fig. 4C and 4D).
One way in which the BRCA1 protein exerts its tumor suppressor function is to regulate the centrosomal number; functional deregulation and amplification which leads to abnormal spindle formation and chromosomal instability, which is turn strongly associated with tumorigenesis (D’Assoro et al. 2002; Fukasawa et al. 1996). Studies have shown that BRCA1 localizes to the centrosome in mitotic cells and binds to γ-tubulin, a major component of centrosomes (Starita et al. 2004). In addition, BRCA1 has been shown to interact with a variety of proteins that regulate centrosome duplication like BRCA2, BubR1, CDK2-cyclin A, CDK2-cyclin E, Gadd45, p21, p53 and Rb) (Chabalier et al. 2006; Weaver et al. 2002). This role has been confirmed in experiments in which embryonic fibroblasts derived from wild-type BRCA1-deficient mice were found to have amplified centrosome numbers. Centrosomal amplification is known to be a hallmark of cells whose BRCA1 expression or function has been disrupted (Birgisdottir et al. 2006; Chen et al. 2006; Weaver et al. 2002). Interestingly, we observed a similar phenomenon of centrosomal amplification in MTA1 over expressing cells (Fig. 6B). That normal centrosomal numbers could be restored by the over expression of BRCA1 in cells stably over expressing MTA1 provides evidence that abnormal centrosomal amplification may be due to the drastic suppression of BRCA1 gene transcription.
In conclusion, our present studies have demonstrated that MTA1, a protein involved in nucleosomal remodeling and transcriptional repression, drastically represses transcription of the BRCA1 gene by associating, via ERα, with the BRCA1 gene promoter in a region containing a previously identified ERE. As a consequence, cells over expressing MTA1 exhibit centrosomal amplification, a phenomenon routinely observed in cells that have lost BRCA1 expression or functions. Although the role played by MTA1 in aggressive and metastatic cancers has been well established, its exact molecular mechanisms and gene targets have yet to be deciphered. Here, we have identified the BRCA1 gene as one of the gene targets whose suppression by MTA1 might be intimately involved in the malignant transformation of normal cells and the appearance of aggressive phenotypes.
MATERIALS AND METHODS
Cell culture and reagents
MCF7 (breast cancer cells) and Hela (cervical cancer cells) were purchased from the American Tissue Culture Collection (Rockville, MD). The salivary gland adenocarcinoma cell line HSY was generously provided by Dr. Fredrick Kay (NIH, Bethesda, MD). Cells were cultured in Dulbecco’s modified Eagle’s medium-F12 (1:1) supplemented with 10% fetal calf serum (FCS). The mouse mammary epithelial HC11 cells were generously provided by Dr. Dan Medina (Baylor College of Medicine, Houston, TX). HC11 cells were cultured in RPMI 1640 medium (Invitrogen) with 10% (v/v) FCS, L-glutamine, 10 ng/ml epidermal growth factor and 5 μg/ml insulin. Thymidine, mouse EGF, insulin and other chemicals were purchased from Sigma Chemical Company (St. Louis, MO). Antibodies against the T7 epitope were purchased from Novagen (Milwaukee, WI); antibodies to actin, vinculin, α tubulin and pericentrin from Sigma (St. Louis, MO); antibody to ERα from Chemicon (Temecula, CA); antibody to ERß from Oncogene Research Products (San Diego, CA); antibody to PARP1 from Santa Cruz Biotechnology (Santa Cruz, CA); antibody to BRCA1 from Calbiochem (San Diego, CA); and antibody to MTA1 from Bethyl (Montgomery, TX). ToPro3 and DAPI were purchased from Molecular Probes. Anti-mouse and anti-rabbit horseradish peroxidase-tagged antibodies were purchased from Amersham Biosciences (Piscataway, NJ).
Generation of stable clones
The stable clones of MTA1 or pcDNA in MCF7 cells and HC11 cells were generated as mentioned elsewhere (Bagheri-Yarmand et al. 2004; Mazumdar et al. 2001). For transient transfection, MCF7/MTA1 cells were grown to approximately 50% confluence, and then transfected with GFP vector or GFP-BRCA1 using the FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN).
Immunofluorescence and confocal microscopy
Cells were grown on glass coverslips in culture medium for 24 h after which cells were synchronized using double thymidine block. After treatment, the coverslips were washed with PBS and fixed and permeabilized with methanol at -20°C for 10 min. In case of GFP transfected cells, post 48 h of transfection, cells were fixed with 4% paraformaldehyde at 37°C for 10 min followed by permeabilization with 0.1% triton X-100 at room temperature for 10 min. Next, the coverslips were incubated with primary antibody and then with secondary antibody conjugated with fluorescent dye. Finally, each coverslip was examined by confocal microscopy under an Olympus FV300 laser scanning confocal microscope in accordance with established methods. Each representative image was examined and digitally recorded at the same cellular level and magnification.
RT-PCR analysis
RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad, CA) and then treated with DNase for 15 min at 37°C. Next, the DNase was inactivated by heating the samples at 65°C for 10 min. The mRNA so isolated was then subjected to RT-PCR analysis using specific primers for BRCA1 (3′-GGAAGGCAAAAACAGAACCA-5′ and 3′-GGATTTGAAAACGGAGCAAA-5′), for PARP1 (3′-ATGGAAAAGTCCCACACTGG-5′ and 3′-ACACCCCTTGCACGTACTTC-5′), and for actin (3′-TCCCTGGAGAAGAGCTACGA-5′ and 3′-GTACTTGCGCTCAGGAGGAG-5′).
Northern blot analysis
Total RNA was extracted from MCF7/pcDNA and MCF7/MTA1 cells using Trizol reagent. 15 μg of RNA from each sample was separated on a 0.8% agarose formaldehyde-formamide gel and transferred to a nitrocellulose membrane. Each blot was probed with an α-[32P]dCTP-labeled BRCA1 RT-PCR product as a probe to detect BRCA1 mRNA
siRNA transfection and MTA1 or ERα knockdown analysis
Small interfering RNA (siRNA) against human MTA1 or human ERα was purchased from Dharmacon (Lafayette, CA). Cells were transfected with control siRNA, MTA1 siRNA or ERα siRNA using 100 nmol/L siRNA and 4 μL oligofectamine in 6-well plates according to the manufacturer’s suggested protocol. Forty-eight hours after transfection, the MTA1 or ER α knockdown status of transfected cells was evaluated by Western blot analysis.
Luciferase reporter assay
Cells were seeded on 6-well tissue culture plates. Twenty-four hours later, when the cells had reached approximately 60% confluence, they were transfected with pGL3 control luciferase or pGL3 BRCA1 promoter luciferase using FuGENE 6 transfection reagent (Roche Applied Science). Luciferase activity was assayed 48 hours later using a luciferase assay kit (Promega, Madison, WI). Each experiment was repeated 3 times.
Site directed mutagenesis
BRCA1 ERE mutants was generated using a QuikChange kit (Stratagene, La Jolla, CA), using pGL3 basic BRCA1 promoter as template. The primers used were 5′-CGTCGGCTGTACATGAGGTC-3′ (BRCA1 ERE-1), 5′-GGTCAGGAGTTCCATTCCAGCCTGACCAAC-3′ (BRCA1 ERE-2), 5′- GAGTTCCATTCCAGCCATACCAACGTGGTGAAAC -3′ (BRCA1 ERE-3).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (Mazumdar et al. 2001). DNA extracted was subjected to PCR analysis using the following primers for the ERE-containing region of the BRCA1 promoter: 3′-CTAGCGATTCTCCTGCCTCA-5′ and 3′-GGGAATCCTCGTGATAGGAA-5′. Primers used to PCR-amplify the phosphofructokinase-muscle-type isoform (PFK-M) gene chromatin were 5′-TCTTCCAGGGAGAAGCTGTGA-3′ and 5′-TGATCCTACACTGGGGCATAGC-3′.
Immunoblot analysis
Cells from stable clones were grown in complete medium. When indicated, the specific HDAC inhibitor trichostatin A (TSA) was added to the culture medium, and the cells were incubated at 37°C for 16 h. Then, cells were washed 3 times with PBS and incubated in a lysis buffer (50 mmol/L Tris-HCl [pH 7.5], 120 mmol/L NaCl, 1% Triton X-100, 1× protease inhibitor mixture (Roche), 1 mmol/L sodium vanadate on ice for 30 min. Cell lysates containing equal amounts of protein were resolved by 8% SDS-PAGE, transferred to nitrocellulose membranes, probed with the appropriate antibodies, and detected by means of enhanced chemiluminescence.
Statistical analysis
Statistical analyses were performed using SPSS version 11.0 software. The samples were analyzed using 2-sided Student T test. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grant CA 098823 and CA65746 to RK
Abbreviations
- MTA1
metastasis- tumor antigen 1
- BRCA1
breast cancer susceptibility gene 1
- NuRD complex
nucleosome remodeling and deacetylase complex
- ERα
estrogen receptor α
- ChIP
chromatin immunoprecipitation
- ERE
estrogen-responsive element
- TSA
trichostatin A
- HDAC
histone deacetylase
Reference List
- 1.Bagheri-Yarmand R, Talukder AH, Wang RA, Vadlamudi RK, Kumar R. Metastasis-associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development. 2004;131:3469–3479. doi: 10.1242/dev.01213. [DOI] [PubMed] [Google Scholar]
- 2.Baker KM, Wei G, Schaffner AE, Ostrowski MC. Ets-2 and components of mammalian SWI/SNF form a repressor complex that negatively regulates the BRCA1 promoter. J Biol Chem. 2003;278:17876–17884. doi: 10.1074/jbc.M209480200. [DOI] [PubMed] [Google Scholar]
- 3.Balasenthil S, Broaddus RR, Kumar R. Expression of metastasis-associated protein 1 (MTA1) in benign endometrium and endometrial adenocarcinomas. Hum Pathol. 2006;37:656–661. doi: 10.1016/j.humpath.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle. 2006;5:1001–1007. doi: 10.4161/cc.5.9.2726. [DOI] [PubMed] [Google Scholar]
- 6.Chen XW, Arciero CA, Godwin AK. BRCA1-associated complexes: new targets to overcome breast cancer radiation resistance. Expert Review of Anticancer Therapy. 2006;6:187–196. doi: 10.1586/14737140.6.2.187. [DOI] [PubMed] [Google Scholar]
- 7.D’Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–6153. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 8.Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Research. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 11.Hamatsu T, Rikimaru T, Yamashita Y, Aishima S, Tanaka S, Shirabe K, Shimada M, Toh Y, Sugimachi K. The role of MTA1 gene expression in human hepatocellular carcinoma. Oncol Rep. 2003;10:599–604. [PubMed] [Google Scholar]
- 12.Indraccolo S, Tisato V, Agata S, Moserle L, Ferrari S, Callegaro M, Persano L, Dalla Palma M, Scaini MC, Esposito G, Fassina A, Nicoletto O, Plebani M, Chieco-Bianchi L, Amadori A, D’Andrea E, Montagna M. Establishment and characterization of xenografts and cancer cell cultures derived from BRCA1-/-epithelial ovarian cancers. European Journal of Cancer. 2006;42:1475–1483. doi: 10.1016/j.ejca.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Jang KS, Paik SS, Chung H, Oh YH, Kong G. MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancers. Cancer Sci. 2006;97:374–379. doi: 10.1111/j.1349-7006.2006.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai M, Tong WM, Sugihara E, Wang ZQ, Fukasawa K, Miwa M. Involvement of poly(ADP-Ribose) polymerase 1 and poly(ADP-Ribosyl)ation in regulation of centrosome function. Mol Cell Biol. 2003;23:2451–2462. doi: 10.1128/MCB.23.7.2451-2462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko MJ, Murata K, Hwang DS, Parvin JD. Inhibition of BRCA1 in breast cell lines causes the centrosome duplication cycle to be disconnected from the cell cycle. Oncogene. 2006;25:298–303. doi: 10.1038/sj.onc.1209028. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Wang RA, Bagheri-Yarmand R. Emerging roles of MTA family members in human cancers. Semin Oncol. 2003;30:30–37. doi: 10.1053/j.seminoncol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Magdinier F, Ribieras S, Lenoir GM, Frappart L, Dante R. Down-regulation of BRCA1 in human sporadic breast cancer; analysis of DNA methylation patterns of the putative promoter region. Oncogene. 1998;17:3169–3176. doi: 10.1038/sj.onc.1202248. [DOI] [PubMed] [Google Scholar]
- 18.Martin MD, Hilsenbeck SG, Mohsin SK, Hopp TA, Clark GM, Osborne CK, Allred DC, O’connell P. Breast tumors that overexpress nuclear metastasis-associated 1 (MTA1) protein have high recurrence risks but enhanced responses to systemic therapies. Breast Cancer Res Treat. 2006;95:7–12. doi: 10.1007/s10549-005-9016-8. [DOI] [PubMed] [Google Scholar]
- 19.Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, Kumar R. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3:30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- 20.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–5863. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 21.Nawa A, Nishimori K, Lin P, Maki Y, Moue K, Sawada H, Toh Y, Fumitaka K, Nicolson GL. Tumor metastasis-associated human MTA1 gene: its deduced protein sequence, localization, and association with breast cancer cell proliferation using antisense phosphorothioate oligonucleotides. J Cell Biochem. 2000;79:202–212. [PubMed] [Google Scholar]
- 22.Ouchi T. BRCA1 phosphorylation - Biological consequences. Cancer Biology & Therapy. 2006;5:470–475. doi: 10.4161/cbt.5.5.2845. [DOI] [PubMed] [Google Scholar]
- 23.Rio PG, Maurizis JC, Peffault dL, Bignon YJ, Bernard-Gallon DJ. Quantification of BRCA1 protein in sporadic breast carcinoma with or without loss of heterozygosity of the BRCA1 gene. Int J Cancer. 1999;80:823–826. doi: 10.1002/(sici)1097-0215(19990315)80:6<823::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Rosen EM, Fan SJ, Ma YX. BRCA1 regulation of transcription. Cancer Letters. 2006;236:175–185. doi: 10.1016/j.canlet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Simbulan-Rosenthal CM, Rosenthal DS, Luo R, Li JH, Zhang J, Smulson ME. Inhibition of poly(ADP-ribose) polymerase activity is insufficient to induce tetraploidy. Nucleic Acids Res. 2001;29:841–849. doi: 10.1093/nar/29.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, Gygi SP, Parvin JD. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol. 2004;24:8457–8466. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starita LM, Parvin JD. The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repair. Curr Opin Cell Biol. 2003;15:345–350. doi: 10.1016/s0955-0674(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 28.Talukder AH, Mishra SK, Mandal M, Balasenthil S, Mehta S, Sahin AA, Barnes CJ, Kumar R. MTA1 interacts with MAT1, a cyclin-dependent kinase-activating kinase complex ring finger factor, and regulates estrogen receptor transactivation functions. J Biol Chem. 2003;278:11676–11685. doi: 10.1074/jbc.M209570200. [DOI] [PubMed] [Google Scholar]
- 29.Toh Y, Kuwano H, Mori M, Nicolson GL, Sugimachi K. Overexpression of metastasis-associated MTA1 mRNA in invasive oesophageal carcinomas. Br J Cancer. 1999;79:1723–1726. doi: 10.1038/sj.bjc.6690274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toh Y, Pencil SD, Nicolson GL. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. 1994;269:22958–22963. [PubMed] [Google Scholar]
- 31.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 32.Weaver Z, Montagna C, Xu X, Howard T, Gadina M, Brodie SG, Deng CX, Ried T. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene. 2002;21:5097–5107. doi: 10.1038/sj.onc.1205636. [DOI] [PubMed] [Google Scholar]
- 33.Xu CF, Chambers JA, Solomon E. Complex regulation of the BRCA1 gene. J Biol Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- 34.Yarden RI, Papa MZ. BRCA1 at the crossroad of multiple cellular pathways: approaches for therapeutic interventions. Molecular Cancer Therapeutics. 2006;5:1396–1404. doi: 10.1158/1535-7163.MCT-05-0471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.