Abstract
The ostrich (Struthio camelus) is the largest extant biped. Being flightless, it exhibits advanced cursorial abilities primarily evident in its characteristic speed and endurance. In addition to the active musculoskeletal complex, its powerful pelvic limbs incorporate passive structures wherein ligaments interact with joint surfaces, cartilage and other connective tissue in their course of motion. This arrangement may enable energy conservation by providing joint stabilisation, optimised limb segment orientation and automated positioning of ground contact elements independently of direct muscle control.
The intertarsal joint is of particular interest considering its position near the mid-point of the extended limb and its exposure to high load during stance with significant inertial forces during swing phase. Functional-anatomical analysis of the dissected isolated joint describes the interaction of ligaments with intertarsal joint contours through the full motion cycle. Manual manipulation identified a passive engage-disengage mechanism (EDM) that establishes joint extension, provides bi-directional resistance prior to a transition point located at 115° and contributes to rapid intertarsal flexion at toe off and full extension prior to touch down. This effect was subsequently quantified by measurement of intertarsal joint moments in prepared anatomical specimens in a neutral horizontal position and axially-loaded vertical position. Correlation with kinematic analyses of walking and running ostriches confirms the contribution of the EDM in vivo.
We hypothesise that the passive EDM operates in tandem with a stringently coupled multi-jointed muscle-tendon system to conserve the metabolic cost of locomotion in the ostrich, suggesting that a complete understanding of terrestrial locomotion across extinct and extant taxa must include functional consideration of the ligamentous system.
Keywords: Struthio camelus, intertarsal joint, ligamentous system, engage-disengage mechanism (EDM), passive locomotor system, locomotion
Introduction
The ostrich (Struthio camelus), the largest extant bird, is a highly cursorial animal and is acknowledged as the fastest biped with the greatest capacity for long-endurance running (Grzimek & Grzimek, 1959; Alexander et al. 1979; del Hoyo et al. 1992; Hallam, 1992). As an inhabitant of the African savannah and steppe, the ostrich relies on its locomotor abilities to cover great distances with feral adults in motion for up to 90% of daylight time while occupying territories in excess of 25 km2 (Bertram, 1992; Williams et al. 1993). With a life expectancy of up to 85 years and a maximum body mass of 150 kg (Deeming, 1999), specialized leg anatomy and locomotor function can be expected.
The musculoskeletal morphology of the ostrich pelvic limb has been the subject of various studies (e.g. Haughton, 1865; Gadow, 1880; Stolpe, 1932; Glutz von Blotzheim, 1958; Alexander et al. 1979; Mellet, 1985; van den Berge & Zweers, 1993; Liswaniso, 1996; Pavaux & Lignereux, 1995; Sales, 1996; Bezuidenhout, 1999; Abourachid & Renous, 2001; Weissengruber et al. 2002; Gangl et al. 2004; Wagner, 2004; Schaller et al. 2005). Predictably high muscle-power output (Smith et al. 2006) is channelled through a multi-jointed system of interconnected bi- and tri-articular muscles functionally connecting proximal and distal leg segments. Evidence of this interconnection can be observed in isolated limbs of anatomical specimens where manual extension of the knee is mandatorily coupled with extension of all distal joints and controlled abduction of the 4th toe (Weissengruber et al. 2003; Schaller et al. 2005, 2007). Muscle distribution further enhances the swing dynamics of the limb. When compared to the emu, the second largest ratite, muscle mass in the ostrich leg is proximally concentrated at the pelvis and relatively short femur while the distal leg segments, worked via long tendons, are comparatively lighter and more elongated to provide a larger action radius (e.g. Schaller et al. 2005). A further functional increase of distal limb length is achieved through permanent elevation of the metatarsophalangeal joint above the ground plane. This unique supra-jointed toe-posture likely increases elastic energy storage and provides shock absorption during fast locomotion (Schaller et al. 2005; Rubenson et al. 2007). As the only extant didactyle bird, the mass of the distal limb is minimized by the absence of the 2nd toe and its associated muscles (e.g. Hallam, 1992). Structurally, the interacetabular distance is relatively the smallest amongst all bird species, resulting in narrow guidance of limb motion at the hip joint and reflecting a common arrangement in highly cursorial species (e.g. Kummer, 1956; Firbas & Zweymüller, 1971).
Although the musculoskeletal system clearly drives limb motion, it is not the only potent constituent of the locomotor apparatus. Ligaments, menisci, cartilage and their interactions with skeletal anatomy play a vital role in all modes of locomotion. The importance of this passive locomotor system has been discussed in only a limited number of studies where constraints to joint movement and their possible influence on the dynamics of ostrich locomotion have been addressed (Bell, 1847; Langer, 1859; Stolpe, 1932; Fuss, 1996; Gangl, 2001; Weissengruber et al. 2003; Hertel & Campbell, 2007).
As typical for avian species, the knee and hip joint in the ostrich are flexed during ground contact as a result of the horizontally positioned femur. However, the intertarsal joint remains in a predominantly extended state to provide columnar support for the significant trunk mass – similar in function to the extended knee joint in standing humans (e.g. Stolpe, 1932; Kummer, 1956; Gatesy & Biewener, 1991). Due to the near-equal lengths of tibiotarsus and tarsometatarsus, the intertarsal joint is located at almost the vertical midpoint of the slender, elongated distal limb (e.g. Alexander, 1983, Gangl, 2001).
The intertarsal joint is distinct from the more proximal hip and knee joint due to the absence of encapsulating musculature. Passive structures such as ligaments, in combination with the morphology of joint anatomy, presumably play a dominant role in the stabilization and guidance of this joint. Functional morphological evidence of the importance of passive structures has been presented for the human knee (e.g. Muller, 1993a,b).
The present study describes the prominent ligamentous system of the intertarsal joint and its interaction with joint surfaces and protrusions to determine its role in ostrich locomotion. In the course of our preliminary manual manipulation experiments with intact distal ostrich limbs, a significant resistance occurred when flexing the intertarsal joint from a fully extended position. Moreover, with the tibiotarsus held immobile, the tarsometatarsus automatically snapped back towards full extension if released when the intertarsal joint was not flexed beyond a point of seemingly highest resistance. If flexed beyond this angle, resistance gradually decreased to a point where the limb automatically snapped towards flexion. We refer to this effect as the engage–disengage mechanism (EDM) where the ‘engaged’ state represents intertarsal joint angles that automatically return to full extension. The ‘disengaged’ state refers to joint angles where the intertarsal joint exhibits unconstrained mobility in the flexion–extension plane after overcoming the point of highest resistance. This is a bi-directional effect with a similar, but less pronounced, resistance when moving the limb from flexion towards extension. The effect remained evident after removal of all muscles and their corresponding tendons, indicating that this joint behaviour is entirely intrinsic to joint anatomy. A related mechanism has been identified in the elbow of various large mammals, where it provides some degree of passive support in the standing posture (e.g. Hultkrantz, 1897; Richter, 1922; Aichel, 1925; Palmgren, 1929; Nickel et al. 1992; Hildebrand, 1995).
To explore the functional implications for locomotion in live ostriches, the inherent resisting forces of the EDM in anatomical specimens were quantified. Subsequently, these data were correlated to kinematics of over-ground walking and running ostriches.
Materials and methods
Nomenclature
The terminology used in the anatomical description concurs with the Nomina anatomica avium (Baumel et al. 1993) with supplementary references to Gangl (2001) and Wagner (2004).
Macroscopic dissection of the intertarsal joint and quantification of joint moments
The anatomical material used in this analysis was obtained from three freshly slaughtered adult ostriches from three local ostrich-breeding facilities. The birds were healthy and kept in accordance with German regulations for ostrich farming. First, maximum flexion and extension angles of the intact intertarsal joint were measured. Subsequently, the skin of the tibiotarsus, the muscles and tendons surrounding the intertarsal joint and the joint capsule were completely removed with only the ligaments, lateral meniscus and articular cartilage remaining intact. The limb was again manipulated and angles measured to determine the extent to which ligaments and cartilage, as passive structures, guide motion.
Manual manipulations with and without the cartilago tibialis, a cartilaginous structure encasing the caudal intertarsal joint surface, had no effect on joint and ligament function (i.e. EDM effect and ligament orientation during motion). As the presence or absence of the cartilago tibialis did not alter joint function and obscured the caudal section of the intertarsal joint, it was removed to make the caudal joint elements visible. The location and function of passive structures of the intertarsal joint were then identified, with special attention given to the interaction between osseous protrusions and ligaments throughout the range of motion. States of tension among the intertarsal joint ligaments were not measured as in Fuss & Gasser (1992) but were instead qualitatively assessed by attempting to move the ligament perpendicular to its long axis at various stages of flexion and extension. In particular, deflection of ligaments by the epicondylus medialis, the cranioproximal and the distal lateral crest created states of tension that rendered manual ligament distortion impossible. This technique determined the angle at which each individual ligament attained its relatively highest state of tension.
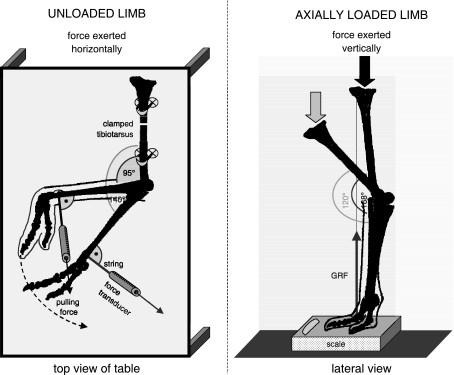
Positional relationships throughout the range of motion were recorded on digital video (Canon MV 550i and Konica KD-500Z) to allow frame-by-frame analysis of joint articulations. Passive (i.e. non-muscular) moments resisting flexion and extension in the intertarsal joint were measured in isolated limbs (sub-knee preparations; Fig. 1). The skin, muscles and corresponding tendons were removed from the tibiotarsus and the intertarsal joint while tarsometatarsus and toes were left intact. Measurements were performed with each limb in two different configurations (Fig. 1).
Fig. 1.
Experimental set-up for quantification of joint moments with the distal limb (tibiotarsus, tarsometatarsus, toes) unloaded (left) and axially loaded (right). GRF, ground reaction force.
Angles of flexion/extension, abduction/adduction and external/internal rotation are coupled during a full course of intertarsal joint motion. The angular displacements measured in this study were derived from the 2-D flexion/extension plane from the lateral view as this is the predominant axis of limb movement in a stride cycle. Degrees of flexion/extension were obtained as angular displacement of the tarsometatarsus relative to the tibiotarsus. Following common kinesiological definition, abduction draws the tarsometatarsus away from the median plane and, conversely, adduction brings the tarsometatarsus back towards the median plane. During internal rotation the long axis of the tarsometatarsus is rotated towards the centre of the body and during external rotation the long axis of the tarsometatarsus is rotated away from the body centre. Detailed kinematic data concerning intertarsal ad-/abduction and internal/external rotation for anatomical specimens and live ostriches can be obtained from Rubenson et al. (2007) Maximum extension angle of the intertarsal joint is presented as 162° in the study by Rubenson and his team (2007) and not at 168° as presented in our study. This discrepancy may arise from different measuring techniques employed in the two studies and potential kinematic cross-talk (e.g. Marin et al. 2003).
In the first experiment, the prepared limbs were oriented horizontally on a dissection table with the tibiotarsus raised above grade and fixed to the table to allow the tarsometatarsus to move freely in a horizontal plane through flexion and extension during manual manipulation. A calibrated mechanical force transducer (100 N max) was attached to the distal end of the tarsometatarsus using a 15-cm non-elastic string. Throughout the experiment, a machinist's square was used to maintain the force transducer in a position perpendicular to the long axis of the tarsometatarsus. Forces were measured while pulling the tarsometatarsus slowly towards flexion and extension (approximately 1° s−1). Multiplying these forces by the distance to the intertarsal joint centre yields the moment required to overcome the resistance in the joint against flexion/extension (without axial loading of the joint) imposed by passive joint structures without gravitational or inertial effects. The opposite of this moment is the passive resistive unloaded moment.
Experiments were filmed with a digital camera (Canon MV 900, 50 frames s−1) and measured forces were vocally reported simultaneously (approximately one reading per 1°) on the audio channel. Afterwards, the angular displacements of the tarsometatarsus relative to the tibiotarsus were accurately calculated from the digitized video frames (Didge, Alistair Cullum, Creighton University, Omaha, USA) and were combined with the recorded moments.
In the second experiment, the preparation was oriented in anin vivo standing position with the toes on a digital scale (100 g calibration, Fig. 1). With the intertarsal joint fully extended (i.e. the ‘engaged’ position as described in the Introduction), a manual downward pushing force was gradually increased on the proximal tibiotarsus to overcome the resistance of the engaged joint to drive the intertarsal joint slowly (about 1° s−1) into flexion. Movements were videotaped in lateral view as in the former experiments. A second camera (Canon MV 500i, 50 frames s−1) was used to simultaneously record the scale reading. Digitization of the lateral views (cf. above) provided positional information of the limb segments. These data, combined with the recorded scale readings (i.e. vertical forces), were used to calculate static equilibrium. In a first step, the entire preparation was considered a free body to obtain the orientation and magnitude of the pushing forces at the tibiotarsus. Next, the equilibrium of the limb segments could be calculated, yielding, amongst other data, the passive resistive moment of the axially loaded intertarsal joint. Measurements were carried out immediately after slaughter to avoid any effect of tissue decay. Manual manipulation experiments were carried out three times each by two experimenters. Averages of these measurements were used in final calculations. In the case of individuals 2 and 3 (Table 1), data of both limbs were pooled as the differences measured between the left and right sides were insignificant. The trials took between 15 and 30 min per experiment. Variability in results between the experimenters amounted to less than 5%. A ligament ‘fatigue’ effect was not observed within the time-frame of the experiments, suggesting that ligaments do not become more pliable after repeated manipulation over the short term. To test whether degradation of ligament structure occurs over a longer time period, experiments were repeated after storing one set of limbs overnight at 15 °C. Resistance decreased by ∼30% from the previous day's quantification, suggesting that only fresh material should be used in studies of this type.
Table 1.
Measurements of tibiotarsus and tarsometatarsus (fresh material)
| Individual age | Sex | Mass (kg) | Side | Mass (kg) Tmt + Ph | Tib. Fl (mm) | Tib. Bd (mm) | Tmt. Fl (mm) | Tmt. Bp (mm) | IT joint max. flex. | IT joint max. ext. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||||
| 15 months | m | 105 | left | 1.9 + 0.9 | 521 | 74 | 477 | 79 | 20.0°[10.5°] | 168.0°[168.0°] |
| 2 | ||||||||||
| 12 months | m | 80 | left | 1.7 + 0.8 | 503 | 70 | 452 | 75 | 19.0°[10.0°] | 168.0°[168.0°] |
| right | 1.7 + 0.8 | 504 | 71 | 451 | 77 | |||||
| 3 | ||||||||||
| 13 months | f | 85 | left | 1.8 + 0.8 | 490 | 69 | 435 | 74 | 19.5°[10.0°] | 168.0°[168.0°] |
| right | 1.8 + 0.8 | 489 | 69 | 434 | 73 | |||||
Tib., tibiotarsus; Tmt., tarsometatarsus; Ph., phalanges. Fl, functional linear length according to Schaller et al. 2005; Bd, distal breadth of tibiotarsus; Bp, proximal breadth of tarsometatarsus according to Von den Driesch (1976) (from the medial to the lateral edge of the articular surfaces of the condyli), all morphometric measurements with cartilage intact. IT joint max. flex./ext., intertarsal joint maximum flexion/extension; unbracketed values indicate intertarsal joint with skin and muscles intact; bracketed values indicate skin and muscles removed with ligaments remaining intact.
2-D Kinematics in live ostriches
The live ostriches used in this study were hand-raised from 10 weeks of age to present day in an outdoor-enclosure (6400 m2) near Heidelberg, Germany. Water was available ad libitum with daily feedings of special ostrich feed dependent on freely available pasturage. The living conditions and enclosure were maintained in accordance with German ostrich-raising standards. To habituate the ostriches to the experimental set-up, they were allowed access to the sampling corridor for increasing periods of time starting 8 weeks in advance of data capture.
To determine excursion angles of the intertarsal joint in live ostriches, kinematic data for walking to intermediate running speeds ranging from 0.5 ms−1 to 3.5 ms−1 were obtained. Experiments were carried out with two 3-year-old females (90 kg) and one 13-year-old male (140 kg) in a level-ground corridor (76 m long and 3 m wide) which was separated from the main outdoor enclosure by coarse wire mesh.
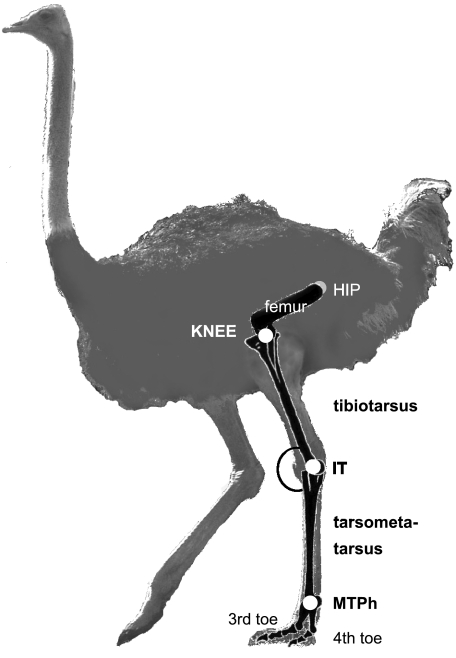
Knee, intertarsal and metatarsophalangeal joints were marked as indicated in Fig. 2 by applying locator marks (4 cm in diameter) with ALUNIC aluminium spray commonly used in animal healthcare as a disinfectant. This spray is clearly visible on video and cannot be removed by the ostriches. Two digital video cameras (Canon MV 500i and 900, 50 frames s−1) were oriented at a 90° angle to simultaneously capture front/back and lateral view. Cameras were installed at a distance of 5 m from the focal zone through which the ostriches passed, and were synchronized using on-screen time-code.
Fig. 2.
Ostrich with joint marker. Left lateral view. IT, intertarsal joint; MTPh, metatarsophalangeal joint.
At least two complete stride cycles were recorded at each pass. Only walks and runs that remained straight for at least three stride cycles were included in the data pool, as verified by the front/rear view camera. Five straight walks (duty factor: ∼0.65; ∼1 ms−1) and five straight runs (duty factor: ∼0.5: 3–3.5 ms−1) were selected for analysis. Movements of knee, intertarsal and metatarsophalangeal joint (lateral view) were digitized frame-by-frame (Didge).
Results
Anatomy
The intertarsal joint consists of the articulation surfaces of the distal tibiotarsus and proximal tarsometatarsus. The joint capsule originates from the periosteum of the distal tibiotarsus, envelops the intertarsal joint laterally, cranially and medially, and inserts at the proximal tarsometatarsus. With the exception of the prominent ligamentum collaterale mediale longum (LCML), all intertarsal joint structures (Table 2) are situated within the intertarsal joint capsule. Caudally, the joint capsule is conjoined with the cartilago tibialis. This prominent cartilaginous structure surrounds the joint caudally and provides a pathway for the tendons of toe flexor muscles.
Table 2.
Intertarsal joint anatomy – identification of ligamentous structures and osseous protrusions
| Structure and abbreviation | Origin | Insertion | Position |
|---|---|---|---|
| m. fibularis brevis, tendinous MFB | On tibiotarsus (Tib.) distally at end of fibula (Fib.) | Proximolateral on plantar surface of tarsometatarsus (Tmt.) | Medial to tendo lateralis of m. fibularis longus (MFL); intracapsular |
| lig. collaterale laterale LCL | Depressio epicondylaris lateralis (DEL) of distolateral Tib. & epicondylus lateralis (El) | Dorsolateral at impressio lig. coll. lat. of proximal Tmt. | Medial to m. fibularis brevis (MFB); intracapsular |
| Meniscus lateralis M lat with cornu craniale C cr & cornu caudale C cd | On proximolateral tarsometatarsal joint surface | At fovea menisci laterale at medioventral tarsometatarsal joint surface | C-shaped at lateral tarsometatarsal joint surface; intracapsular |
| lig. menisco-metatarsale caudale m1 | fovea menisci lateralis | At lig. meniscometatarsale craniale (m2) | Medial to area intercotylaris, intracapsular |
| lig. menisco-metatarsale craniale m2 | Lateral to eminentia intercotylaris | Craniomedial rim of cornu craniale (C cr) | intracapsular |
| caudomedial ligament m3 | Caudomedial rim of cornu caudale (C cd) | Medial at cartilago tibilais | intracapsular |
| caudolateral ligament m4 | Caudolateral rim of cornu caudale (C cd) | Lateral at cartilago tibialis | intracapsular |
| lig. collaterale mediale longum LCML | Triangular attachment at lateral distomedial Tib. | Fan-shaped at proximomedial Tmt. | Caudal to LCM; extracapsular |
| Broad ligament BL | Transition of epicondylus medialis (Em) to depressio epicondylaris medialis (DEM) | Caudal rim of LCML over 40 mm | Caudomedial/medial joint space; intracapsular |
| lig. collaterale mediale LCM | depressio epicondylaris medialis (DEM) of distomedial Tib. | Dorsomedial at impressio lig. col. lat. of proximal Tmt. | Medial to LCML; intracapsular |
| epicondylus medialis Em | Distomedial Tib.; intracapsular | ||
| Cranioproximal crest Cr1 | Cranial rim of condylus lateralis (ConL); intracapsular | ||
| Distal crest Cr2 | Distal rim of condylus lateralis (ConL); intracapsular | ||
| Extension-limiting protrusion S | Distocaudally at medial tibiotarsal condyle (ConM); intracapsular |
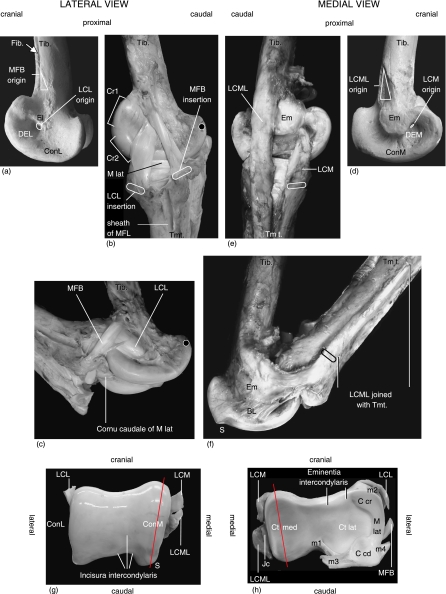
While fully developed in many avian species, the m. fibularis brevis (MFB) in Struthio camelus is reduced to a tendon and essentially functions as a ligament. The tibiotarsal attachment of the MFB lies distal to the end of the fibula where it is 6 mm wide (Fig. 3b,c). In the extended intertarsal joint, the MFB is 75 mm long (± 3 mm), runs distocaudally, widens to 10 mm, crosses the origin of the ligamentum collaterale laterale (LCL) laterally and inserts proximolaterally at the plantar surface of the tarsometatarsus. In cross-section, the MFB is slightly ovoid. When the joint is fully extended, the MFB is covered entirely by the lateral end-tendon of the m. fibularis longus with which it shares an aponeurosis. This tendo lateralis is firmly secured by a tendon sheath and is additionally enveloped by a strong fascia where the tendon crosses the lateral intertarsal joint space. Because of this stiff encasement, it was not possible to displace the tendo lateralis or the MFB in our intact specimens when the intertarsal joint was extended.
Fig. 3.
Anatomical photo reference in accordance with Table 2, left limb. (a) Macerated distal tibiotarsus, lateral view. (b) Intertarsal joint at maximum extension, lateral view. Black dot on trochlea cartilaginis tibialis indicates reference point. (c) Intertarsal joint in flexion. Black dot indicates reference point (see b). (d) Macerated distal tibiotarus, medial view. (e) Intertarsal joint at maximum extension, medial view. (f) Intertarsal joint in flexion with osseous protrusion (S) to prevent hyperextension and tensed broad ligament (BL) preventing LCML from sliding over Em. (g) Tibiotarsal joint surface with LCL, LCM and LCML severed. (h) Tarsometatarsal joint surface with LCL, MFB, LCM and LCML severed. Joint capsule (Jc) associated with LCML.
The origin of the ligamentum collaterale laterale (LCL) lies in the depressio epicondylaris lateralis, with some of its fibres also originating at the epicondylus lateralis (Fig. 3a). In its course, the LCL broadens to 15 mm at the joint space and attaches to the tarsometatarsus cranioproximally at the impressio lig. collaterale laterale. At its origin, the LCL is round in cross-section but flattens after a short distance and remains largely flattened to its insertion. In a full course from extension to flexion, the MFB traverses the LCL with ligaments crossed at full flexion (Fig. 3c). At full joint extension, the length of the LCL is 45 mm (± 2 mm).
On the medial side, the ligamentum collaterale mediale (LCM) originates entirely in the depressio epicondylaris medialis of the distal tibiotarsus, broadens to 15 mm, and after a straight course inserts proximomedially at the tarsometatarsus and fuses internally with the joint capsule (Fig. 3e). At its origin, the LCM is round in cross-section, flattens towards the joint space and remains falcate to its insertion. At full joint extension, the length of the LCM is 36 mm (± 2 mm).
Caudal to the LCM runs the prominent ligamentum collaterale mediale longum (LCML). It originates at a distinct 40-mm-long triangular attachment on the distal tibiotarsus (Fig. 3d). In the extended joint, this ligament is 265 (± 5 mm) long, is oriented distally parallel to the LCM (Fig. 3e) and inserts in a fanned shape over 70 mm at the proximomedial tarsometatarsus. It is 10 mm wide and flat-elliptical in cross-section. At the area of the joint space, the LCML is connected to a broad, intracapsular ligament. This ligament originates at the medial depressio epicondylaris and becomes gradually visible with increasing joint flexion (see Functional analysis). As opposed to the ligaments on the lateral side, LCML and LCM do not cross each other during a full course from extension to flexion (Fig. 3f). A central ligamentum anticum as found in many bird species is absent.
During a motion cycle, MFB, LCL, LCM and LCML are deflected by (1) vaulted contours situated on the lateral joint rim (cranioproximal lateral crest and distal lateral crest, Fig. 3b,c) and (2) medially by the protruding epicondylus medialis (Fig. 3d–f). For the upcoming functional analysis it is important to note that the epicondylus medialis has a plateaued surface contour. While less evident in macerated bone samples, all osseous protrusions present on the intertarsal joint surfaces gain additional height and contour when articular cartilage remains intact, as seen in the fresh specimens used in this study.
Many bird species possess two menisci, whereas the ostrich has only one C-shaped meniscus located at the lateral tarsometatarsal joint surface (Fig. 3h). The meniscus is thickened at its outer edge where the cartilaginous cornu craniale and the more prominent cornu caudale are situated. In the space between the two cornua, the meniscus is flattened and with a compressed triangular shape in cross-section (Fig. 3h). The meniscus is connected to the ligg. collaterale laterale and mediale and to the cartilago tibialis via small ligaments (m1–m4 in Fig. 3g). Due to these connecting ligaments, the position of the meniscus is coordinated with movement of the joint surfaces through the full motion cycle to ensure lateral joint surface closure and to compensate for incongruent joint surfaces (Fig. 3g,h).
The lateral tibiotarsal condyle is oriented on a largely straight longitudinal/craniocaudal axis. The tibiotarsal incisura is unequally shaped and incised more towards the mediocaudal joint surface (Fig. 3g), resulting in the higher contour of the caudal portion of the medial condyle. When compared to the lateral condyle, the condylus medialis is slightly more elevated and oriented on an oblique axis (Fig. 3g, indicated by red line). The corresponding tarsometatarsal cotyla medialis features a defined mating groove (Fig. 3h, indicated by red line) and is smaller than the lateral cotyla, the latter having a rather flat aspect.
Functional analysis
The intertarsal joint is a synovial roll-and-glide joint with the great majority of motion range occurring in the flexion/extension plane. During a full motion cycle, flexion of the tarsometatarsus is automatically coupled with tarsometatarsal abduction and external rotation, while extension leads to tarsometatarsal re-adduction/internal rotation. With the joint fully extended (Figs 1, 2), tibiotarsus and tarsometatarsus form a straight axis from the cranial view. In this position, the strong ligamentous system prevents tarsometatarsal rotation and the intertarsal joint resists flexion and cannot be hyper-extended due to the presence of an extension-limiting protrusion that mates with the caudal rim of the proximal tarsometatarsus (Fig. 3g). As seen in the lateral views of full extension in Fig. 3b,e and Fig. 4, this position results in maximum surface contact between tarsometatarsal and tibiotarsal joint surfaces. As the degree of flexion increases, surface-to-surface contact decreases (Fig. 4). Due to their complanate shape, the lateral articulation surfaces are minimally involved in motion guidance. Only the medial articulation surfaces direct the degree of ab-/adduction of tarsometatarsus to tibiotarsus in response to flexion angle. Given the largely featureless joint surfaces, the ligamentous system provides the primary guiding function and ensures joint coherence throughout the range of motion (Fig. 3h).
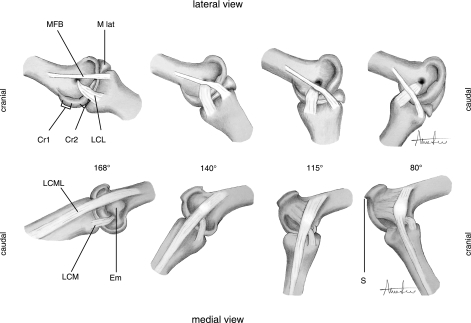
Fig. 4.
Ligament interactions with osseous protrusions in the course of flexion. Abbreviations as in Table 2.
Ligament interplay with protrusions in the course of flexion
168°
At maximum extension (Fig. 4) the intertarsal joint is in an engaged position under the conditions of the engage–disengage mechanism (EDM). With the tibiotarsus held immobile, force has to be exerted to overcome resistance and move the tarsometatarsus towards joint flexion. In addition, when the joint is flexed up to 130° by moving the tarsometatarsus and subsequently releasing it, the tarsometatarsus rapidly snaps back to the maximally extended joint position of 168°. At maximum extension, the LCML rests caudal of the medial epicondyle (Em) and, like the other three ligaments, is in a tensed state. Internal/external rotation of the tarsometatarsus is not possible in this position.
Over-extension of the intertarsal joint beyond 168° is not possible due to an elevated, distinctly shaped process (S) on the medial condyle of the distal tibiotarsus that mates with the corresponding shape of the caudal rim of the tarsometatarsal joint surface. This osseous process functions as a structural limiter to the maximum angle of extension when all ligaments are intact (S in Fig. 3f,g).
140°
On the lateral side of the joint, the MFB now runs over the distal condylar crest (Cr2). As a result of this deflection, the MFB remains tensed despite the shortened distance between its origin and insertion areas (Fig. 4). Simultaneously, the MFB begins to interact with the LCL, further influencing the tension of the MFB to maintain joint surface cohesion.
On the medial side of the joint, the LCML is now situated over the medial epicondyle. As seen on the lateral side, the tension of this ligament is maintained by a protrusion – in this case the epicondyle – despite increased flexion.
120° ± 5°
In this range of excursion, the joint is in a state of transition. In manual manipulations, releasing the tarsometatarsus within this angle bracket results in rapid movement of the joint towards either a flexed or extended position. This automatic transitional effect can be explained by the particular ligament arrangement at this stage where the LCML on the medial side is positioned on top of its corresponding epicondyle while, on the lateral side, the MFB is positioned on top of its corresponding distal condylar crest. As a result of this deflection by protrusions, these ligaments simultaneously achieve their relatively highest state of tension throughout the range of excursion at ∼120°. To resolve these tensile stresses the relative angle of tibiotarsus to tarsometatarsus shifts to allow the MFB to descend its crest. Ligament stress is reduced in the course of flexion as the areas of origin and insertion of the LCML move closer together. During the transition at 125°–115°, the lateral LCL and MFB interact at the interface of the intertarsal joint space and horizontally oppose the medially originating forces generated by the LCML/epicondyle interaction.
115° (Transition point of the engage–disengage mechanism)
At this critical angle, the LCML covers the plateaued surface of the medial epicondyle completely and the broad connective ligament between condylar rim and LCML begins to stretch (Fig. 4). On the lateral side of the joint, the MFB has moved past the distal condylar crest and tension appears reduced. However, LCL and medial LCM remain tensed over their respective condylar rim.
When the tarsometatarsus is released in this position, it rapidly snaps towards either flexion or extension depending on the prevailing external force (i.e. gravity or inertia).
80°
The MFB, now fully overlapping the LCL, is under less tension. On the medial side, the broad connective ligament between condylar rim and LCML is clearly stretched. This ligament does not restrict further flexion but instead anchors the LCML, preventing it from traversing the epicondyle completely, where it would be ‘trapped’ on the cranial side of the joint and would prevent the re-establishment of an extended position.
Re-extension
When re-extending the intact joint the lateral MFB and the LCL essentially reverse their interplay sequence as described for flexion, except that resistance encountered by the MFB is provided by the comparatively less prominent cranioproximal condylar crest. The maximum resistance against extension occurs at 95°. Medially, the sequence is simply reversed, with the LCML starting its descent from the epicondyle at 140°. As above, the tarsometatarsus will snap towards flexion or extension depending on the joint angle at point of release within a transitional range of 105°–115°.
The anatomically similar LCL and LCM provide joint surface coherence throughout the entire articular range. These two ligaments are comparatively short and their areas of origin comply with the centre of joint axis rotation, resulting in less ligament distortion throughout the motion range. The LCL and LCM essentially serve as clamps to maintain closure of the joint surfaces during the roll-and-glide motion sequence. LCML and MFB provide a critical guiding function, particularly as intertarsal flexion increases. This relationship became obvious in anatomical specimens where ligaments were cut after experiments were concluded with only LCL and LCM remaining intact. When fully extended, the joint surfaces still appeared coherent with ab-/adduction and internal/external rotation of the tarsometatarsus greatly limited. Increasing degrees of flexion caused a gap to occur between the dorsal joint surfaces and the tarsometatarsus was no longer bound to its arc of motion. Cutting either the medial LCML or the lateral MFB – while leaving the respective counteracting ligament intact – completely eliminated the effects of engagement and disengagement.
In summary, manual manipulations of the intertarsal joint have identified three distinct stages during joint articulation. In the extended position (stance default angles of 168° through to 140°), the joint is in an engaged condition where resistance increases as the joint is flexed towards the transition stage. During the transition stage (125°–105°) resistance has been overcome and the joint is in a volatile state with ligaments at their relatively highest state of tension (∼115°). This condition is resolved by rapid movement towards either flexion or extension. In the flexed position (95°–20°), the joint is in a disengaged state where resistance is increased as the joint is extended back towards the transition stage.
Quantification: passive moments of the intertarsal joint
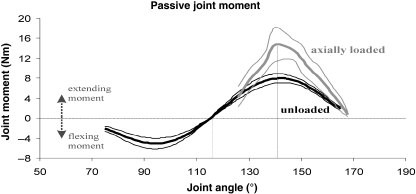
Resistance against flexion and extension: the unloaded joint
Graph 1 presents the results of the measurements with the limbs in the horizontal plane (Fig. 1). Starting from full extension, the resistive moment builds up gradually to become maximal (∼8 Nm) at a flexion angle of 140°. When flexed further, the resistance against flexion decreases, falling to 0 Nm at 115°. Beyond this angle, passive joint moments become flexing moments and the tarsometatarsus snaps rapidly towards maximum flexion. During manipulation towards extension, a similar but opposite passive behaviour of the joint was observed. Resistance against extension grew to a maximum of ∼5 Nm at 95° and decreased as it made a transition into an extending moment when crossing the 115° boundary.
Graph 1.
Passive joint moments in the unloaded (black line) and the axially loaded limb (grey line). Line at 140° indicates point of highest resistance; line at 115° indicates EDM transition point. Bold lines represent the mean of the three specimens (cf. Table 1); thin lines the mean ± 1 SD.
Resistance against flexion: axial loading in the standing limb
Similar to the experiment with the unloaded limb, the moment at maximum extension is ∼1.5 Nm with the highest values against flexion occurring at 140°. When compared to the horizontally oriented limbs (unloaded), the moments in the loaded limbs at peak are approximately twice as high in value at ∼16 Nm. However, the transition point remains consistent at 115° once resistance is overcome (Graph 1).
Correlation of EDM with real-time kinematic data
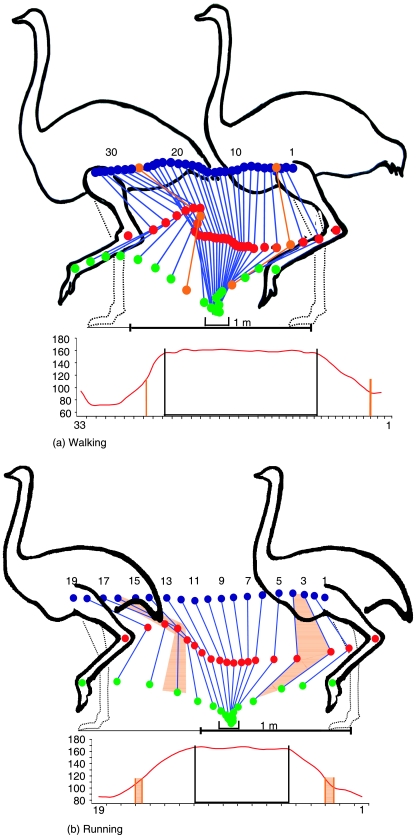
Figures 5a and b provide kinematic data for intertarsal joint excursion throughout one complete stride in walking (a) and running (b). This allows correlation of manual manipulation data with flexion/extension data in the live bird. This chart also superimposes the location of the EDM transition zone within the stride cycle (orange lines/areas). In accordance with data from manually manipulated hind limbs, the maximum extension angle of the intertarsal joint in walking and running is 168°. During the entire period of ground contact (indicated by black bracket/lines) in both walking and running the intertarsal joint remains nearly fully extended with flexion angles never below 164° and 160°, respectively.
Fig. 5.
(a,b) Kinematic analysis of stride cycle and EDM in walking and running. Blue points = knee joint, red points = intertarsal joint, green points = metatarsophalangeal joint. Orange lines/areas indicate the limb configuration where EDM transition occurs (either in extension before touch-down or in flexion after toe-off) during the swing phase of the stride cycle. One point/number represents one video frame (25 frames = 1 s). The black brackets show the period of ground contact. The corresponding graphs at bottom (not to same scale) show flexion/extension angles of the intertarsal joint over a full motion cycle; black brackets indicate ground contact time, orange lines/areas indicate EDM transition.
Although unable to gather first-hand data on ostriches running at high speed over ground, lateral view film footage of feral ostriches in the Serengeti was used to estimate maximum flexion and extension angles of the intertarsal joint (Grzimek & Grzimek, 1959). This male adult ostrich was reported with a running speed approximating 60 km h−1 (16.4 ms−1). Maximum flexion reached 45° during the mid-swing phase and maximum extension (168°) was recorded during stance phase. Maximum flexion during ground contact occurred at mid-stance phase and was measured at 140°. This indicates that the point of highest resistance against flexion as determined in the experiments (i.e. 140°) is only achieved during high-speed running and further establishes that the EDM is in the engaged state during all stages of ground contact (Fig. 5b).
In walking, an abrupt upward vertical trajectory of the intertarsal joint occurs after toe-off (Fig. 5a). This coincides with the exact angle at which the intertarsal joint disengages towards flexion when releasing the tarsometatarsus in the experiment with loaded and unloaded limbs (see above). In running, a forward thrust of the intertarsal joint occurs just prior to touch-down in the course of joint extension (Fig. 5b). This effect also coincides with the joint angle at which the mechanism engages as described in the experiments performed with anatomical specimens (see above). Given these results, it can be accepted that the transition point of the EDM as qualified in anatomical specimens exists in the live animal during locomotion.
Discussion
Functional anatomy
Maintaining ligament tension
As the intertarsal joint surfaces provide little constraint to rotational movement, the ligamentous system limits the roll-and-glide articulation of the joint to the highly constrained 3-D path observed in anatomical specimens and live ostriches. The reductive dissection showed that the active locomotor system, comprising muscles and tendons, is uninvolved in guidance and segment axis alignment of tibiotarsus and tarsometatarsus. To maintain ligament tension throughout the range of coupled/3-D motion, ligaments are deflected by protrusions present in the tibiotarsal joint surface. The distinct locations of the two protruding lateral condylar crests are presumably related to the degree of external rotation/abduction of the tarsometatarsus during flexion. The distance covered by the joint surfaces on the medial and lateral sides is not equal and likely requires compensation to maintain steady ligament tension. Laterally, the distal crest counteracts towards flexion and the cranioproximal crest counteracts towards extension by deflecting the LCL and MFB. The location and domed shape of these crests may provide a tension pre-load modulator to slope-up the stabilizing properties of the lateral ligaments in advance of the major shear force that originates on the medial side as the LCML is increasingly tensed by the protruding medial epicondyle. This arrangement could ensure additional lateral joint stability prior to the EDM transition point of 115° in each direction (Graph 1). This principle is also present in the avian knee where deflection of ligaments by protrusions leads to ‘a greater and lesser state of tension’ but does not allow ‘distinct differentiation between a tensed and a relaxed state’ (Fuss & Gasser, 1992). The relatively constant state of tension of all intertarsal ligaments may also work in tandem with the elastic properties of the articular cartilage of the joint surfaces that compress somewhat under pressure (e.g. Aichel, 1925; Palmgren, 1929).
Prevention of hyperextension
The ligaments prevent hyperextension of the fully extended joint in combination with a distinctly shaped osseous protrusion located caudally at the medial tibiotarsal condyle (Fig. 3f,g). This mechanism is likely important at the end of swing phase when the extensors pull at the tarsometatarsus before touch-down to extend the distal limb. With maximum extension clearly defined by the passive ‘stopper’, there is no need to counteract with flexors. This also allows muscle power to be directed through the limb at push-off without applying compensatory muscle action to avoid hyperextension. Functionally similar, but anatomically different, mechanisms have been found in wading birds where they have been proposed to be important during extended durations of standing in place (Stolpe, 1932).
Role of the meniscus lateralis
To balance the incongruent joint surface of the distal tibiotarsus (Fig. 3g,h), the ostrich possesses a prominent meniscus lateralis which presumably acts as a guiding rim for the predominantly flat lateral joint surfaces. The lateral meniscus has been anatomically and histologically described by Stornelli et al. (2001) and Wagner (2004). Wagner (2004) examined the age-related degree of ossification of the cornu caudale, which is cartilaginous at the age of 6 months and entirely ossified after 1.5 years. Stolpe (1932) suggests that the ossified cornu caudale of the meniscus functions as an articular shock absorber in heavy S. camelus.
This is similar to the human knee where menisci have been found to be important for load transmission (Boyd & Myers, 2003). A medial meniscus and central ligamentum anticum, commonly present between the joint surfaces of many other bird species, e.g. chicken (Gallus gallus) and parrots (Psittaciformes), are missing at the tarsometatarsal joint surface of the ostrich. As medial rotation of the ostrich tarsometatarsus is limited to the screw-back arc at full extension, a medial meniscus is probably not required. When compared to parrots, distal limb movement in the heavy cursorial ostrich is highly concentrated in the fore–aft plane with limited internal rotation/abduction. Limbs are used solely for locomotion and to provide columnar support to the trunk and lack the agility seen in other species that employ hind limbs and phalanges for more complicated articulations. Furthermore, the ostrich tarsometatarsal medial cotyle has a deeper and more defined groove for the medial tibiotarsal condyle than its lateral counterpart, which might also decrease the need for a medial meniscus. The central lig. anticum may have been fully reduced because it would hinder movement through the large flexion/extension radius of ∼150° in the distal limb of the ostrich (Stolpe, 1932).
Load management and intertarsal posture
During stance phase, the intertarsal ligaments presumably play an essential role in acting against lateral and medial shear forces. Compensation for transverse forces is especially important in running when body mass is accelerated and its impact on the joints at touch-down is increased in comparison with walking gaits. Herein, the lateral MFB supports the fully developed m. fibularis longus, functioning as an important extensor of the intertarsal joint (Gadow, 1880; Stolpe, 1932; Gangl, 2001; Weissengruber et al. 2003). Due to its insertion at the proximolateral tarsometatarsus, it is the only muscle that acts directly on the lateral side of the intertarsal joint with its lateral end-tendon entirely overlapping the MFB.
In the absence of musculature inserting at the medial joint surface, the tensed LCML counteracts shear forces during stance phase in support of the extended joint. The prominence of this major medial ligament is presumably linked to the mass and dimensions of the ostrich. In our analysis of skeletal material, all specimens of ostrich, emu, rhea and cassowary showed osseous depressions associated with the LCML (own unpublished data). The distal tibiotarsi of extinct moa skeletons (Dinornis maximus) also showed a deep 8-cm-long triangular groove for insertion of the LCML, as well as the protruding epicondylus medialis, which plays a major role in the EDM. In this biped weighing ∼300 kg (Alexander, 1983), it can be expected that a stabilizing mechanism of the intertarsal joint would have been beneficial in supporting body mass on an extended distal limb. The New Zealand kiwi, as the smallest member of the ratite family, does not feature the prominent groove associated with the LCML. It stands to reason that this chicken-sized bird (up to 3 kg) would not require, or derive benefit from, this stabilizing mechanism as the kiwi's intertarsal joint is flexed to a higher degree during ground contact than in large ratites (e.g. Abourachid & Renous, 2000). To test whether body mass and limb posture might correlate to structures associated with the EDM, the chicken (Gallus gallus) was included in comparative skeletal analyses. Skeletal material did not show the tibiotarsal depressions of the LCML and the epicondylus medialis was of comparatively limited size and protruded less than in large ratites. In limb manipulation experiments with a fresh free-range chicken (2.6 kg), no resistance during flexion or extension was detected and subsequent dissection confirmed the absence of the LCML. This dissection also showed that the lateral tendon of the fully developed MFB slid freely over the distal lateral condyle of the tibiotarsus in the absence of elevated crests.
In the ostrich, the dynamics of the EDM are certainly derived from the joint's requirement for sufficient ligament tension to establish joint coherence and compensate for 3-D moments and shear stresses acting on the joint during stance. However, it is not possible to separate the function of the ligaments from the resultant effects of the EDM – the structures and their effects are intertwined. As there is a direct correlation between the angle of maximum resistance to flexion in the isolated hind limb and the maximum angle of flexion under load in the live animal, the absolute correspondence of anatomical effect with kinematic expression strongly suggests that the role of ligaments at the joint extends beyond maintenance of joint coherence.
Role of the MFB
In the ostrich, the participation of the MFB in the intertarsal joint system is particularly interesting and, to our knowledge, has not been previously clarified in the existing literature. Pavaux & Lignereux (1995) correctly termed it a second lateral ligament, but Liswaniso (1996) described it as rudimentary and probably functionless and George & Berger (1966) erroneously declared it absent in all large, extant ratites. In the majority of avian species the m. fibularis brevis is fully developed and contains a venter (e.g. George & Berger, 1966). These species are much smaller and lighter than ratites and use legs and toes for complex actions not exclusively dedicated to terrestrial locomotion. In most birds the developed m. fibularis brevis supports tarsometatarsal control and manoeuvrability in the lower limb (Stolpe, 1932). Degrees of intertarsal flexion may be another crucial factor for reduction of the MFB and its integration with the ligamentous system. As discussed above, many smaller bird species exhibit a flexed intertarsal joint during stance postures that might require the muscle control provided by a developed m. fibularis brevis. In contrast, large ratites use their vertically extended legs primarily for terrestrial locomotion in a planar environment. The reduction of a muscle intended for fine movement unnecessary to the ostrich presumably saves muscle mass and concentrates energy for limb motion in the fore–aft plane. We assume that this led to the gradual reduction of this muscle's venter to its present form as an important ligament/tendon contributing to joint surface coherence, motion guidance and the dynamics of the engage–disengage mechanism. The MFB of the ostrich provides a good example of a structure whose function might be misinterpreted as rudimentary due to reduction but which is in fact necessary for the gross functionality of the organism.
In summary, the morphology of intertarsal ligaments and joint surfaces in combination with lateral condylar crests and contours of the medial epicondyle provide a coherent system of joint stability and motion range control. In addition, this joint complex provides the structural basis for the dynamic expression of an engage–disengage mechanism that may deliver locomotor benefits that exceed any associated metabolic cost.
Prior descriptions of snap joints
Following earlier descriptions (e.g. Langer 1859), Stolpe (1932) dissected ostrich hind limbs but did not encounter resistance through the motion arc in the intertarsal joint. It is most likely that specimen quality may have been the main factor in Stolpe's (1932) inability to detect a ‘snapping motion’ during his limb examinations. This is supported by our findings, where the effect had decreased significantly 24 h post mortem as a probable result of tissue degradation (Materials & methods, last paragraph).
Bell (1847) and Langer (1859) explicitly describe a ‘Schnappbewegung’ (snapping motion) in the intertarsal joint of isolated ostrich hind limbs. Their anatomy-based interpretations identify it as the mechanism responsible for the springy walking gait typical of many long-legged avian species. However, the authors did not provide quantification for the stabilizing effects of this mechanism during stance phase and neither description clearly correlated intertarsal joint behaviour with simultaneously occurring medial and lateral ligament interactions.
Bell (1847) posits that a ‘prominent medial ligament’ (presumably the LCML) is the single constituent driving snapping motion, stating that this ligament is in a relaxed state in the depression caudal to the medial epicondyle when the intertarsal joint is extended, stretches during flexion when gliding over the epicondyle and returns to a relaxed state in the cranial position after passing the epicondyle. As shown in our results, this interpretation is incorrect, as the LCML remains tensed in the extended intertarsal joint to provide distal limb stability during stance phase. Furthermore, if the LCML were to completely traverse the epicondyle in the course of flexion as reported by Bell (1847), it would be trapped in an irretrievable location. To prevent this condition, the central part of the LCML is secured from over-travel by the broad ligament originating at the medial epicondyle of the distal tibiotarsus.
Langer (1859) dismissed Bell's interpretation (1847) as he observed the springy gait in bird species (stork, flamingo, heron) that did not feature a prominent medial epicondyle. In the ostrich, Langer (1859) instead proposed an unspecified lateral ligament (presumably the MFB) in interaction with a condylar crest as the main factor underlying the snapping effect and visually identifies maximum resistance at 125°, which is higher than measured in our experiments.
However, the existence of a snap mechanism in wading birds without the elevated medial epicondyle and interacting LCML might be explained by their much smaller size and mass in comparison with ratites. In a 5-kg stork, the arrangement of ligaments alone may be sufficient to support the extended intertarsal joint and to prevent over-flexion. In a 150-kg ostrich with longer effective limb length and higher centre of mass, more robust intertarsal joint structures (LCML/medial epicondyle and lateral ligaments/crests) and their rather complicated interaction are required to augment muscular trunk support. Similar findings of snap joints in the elbow and ankle of large cursorial mammals (e.g. horse) have also been interpreted in the context of economic stance postures (e.g. Hultkrantz, 1897; Richter, 1922; Palmgren, 1929; Nickel et al. 1992; Hildebrand, 1995).
Biomechanical analysis
Influence of body dimensions and joint anatomy on EDM expression
To investigate the potential stabilizing properties of the ostrich snap joint (the EDM) we quantified the resistance present during manual extension and flexion of the prepared intertarsal joint. The data of the three individuals resulted in similarly expressed curves but showed different peak passive joint moments at the highest point of resistance. It is likely that body mass and limb segment dimensions affect the resistance provided by the EDM. Future investigations should incorporate a more diverse sample group including juvenile and older ostriches (< 3 years). Bone segment lengths will change according to specimen age in tandem with increasing ligament calcification, requiring comparative analyses of ligament fibre properties. In addition, the closely related large ratites should be part of an inter-specific comparison employing identical methodologies to establish species-specific characteristics of the EDM.
The experiment with the unloaded horizontally positioned limb eliminated gravitational effects and showed that resistance from extension to flexion was greater than resistance from flexion to extension (Graph 1). This difference is primarily caused by the LCML in relation to the protruding medial epicondyle where, in full extension, it is held in position (engaged) by this protrusion and undergoes an increase in tension as it ascends the elevated epicondyle during movement towards flexion. When moving from flexion to extension, this ligament simply descends from its secured position atop the epicondyle and registers less resistance. These medial dynamics occur in tandem with the interplay of lateral ligaments and condylar crests. Here, when moving from extension to flexion, i.e. towards a disengaged state, the tendinous MFB is deflected by the distinctly elevated distal lateral crest, compounded by the resistance arising from its interaction with the LCL. The smoother, comparatively less-elevated lateral cranioproximal crest is more easily negotiated by the MFB/LCL when in transition towards the extended engaged state.
An approximate two-fold increase in passive joint moments occurred in the axially loaded limb at 140° when moving from extension to flexion compared to the unloaded limb. With the axially loaded limb reflecting standing posture of the distal leg, we propose that in vivo intertarsal joint stability is passively augmented during all phases of ground contact given that this joint never undergoes flexion below 140°. To test our hypothesis, we correlated the results from these experiments with kinematic data of walking and moderate-speed running ostriches.
Expression of the EDM in the live ostrich
EDM during stance phase
In both walking and intermediate speed running, the extended intertarsal joint exhibits almost no deviation from the maximally extended angle of 168° (Fig. 5). In high-speed running (14.6 ms−1) under increased load the extension angle falls to a momentary low of 140° during mid-stance (Grzimek & Grzimek, 1959). This corresponds exactly to the angle of maximum resistance against flexion provided by the EDM. Thus, at any given moment of ground contact in live ostriches, the intertarsal joint never flexes beyond the minimum angle that ensures the engaged state of the EDM.
With the distal limbs of live ostriches vertically extended, the moment arm of the ground reaction force acting on the intertarsal joint is very small. This significantly reduces the flexing moment of the forces acting on this joint. Hence, moments required to neutralize this flexing action can be equally small, imparting even greater significance to the engaged state in vivo. In other words, although passive resistive moments against flexion are rather small at the fully extended joint (Graph 1) it can be anticipated that they contribute considerably to joint stabilization.
When compared to the unloaded limb, the axially loaded ostrich limb exhibited a two-fold increase at the point of highest resistance (140°) against flexion despite the corresponding reduction in columnar support present in the now partially flexed joint. It is possible that passive extending moments may be even higher in vivoconsidering that our experiments were performed with all tibiotarsal muscles removed and tendons running over the intertarsal joint severed. A potentially important factor for higher in vivo passive extending moments in the intertarsal joint is the tensed state of the m. fibularis longus. This prominent muscle forms the cranial contour of the shank and has been acknowledged as a powerful extensor of the intertarsal joint in detailed studies about ostrich limb muscle function (Gadow, 1880; Stolpe, 1932; Weissengruber et al. 2003; Gangl et al. 2004). In the extended/engaged intertarsal joint, the tendo lateralis presses the MFB against the lateral joint surface, thus constricting the MFB's ascent of the distal lateral crest.
This would offer additional lateral support to the engaged joint in vivo and potentially increase the force required to enable the LCML to ascend the protrusion of the medial epicondyle. When the joint flexes in swing phase, the tendo lateralis attains a relaxed state to allow easy disengagement.
This interplay between passive and active locomotor systems suggests that EDM stabilizing properties may actually increase in scaled response to loads encountered during stance phase. In vivo analyses of the m. fibularis longus muscle in ground-dwelling species (Numida meleagris and Meleagris gallopavo) show a direct correlation between muscle power output and applied load during stance phase (Gabaldon et al. 2004; Ellerby & Marsh, 2006). It can be deduced that tension of the tendo lateralis in the ostrich also increases in direct proportion to limb loading, with a resultant increase in EDM stabilization. This proposal will certainly require further research and should include the role of the extensors within the framework of the multi-jointed muscle–tendon complex.
EDM within the mandatorily combined chain of limb motion
A parallel beneficial characteristic of the extended, EDM-supported distal limb becomes apparent when focusing on metatarsophalangeal joint (MTPh) posture during stance phase in running ostriches. Our samples show that this joint becomes increasingly extended, i.e. is positioned closer to the ground, as speed increases (Fig. 5b). The toe flexor tendons running caudally over the metatarsophalangeal joint are proposed as the prime locus of elastic energy storage and release with concentration of elastic energies reinforced by the engaged intertarsal joint (e.g. Schaller, 2008). This suggestion is supported by Rubenson et al. (2007) who also observed the stiff condition of the intertarsal joint throughout stance phase in over-ground running ostriches at speeds of 3.5 ms−1.
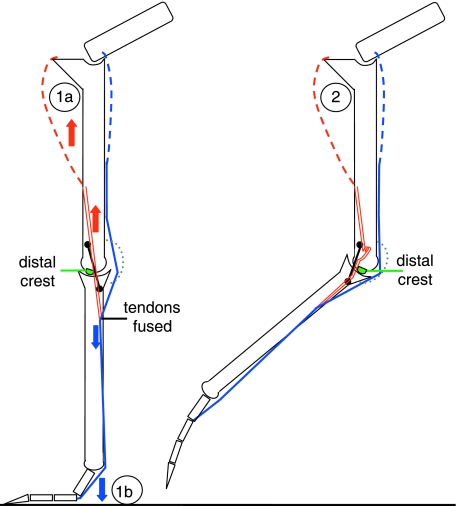
The EDM should be further contextualized within the limb's automated chain of motion where flexion and extension of knee, intertarsal and MTPh joint is mandatorily coupled in an interconnected multi-articular muscle–tendon system (e.g. Weissengruber et al. 2003). When integrating the intertarsal joint's MFL/MFB interaction with proximal muscular activity and distal MTPh joint extension during ground contact, it becomes apparent that EDM stabilization may scale relative to force inputs. As shown by the red arrows in Fig. 6, the tendo lateralis is tensed by contraction of the m. fibularis longus. The m. flexor perforatus digiti III (m. flex. III), acts as both intertarsal joint extensor and 3rd toe flexor (e.g. Gadow, 1880; Weissengruber et al. 2003). Its tendon is tensed by venter contraction and MTPh/interphalangeal joint extension (blue arrows). The m. flex. III originates at the distocaudal femur and becomes tendinous at the distal half of the tibiotarsus. Its tendon runs through the cartilago tibialis at the caudal intertarsal joint and fuses with the tendo lateralis of the MFL at the proximal tarsometatarsus. After crossing the MTPh joint on the plantar side, it inserts plantar to the 3rd toe (Gangl et al. 2004).
Fig. 6.
Schematic of left limb, lateral view. Tendo lateralis of m. fibularis longus (MFL, double red line) interacts with MFB (black line); m. flexor perforatus digiti III (m. flex. III, single blue line) joined to MFL; green spot, distal lateral crest; grey dotted line, Cartilago tibialis. (1a) During extension/stance, the IT extensor m. fibularis longus is highly active. Its tensed tendo lateralis presses the MFB against the lateral joint to further constrain MFB ascent of the distal lateral crest. As a result, EDM joint stabilization is augmented by an extensor muscle in the engaged state. The interplay of MFL/MFB is activated proximally of the IT joint with direction of tension indicated by red arrows. (1b) Ground reaction forces increase tension of the m. flex. III (blue) which fuses with the MFL distal to the IT joint, further increasing pressure on the MFB during stance. Interplay of m. flex III tendon/tendo lateralis during MTPh joint extension provides a load-based component to scale EDM stabilization in response to the tensed toe flexor tendon. This interplay is activated distally of the IT joint with direction of tension indicated by blue arrows. (2) During flexion/swing phase, the m. fibularis longus is relaxed. The tendo lateralis forms a loop and exerts no pressure on the MFB, which easily overcomes the distal lateral crest to achieve the disengaged state.
The conjoined tendons of MFL and m. flex. III might actually increase EDM stability under load by increasing pressure on the MFB to provide additional resistance to traversal of the distal lateral crest. The contraction of muscle above the IT joint during stance phase tenses the MFL, while the high degree of extension in the MTPh joint during running causes the m. flex. III to become loaded and exert tension on the tendo lateralis from below. This anatomical arrangement, influenced by both muscle activity and ground reaction forces, could provide a direct increase of EDM-based stability in the extended intertarsal joint at higher speeds when loads/3-D forces are concurrently increased, resulting in a feedback loop between speed and IT joint stability.
EDM during swing phase
While the axially loaded limb represents limb posture during stance, the unloaded limb experiments can be compared to joint dynamics seen during swing phase. Results indicate that the unloaded joint becomes relatively free to attain flexion, or regain extension, when the phalanges are lifted off the ground.
In kinematic measurements of swing phase in walking ostriches (Fig. 5a) the intertarsal joint passes through the EDM transition point shortly after toe-off, resulting in a rapid and pronounced upwards trajectory of the intertarsal joint. Evidence of this effect is also present at the end of the curve in Graph 1 at 126° where rapid transition from engaged to disengaged state automatically elevates distal limb elements off the measuring scale as flexion is induced. The effect of abruptly lifting the distal limb elements vertically off the ground might prevent dragging the limb through high steppe grass when walking and presumably leads to the distinct springy gait often noted in observations of ostrich locomotion (e.g. Bell, 1847; Langer, 1859).
EDM transition was less noticeable in running than in walking and EDM effects seen during swing phase – most notably the rapid snapping towards extension – may be overshadowed by the fast-working, powerful musculature. It could even be argued that any benefit derived from resistance to flexion during stance becomes an energetic disadvantage as resistance must be overcome twice during each stride cycle. Under these conditions, increased requirements for muscle power would contradict an essential precondition for long endurance running: the efficient transmission of metabolic energy through the locomotor system towards the substrate. However, it stands to reason that inertial effects in the live ostrich limb resulting from the accelerated tarsometatarsus and toes, particularly at running speeds, might be more than sufficient to overcome any resistance towards flexion and extension when unloaded. This hypothesis can be derived from the reduced resistance measured in the unloaded vs. axially loaded intertarsal joint. Thus, limb muscles would not be required to increase power output to overcome EDM resistance and the ostrich would still profit from the engaged stage during stance phase.
The passive locomotor system in the ostrich hind limb
The benefit of passively supported limb joints becomes apparent when considering that ostriches stand or walk very slowly for extended periods of time. Maintaining standing posture solely by muscle power would be energetically disadvantageous. A striking illustration of passive limb support in the ostrich was seen in the course of a separate anatomical analysis. During reductive dissection of the pelvis with limbs attached, all muscles and tendons had been removed, with only skeleton and ligamentous system remaining intact. When oriented as if standing with phalangeal and distal joints extended and knee and hip flexed, the specimen had the requisite stability to stand erect when laterally balanced by hand.
Structurally, the relatively short femur and its predominantly horizontal position during standing results in a rather small moment arm acting on the hip and knee and requires relatively little muscle force to act against over-flexion (e.g. Stolpe, 1932; Gatesy & Biewener, 1991). The hip joint itself is largely constrained within its 3-D motion range by a strong ligamentous apparatus (Firbas & Zweymüller, 1971). Even with all acetabular ligaments cut, the interaction of trochanter and antitrochanter remains an osseous barrier against further over-flexion (Hertel & Campbell, 2007). The complex guiding qualities of the cruciates in the knee have been functionally described by Fuss & Gasser (1992) with detailed information about the importance of ligaments within the tibio-fibular junction given by Fuss (1996). The functional inter-relationships of the intertarsal ligaments and resulting effects on joint behaviour have been illustrated in the present study. Finally, the supra-jointed metatarsophalangeal posture is maintained primarily by ligaments, as observed in the axially loaded limb. The distinctly oriented metatarsophalangeal joint remained elevated during all phases of loading despite the removal of all muscles and associated tendons.
When integrated into the context of locomotion, the guiding and constraining function of the passive locomotor system in the ostrich hind limb gains even greater relevance. In isolated intact limbs, manual extension of the knee also extended intertarsal, metatarsophalangeal and phalangeal joints with controlled abduction of the 4th toe (e.g. Weissengruber et al. 2003; Schaller et al. 2007). Before touch-down, this multi-jointed muscle-tendon complex re-establishes the EDM-supported limb column in advance of loading and provides proper orientation of the 3rd and 4th toe to begin the next stride cycle. Taken as a whole, it is clear that a significant amount of ‘work’ is managed by passive structures which, if absent, would require active muscle power at additional metabolic cost.
Conclusions
The intertarsal joint, located at the junction of the longest segments of the limb – potentially a weak-point in engineering terms – is stabilized by the complex interplay of the structural components of the engage–disengage mechanism (EDM). During stance phase, the extended joint is sustained in the engaged state to provide additional support for trunk mass while focusing ground reaction forces within the elastic energy storage structures of the supra-jointed metatarsophalangeal joint. The stabilizing qualities of the EDM may actually increase in proportion to load when ligament tension is augmented by an intertarsal extensor muscle which closely interacts with ligamentous structures. The stiffened condition present during ground contact has negligible influence on the freedom of intertarsal joint flexion during swing phase; once the phalanges are lifted off the ground and the joint is unloaded, the arrangement of ligaments relative to the position of joint surface protrusions combines with inertial effects to allow easy disengagement in continuance of the stride cycle.
The qualities of the ligamentous system in combination with skeletal features in the avian leg should be afforded greater consideration in the analysis and interpretation of terrestrial locomotion. Especially when contemplating the reconstruction of fossil taxa, functional integration of the ligaments as important contributors to bipedal locomotion would likely influence existing models that compute locomotor behaviour based largely on skeletal structure and projected muscle mass.
Considering the relationship of modern birds to their theropod ancestors, it might be interesting to imagine how we would reconstruct an ostrich if we had only the bones. Could we have known about the supra-jointed toe-posture and its advantages? Would we have predicted the mandatorily combined extension/flexion system of knee, intertarsal, metatarsophalangeal and phalangeal joints? Could we have deduced the existence of the intertarsal engage–disengage mechanism? Each of these structural attributes in the kinematic chain results in a conservation of locomotor energies. We should remain open to the idea that structures traditionally regarded as ‘passive’ contribute to locomotor efficiency and can provide significant biomechanical advantage without the consumption of additional metabolic energy.
Acknowledgments
N.U.S. thanks J. Gass for providing the outdoor enclosure and dedicated ostrich keeping, the ostrich farms Donaumoos, Hermersberg, and MHOU, Rülzheim, all in Germany, for providing specimens and fresh material; the Royal Ontario Museum, Toronto, Canada, for access to skeletal material in the ornithological and palaeontological collections. The authors offer great thanks to two anonymous referees for their insightful suggestions and valuable criticism, Prof. G. E. Weissengruber, Department of Anatomy, University of Veterinary Medicine, Vienna, Austria for his scientific advice, and the Cusanuswerk Foundation, Bonn, Germany (Bischöfliche Studienförderung) for financial support.
References
- Abourachid A, Renous S. Bipedal locomotion in ratites (Paleognatiform): examples of cursorial birds. Ibis. 2000;142:538–549. [Google Scholar]
- Aichel O. Über die Wirkung der Ligamenta collateralia nebst Bemerkungen über die sogenannten Schnappgelenke. Z Anat Entwicklungsgesch. 1925;76:1–15. [Google Scholar]
- Alexander RM. Allometry of the leg bones of moas (Dinornithes) and other birds. J Zool Lond. 1983;200:215–231. [Google Scholar]
- Alexander RM, Maloiy GMO, Njau R, Jayes AS. Mechanics of running in the ostrich (Struthio camelus. J Zool Lond. 1979;187:169–178. [Google Scholar]
- Baumel JJ, King AS, Breazile JE, Evans HE, van den Berge JC, editors. 2nd edn. Cambridge, MA: Publications of Nuttal Ornithological Club; 1993. Nomina Anatomica Avium. [Google Scholar]
- Bell C. Expedition der Wochenbände. Stuttgart: Scheibele, Rieger & Sattler; 1847. Die Hand und ihre Eigenschaften. Translated from the English by F Kottenkamp, original: The hand, its mechanism and vital endowment as envincing design. [Google Scholar]
- Bertram BCR. The Ostrich Communal Nesting System. Princeton: Princeton University Press; 1992. [Google Scholar]
- Bezuidenhout AJ. Anatomy. In: Deeming DC, editor. The Ostrich – Biology, Production and Health. Cambridge: University Press; 1999. pp. 13–49. [Google Scholar]
- Boyd KT, Myers BT. Meniscus preservation; rationale, repair techniques and results. Knee. 2003;10:1–11. doi: 10.1016/s0968-0160(02)00147-3. [DOI] [PubMed] [Google Scholar]
- Deeming DC. Cambridge: University Press; 1999. The Ostrich – Biology, Production and Health. [Google Scholar]
- del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol. 1. Barcelona: Lynx Edicions; 1992. [Google Scholar]
- Ellerby DJ, Marsh RL. The energetic costs of trunk and distal-limb loading during walking and running in guinea fowl Numida meleagris: II. Muscle energy use as indicated by blood flow. J Exp Biol. 2006;209:2064–2075. doi: 10.1242/jeb.02227. [DOI] [PubMed] [Google Scholar]
- Firbas W, Zweymüller K. Über das Hüftgelenk der Ratiten. Gegenbaurs Morphol Jahrb. 1971;116(1):91–103. [PubMed] [Google Scholar]
- Fuss FK. Tiobiofibular junction of the South African Ostrich (Struthio camelus australis. J Morphol. 1996;227:213–226. doi: 10.1002/(SICI)1097-4687(199602)227:2<213::AID-JMOR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Fuss FK, Gasser CR. Cruciate ligaments of the avian knee: insight into a complex system. J Morphol. 1992;214:139–151. doi: 10.1002/jmor.1052140204. [DOI] [PubMed] [Google Scholar]
- Gabaldon AM, Nelson FE, Roberts TJ. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J Exp Biol. 2004;207:2277–2288. doi: 10.1242/jeb.01006. [DOI] [PubMed] [Google Scholar]
- Gadow H. Zur vergleichenden Anatomie der Muskulatur des Beckens und der Hinteren Gliedmasse der Ratiten. Jena: Verlag Gustav Fischer; 1880. [Google Scholar]
- Gangl D. Die Muskeln der Hinterextremität des Strausses (Struthio camelus Linné 1758) Veterinärmedizinische Universität Wien: 2001. Thesis. [Google Scholar]
- Gangl D, Weissengruber GE, Egerbacher M, Forstenpointner G. Anatomical description of the muscles of the pelvic limb in the ostrich (Struthio camelus. Anat Histol Embryol J Vet Med Series. 2004;C33:100–114. doi: 10.1111/j.1439-0264.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Biewener AA. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J Zool Lond. 1991;224:127–147. [Google Scholar]
- George JC, Berger AJ. Avian Myology. New York: Academic Press; 1966. [Google Scholar]
- Glutz von Blotzheim U. Zur Morphologie und Ontogenese von Schultergürtel, Sternum und Becken von Struthio, Rhea und Dromiceius. Freiburg: Arb Zool vergl-anat Inst. Universität Freiburg; 1958. [Google Scholar]
- Grzimek B, Grzimek M. Serengeti Shall Not Die. West Germany: Asta Motion Pictures; 1959. [Google Scholar]
- Hallam MG. The Topaz Introduction to Practical Ostrich Farming. Harare, Zimbabwe: Hallam MG; 1992. [Google Scholar]
- Haughton S. Notes on animal mechanics. No. III: On the muscular mechanism of the leg of the ostrich. Proc Roy Irish Acad. 1865;9:50–61. [Google Scholar]
- Hertel F, Campbell KE., Jr The antitrochanter in birds: form and function in balance. Auk. 2007;124(3):789–805. [Google Scholar]
- Hildebrand M. Analysis of Vertebrate Structure. 4th edn. New York: John Wiley & Sons; 1995. [Google Scholar]
- Hultkrantz JW. Das Ellenbogengelenk und seine Mechanik. Jena: Verlag Gustav Fischer; 1897. [Google Scholar]
- Kummer B. Bauprinzipien des Säugerskeletts. Stuttgart: Georg Thieme Verlag; 1956. [Google Scholar]
- Langer K. Ueber die Fußgelenke der Vögel. Denkschr KaiserAkad Wissenschaften Wien. 1859;16:93–130. [Google Scholar]
- Liswaniso D. A morphological and diagnostic imaging study of the distal pelvis limb of the ostrich (Struthio camelus) University of Glasgow: 1996. Msc Thesis. [Google Scholar]
- Marin F, Mannel H, Claes L, Durselen L. Correction of axis misalignment in the analysis of knee rotations. Hum Mov Sci. 2003;22:285–296. doi: 10.1016/s0167-9457(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Mellet FD. The ostrich as meat animal – anatomical and muscle characteristics. University of Stellenbosch: 1985. Msc Agric Thesis. [Google Scholar]
- Muller M. The relationship between the rotation possibilities between femur and tibia and the lengths of the cruciate ligaments. J Theor Biol. 1993a;161:199–220. doi: 10.1006/jtbi.1993.1050. [DOI] [PubMed] [Google Scholar]
- Muller M. The angles of femoral and tibial axes with respect to the cruciate ligament four-bar system in the knee joint. J Theor Biol. 1993b;161:221–230. doi: 10.1006/jtbi.1993.1051. [DOI] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E, Frewein J, Wille K-H, Wilkens KH. Lehrbuch der Anatomie der Haustiere. 6th edn. Vol. 1. Berlin: Paul Parey; 1992. [Google Scholar]
- Palmgren A. Zur Kenntnis der sogenannten Schnappgelenke. I. Die Ursache des Federungsphänomens nebst einigen Bemerkungen über die Fossae nudatae des Ellenbogengelenkes beim Pferde. Anat Embryol. 1929;89:710–745. [Google Scholar]
- Pavaux C, Lignereux Y. Une dissection myologique de la Jambe et du Pied de l’Autruche (Struthio camelus. Anat Histol Embryol J Vet Med Series C. 1995;24:127–131. doi: 10.1111/j.1439-0264.1995.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Richter H. Die Bedeutung der federnden Gelenke oder Schnappgelenke. Schweiz Arch Tierheilkd. 1922;64:76–87. [Google Scholar]
- Rubenson J, Lloyd DG, Besier TF, Heliams DB, Fournier PA. Running in ostriches (Struthio camelus): three-dimensional joint axes alignment and joint kinematics. J Exp Biol. 2007;210:2548–2562. doi: 10.1242/jeb.02792. [DOI] [PubMed] [Google Scholar]
- Sales J. Histological, biophysical, physical and chemical characteristics of different ostrich muscles. J Sci Food Agric. 1996;70:109–114. [Google Scholar]
- Schaller NU. Structural attributes contributing to locomotor performance in the ostrich (Struthio camelus) Ruprecht-Karls-Universität Heidelberg: 2008. PhD thesis. [Google Scholar]
- Schaller NU, D’Août K, Herkner B, Aerts P. Structural attributes contributing to locomotor performance in the ostrich (Struthio camelus. J Morphol. 2007;268:1129. [Google Scholar]
- Schaller NU, Herkner B, Prinzinger R. Proceedings of the 3rd International Ratite Science Symposium. Madrid, Spain: 2005. Locomotor characteristics of the ostrich (Struthio camelus). I: Morphometric and morphological analyses; pp. 83–90. [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, Payne RC. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus. J Anat. 2006;209:765–779. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolpe M. Physiologisch-anatomische Untersuchungen über die hintere Extremität der Vögel. J Ornithol. 1932;80:161–247. [Google Scholar]
- Stornelli MR, Ricciardi MP, Giannessi E, Coli A. Osservazione morfo-strutturali sul menisco dell’articolazione tibio-metatarsica di struzzo (Struthio camelus. Ann Fac Med Vet Pisa LIV. 2001:361–372. [Google Scholar]
- van den Berge JC, Zweers GA. Myologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, van den Berge JC, editors. Handbook of Avian Anatomy: Nomina Anatomica Avium. 2nd edn. Cambridge, MA: Publications of Nuttal Ornithological Club; 1993. pp. 189–247. [Google Scholar]
- Von den Driesch A. Messstrecken am Vogelskelett. Das Vermessen von Tierknochen aus vor- und frühgeschichtlichen Siedlungen. 1976:91–113. Aus dem Institut für Paläoanatomie, Domestikationsforschung und Geschichte der Tiermedizin. [Google Scholar]
- Wagner M. Die Osteologie der Hinterextremität und des Beckengürtels beim Afrikanischen Strauss. Veterinärmedizinische Universität Wien: 2004. (Struthio camelus Linné 1758) Thesis. [Google Scholar]
- Weissengruber GE, Gangl D, Forstenpointner G, Probst A. Proceedings of the XXIV Congress of the European Association of Veterinary Anatomists. Brno, Czech Republic: 2002. Morphological features of the patellae of the ostrich (Struthio camelusLinné 1758) p. 66. [Google Scholar]
- Weissengruber GE, Forstenpointner G, Gangl D. Gut zu Fuß– funktionell-anatomische Aspekte des bipeden Laufens beim Afrikanischen Strauß (Struthio camelusLinné, 1758) Vet Med Austria Wien Tierärztl Mschr. 2003;90:67–78. [Google Scholar]
- Williams JB, Siegfried WR, Milton SJ, Adams NJ, Dean WRJ, du Plessis MA, Jackson S. Field metabolism, water requirements, and foraging behaviour of wild ostriches in the Namib. Ecology. 1993;74:390–404. [Google Scholar]