Abstract
The several linked polymorphic genes of the MHC, which has been proposed as a prime determinant of sensed genetic individuality within species, is known to operate in mice by olfactory recognition in aspects of reproductive behavior that concern mate selection, thereby favoring outbreeding and heterozygosity, and also concern the maintenance of pregnancy. A single base-change can alter an individual MHC odortype, and the potential range of combinatorial MHC-determined odortypes is clearly vast. Following our findings that newborn mice already express their MHC odortype (which is detectable at 9 days of gestational age), we sought to determine whether MHC is involved in behavioral aspects of early development, such as rearing. In the studies presented herein, we report the ability and proclivity of mothers to recognize and preferentially retrieve syngeneic (genetically identical) pups from other pups differing only for MHC. Reciprocally, we report the ability of pups to recognize their familial environment, regardless of whether they had been nursed by their biological mothers or by foster mothers. Early learning experiences of the MHC environment are apparently a key element in survival, assuring maternal protection and promoting outbreeding.
Keywords: chemosensation, olfaction, mammalian pheromones, infant
The MHC comprises a family of approximately 50 genes, best known for their crucial role in cell–cell recognition (1, 2). The extreme diversity of these genes suggests an evolutionary investment in mechanisms that promote it. One such mechanism is mating preferences that favor outbreeding and MHC disparity (3–7). Such a mechanism implies that MHC types must be detectable by conspecifics.
Lewis Thomas (8) proposed that MHC's ancestral role was as self-identifier and that this role evolved into the odor marking of individuals of a species. This hypothesis has since been substantiated in mice (9–11), rats (12, 13), and perhaps humans (14, 15); and the term odortype was coined to denote the genetically programmed body odors that uniquely distinguish individuals (16).
Our initial observations (3) of negative assortative mating according to MHC (called H-2 in mice) type in inbred mice have been extended by several investigators (but see Eklund et al., ref. 17). In particular, Potts and colleagues (6) have shown that, under seminatural conditions, the tendency of mice to mate with H-2-dissimilar individuals can account for the maintenance of heterozygosity in natural populations. Manning et al. (18) demonstrated another context in which H-2-determined odortypes are most likely involved: females nest with other females that are MHC-similar.
Parent–infant recognition based on individual-specific odors is well documented among many genera (19–21), but no genetic basis has yet been elucidated. We have found (22) that H-2 odortypes are evident in mice as young as 1 day of age, raising the possibility that a dam might thereby identify her offspring. Moreover, because olfactory function is well developed in mouse pups as young as a few days of age (23), they might reciprocally recognize and prefer their mother's MHC type.
The two studies reported herein were designed to test these reciprocal hypotheses and determine whether MHC plays a role in early behavioral development.
Materials and Methods
Mice.
C57BL/6 (B6; H-2b) and the congenic strain B6-H-2k, which differs from B6 only for H-2, were bred and maintained in the same animal room on a 12:12-h light:dark cycle. At 2 mo of age, virgin females were mated with same-strain males and examined daily for progeny. Date of birth was day 0. Lactating females (18 B6; 19 B6-H-2k) serving as subjects in study 1 continued to live in their family groups throughout the experiment except during the test period.
There were 19 B6 and 22 B6-H-2k litters (3–8 pups/litter) that served as subjects in study 2A. In study 2B, 20 B6 litters fostered on B6-H-2k parents, and, reciprocally, 15 B6-H-2k litters fostered on B6 parents served as subjects. These litters had been removed from their natal environment within 16 h of birth and fostered on adoptive parents whose own same-age litters had been removed simultaneously for fostering.
Apparatus.
For study 1, testing was conducted in the mother's home cage (17.5 × 28.5 × 12-cm clear polycarbonate with cedar shavings as nest material). For study 2, a modified Y maze constructed of clear Plexiglas was used. A clean sheet of paper served as the bottom of the maze. Air, drawn by a fan, was conducted through the left and right arms of the maze, both fitted with transparent lids. Each arm had a perforated plastic box (10 × 8 × 5-cm) containing the odor source, which was the soiled cedar wood shavings taken from the cages, left unchanged for 3–5 days, in which the odor-source mice had been housed. Each arm of the maze had a perforated plastic screen that prevented the pups from directly touching the odor source material. The starting-box end of the maze was connected to an exhaust fan.
Procedures.
All handling of mice was conducted with disposable vinyl gloves to obviate odor contamination. Testing in study 1 was after Chantry and Jenkins (24): both parents and litter were removed from the home cage. After 10 min, three syngeneic pups and three congenic pups, all of the same age, were intermingled and placed in the test mother's home cage at the furthest end from her nest. The test mother was then returned to her cage and the order in which she retrieved the pups during a 3-min trial was noted. Each mother was tested twice, with a 40-min interval between tests. All litters were 2–7 days old at the time of testing (median age = 4).
For study 2, litters were transferred from their home cage to a new cage, which was placed on a heating pad, and left undisturbed for 10 min. Each pup in each litter was tested individually in the Y maze on 4 days at ages 15–21 days. The left–right placement of the perforated plastic box containing soiled bedding was governed by a series of random numbers. Each pup was left in the maze for 4 min, and time the pup spent in each arm of the maze was recorded. Procedures were the same whether the litters had been raised by their natal parents (study 2A) or by foster parents (study 2B).
Statistical Analyses.
In study 1, two approaches were used for testing whether the H-2 type of the mothers and pups influenced the order of pup retrieval. Because most test mothers retrieved all six pups by the end of the test, we examined pups retrieved early in the test to evaluate discrimination as follows. We noted whether the very first pup retrieved differed from or was identical to the mother as a function of H-2 type. We also similarly determined the H-2 type of the first two pups that were retrieved in each of the two tests. This evaluation, based on a total of four pups retrieved, determines whether retrieval deviates from random (random value: two B6 and two B6-H-2k).
In study 2, the mean number of seconds each pup spent in each side of the Y maze over each of the four trials was recorded. Of the possible 240 s (4 min trial), the mice spent an average of 151 s in both arms combined. Because it is appropriate to analyze the litter as the unit for statistical purposes, the mean time each pup in a litter spent in the B6-odor-scented arm relative to the B6-H-2k arm (s with B6 divided by s with B6 + s with B6-H-2k) was computed, and these proportions were averaged across the entire litter, giving a single average proportion for each litter. Preliminary analyses revealed no significant changes in these values across the 4 days of testing for each litter; thus, the means on each of the 4 days were averaged. This resulted in a single proportion for each litter reflecting the average time spent in the B6 arm of the maze. We then calculated whether time spent in each arm of the maze was impacted by the genotype of the subject (sensor) litter and/or by the odor source.
Results
Study 1: Mothers Preferentially Retrieve Syngeneic Young.
When presented with admixed B6 and congenic B6-H-2k pups of the same age as her own litter, mothers retrieve pups of the same genotype as herself in preference to congenic pups (Table 1), the sole genetic criterion in this discrimination being H-2.
Table 1.
Nursing mothers preferentially retrieve syngeneic pups
| Mothers | First Choice*
|
Number of B6 pups in first four pups retrieved†
|
||
|---|---|---|---|---|
| B6 | B6-H-2k | Mean ± SE | Median | |
| B6 (n = 18) | 12 | 6 | 2.56 ± 0.22 | 3 |
| B6-H-2k (n = 19) | 5 | 14 | 1.47 ± 0.29 | 1 |
P < 0.02, Fisher's exact text.
P < 0.01, Mann–Whitney U test.
Study 2: Pups Preferentially Select Familiar Odortypes.
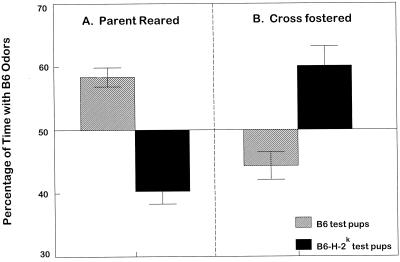
For study 2A, Table 2 and Fig. 1 show that B6 pups, reared in a syngeneic environment, spend more time in the arm of the Y maze scented with B6 bedding (average litter preference 58.7 ± 1.6%), and 17 of 19 litters had a mean preference of >50%. For B6-H-2k litters syngeneically reared, more time was spent in the Y maze arm scented with B6-H-2k bedding (mean preference for B6 bedding was 39.9 ± 2.0%, and 19 of 22 litters had a mean preference of less than 50%).
Table 2.
Mouse pups preferentially gravitate to the H-2 odortype in which they were reared
| Study | Pup (no. of litters) | Familial Type | Preference for Y maze arm scented with
|
|||
|---|---|---|---|---|---|---|
| Per litter
|
Per pup
|
|||||
| B6 | B6-H-2k | B6 | B6-H-2k | |||
| A† | B6 (19) | B6 | 17 | 2** | 76 | 29** |
| B6-H-2k (22) | B6-H-2k | 3 | 19** | 36 | 86** | |
| B‡ | B6 (20) | B6-H-2k | 5 | 15* | 40 | 62* |
| B6-H-2k (15) | B6 | 12 | 3* | 50 | 29* | |
, Greater number in each group considered separately preferring (spending more than 50% time) with B6 or B6-H-2k, P < 0.05;
, P < 0.001.
For litters χ2 = 20.4, P < 0.001; for pups χ2 = 38.1, P < 0.0001.
For litters χ2 = 8.29, P < 0.01; for pups χ2 = 9.38, P < 0.01.
Figure 1.
Percentage of time pup litters (B6 and B6-H-2k) spent in the arm of the Y maze scented by B6-soiled bedding relative to B6-H-2k-soiled bedding. (A) Litters reared by their biological, syngeneic parents (t comparing percentages = 7.3; df = 39; P < 0.0001). (B) Litters removed within 16 h of birth and fostered onto congenic parents (t = 4.04; df = 33; P < 0.005).
In study 2B, as shown in Table 2 and Fig. 1, cross-fostering reversed the tendencies exhibited in study 2A, above. Thus, B6 litters fostered on B6-H-2k preferred the scent of B6-H-2k (average preference for B6 was 46.1 ± 2.1%, and 5 of 20 litters had a mean preference of >50%). Similarly, B6-H-2k litters fostered on B6 preferred the B6 arm of the Y maze (average preference for B6 was 60.1 ± 3.0%, and 12 of the 15 litters had a mean preference for B6 of >50%).
Discussion
The data presented herein show that MHC is a prime determinant of parent–progeny interaction. In the first study, mothers recognized and retrieved pups of their own (familiar) MHC type, in preference to otherwise identical pups bearing a different MHC type. Although the medium of recognition is likely olfaction, the paradigm used in this study does not exclude other possible modes of communication, for example ultrasound (25, 26). If such were the case, these ultrasonic emissions must themselves modulate according to MHC type to produce the results reported in study 1, and such variations have not in fact been reported thus far. However, we have shown previously that MHC odortypes become expressed in mice as early as 9 days of gestational age and are present and operative at birth, allowing for gene-based recognition (27).
Study 2 confirms olfaction as the mode of communication. In the Y maze apparatus, the subject pups could differentiate between odors only. When given the choice of bedding removed from the home cage of syngeneic mothers and pups or bedding removed from a cage housing MHC congenic mothers and pups, the subject pups preferentially selected the odor in which they were reared. This preference was obtained even when the pups were reared by foster mothers of an MHC type different from their own; the operative preference was for the parental-familiar rather than for self.
There was indication that preference was not entirely reversed by fostering. When the data from each pup is considered (right columns, Table 2), a greater proportion preferred familiar odor in study A relative to study B (χ2 = 5.53; P < 0.02). This difference suggests that prenatal or very early postnatal learning (28) or self referral (29) could be involved in modulating choice.
Hepper (23), who did not study directly the role of MHC genes, has reported that siblings recognize each other as individuals by odor. Among animals on a constant diet, both prenatal and postnatal experiences acted to familiarize rat siblings with each other's odors. However, in studies of semi-wild Mus musculus with known MHC types, Manning et al. (30) failed to find MHC-based pup discrimination in recognition assays, in contrast to study 1 reported herein. These disparate results could be because of methodological and/or strain differences. It is possible that variation in other loci in the Manning study could have obscured an MHC effect, whereas our data, obtained with genetically controlled inbred mice, clearly demonstrated discriminatory behavior by the retrieving mothers. The vast majority of mothers in the Manning study were tested only 2 or 3 days after parturition (31 of 36 tested), which may be too early for preference to be established, even though distinctive odors are present at this time in inbred mice (see above). In contrast, the majority of pups we tested were slightly older.
Brown and Schellinck (31) briefly refer to an unpublished study that suggests that mouse pups (of unstated age) were not differentially attracted to their own mother's feces compared with feces of congenic lactating females, but that diet was the main attractant in feces, as has been demonstrated (32). Although an MHC effect was apparently not significant, it may be noteworthy that, based on the graphical data presented in their review, in all four cases studied, the mean preference for the mother's feces was greater than that for congenic females' feces, as we would predict. Brown and Schellinck did not report on preferences in urine, which, as is known, is an excellent conveyor of MHC odor.
We and others have shown that mating preferences according to MHC type favor dissimilar choice, presumably to enhance outbreeding and H-2 heterozygosity, promote diversity of H-2 genes, and edit spontaneous mutations. MHC diversity then in turn enlarges the repertoire of responses to pathogenic assault (2). In contrast, familial choice favors H-2 similarity, thus ensuring appropriate mother–young attachment and hence survival. However, both selective mating and parent–pup preference are influenced by perinatal acclimatization, which plays a key role in both behaviors. In the former, early imprinting promotes selection of the unfamiliar in subsequent mate choice; in the latter, the biologically familiar is favored. The fact that these behaviors are learned responses to MHC type is supported by the fact that both mate choice and pup preference are amenable to manipulation by foster nursing (ref. 33 and current study).
Although the current studies have dealt exclusively with inbred MHC-congenic mice, it seems likely that MHC-determined odors play a role in familial interactions in many species. MHC odortypes have been demonstrated in mice, rats and humans (see introduction). Investigation of a role for MHC in mother–young recognition in humans, which is known to be mediated in part by olfaction (34, 35), would now seem warranted.
Acknowledgments
This work was supported in part by National Science Foundation Grant NSF 9728787.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180320997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180320997
References
- 1.Rammensee H-G, Falk K, Rotzschke O. Cur Opin Immunol. 1993;5:35–44. doi: 10.1016/0952-7915(93)90078-7. [DOI] [PubMed] [Google Scholar]
- 2.Parham P, Ohta T. Science. 1996;272:67–74. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki K, Boyse E A, Mike V, Thaler H T, Mathieson B J, Abbott J, Boyse J, Zayas Z A, Thomas L. J Exp Med. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki K, Yamaguchi M, Andrews P W, Peake B, Boyse E A. Immunogenetics. 1978;6:253–259. [Google Scholar]
- 5.Egid K, Brown J L. Anim Behav. 1989;38(3):548–549. [Google Scholar]
- 6.Potts W K, Manning C J, Wakeland E K. Nature (London) 1991;352:619–621. doi: 10.1038/352619a0. [DOI] [PubMed] [Google Scholar]
- 7.Ober C, Weikamp L R, Cox N, Dytch H, Kostyu D, Elias S. Am J Hum Genet. 1997;61:497–504. doi: 10.1086/515511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas L. In: Fourth International Congress of Immunology. Neter E, Milgrom F, editors. Basel: Karger; 1975. p. 2. [Google Scholar]
- 9.Boyse E A, Beauchamp G K, Bard J, Yamazaki K. In: Psychoneuroimmunology-II. Ader R, Felter D L, Cohen N, editors. New York: Academic; 1991. pp. 831–846. [Google Scholar]
- 10.Brown J L, Eklund A. Am Nat. 1994;143:435–461. [Google Scholar]
- 11.Penn D J, Potts W K. Physiol Behav. 1998;63:235–243. doi: 10.1016/s0031-9384(98)00052-3. [DOI] [PubMed] [Google Scholar]
- 12.Brown R E, Singh P B, Roser B. Physiol Behav. 1987;40:65–73. doi: 10.1016/0031-9384(87)90186-7. [DOI] [PubMed] [Google Scholar]
- 13.Singh P B, Brown R E, Roser B. Nature (London) 1987;327:161–164. doi: 10.1038/327161a0. [DOI] [PubMed] [Google Scholar]
- 14.Ferstl R, Eggert F, Westphal E, Zavazava N, Muller- Ruchholtz W. In: Chemical Signals in Vertebrates VI. Doty R L, editor. New York: Plenum; 1992. pp. 205–211. [Google Scholar]
- 15.Wedekind C, Seebeck T, Bettens F, Paepke A J. Proc R Soc London Ser B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 16.Boyse E A, Beauchamp G K, Yamazaki K. Trends Genet. 1987;3:97–102. [Google Scholar]
- 17.Eklund A, Egid K, Brown J L. Anim Behav. 1991;42:693–994. [Google Scholar]
- 18.Manning C J, Wakeland E K, Potts W K. Nature (London) 1992;360:581–583. doi: 10.1038/360581a0. [DOI] [PubMed] [Google Scholar]
- 19.Beauchamp G K, Doty R L, Moulton D G, Mugford R A. In: Mammalian Olfaction, Reproductive Processes, and Behavior. Doty R L, editor. New York: Academic; 1976. pp. 143–160. [Google Scholar]
- 20.Halpin Z T. Biol Behav. 1980;5:233–248. [Google Scholar]
- 21.Johnston R E, Muller-Schwarze D, Sorensen P W. Advances in Chemical Signals in Vertebrates. New York: Klewer/Plenum; 1999. [Google Scholar]
- 22.Yamazaki K, Beauchamp G K, Imai Y, Boyse E A. Proc Natl Acad Sci USA. 1992;89:2756–2758. doi: 10.1073/pnas.89.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hepper P G. Anim Behav. 1983;31:1177–1191. [Google Scholar]
- 24.Chantrey D F, Jenkins B A B. Anim Behav. 1982;30:881–885. [Google Scholar]
- 25.Wysocki C J. Neurosci Biobehav Rev. 1979;3:301–341. doi: 10.1016/0149-7634(79)90015-0. [DOI] [PubMed] [Google Scholar]
- 26.Bean J J. Physiol Behav. 1982;28:31–37. doi: 10.1016/0031-9384(82)90097-x. [DOI] [PubMed] [Google Scholar]
- 27.Beauchamp G K, Yamazaki K, Curran M, Bard J, Boyse E A. Immunogenetics. 1994;39:109–113. doi: 10.1007/BF00188613. [DOI] [PubMed] [Google Scholar]
- 28.Polan H J, Hofer M A. Dev Psychobiol. 1999;34:269–279. doi: 10.1002/(sici)1098-2302(199905)34:2<269::aid-dev3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Johnston R E, Derzie A, Chiang G, Jernigan P, Lee H. Anim Behav. 1993;45:1061–1070. [Google Scholar]
- 30.Manning C J, Dewsbury D A, Wakeland E K, Potts W K. Anim Behav. 1995;50:741–751. [Google Scholar]
- 31.Brown R E, Schellinck H M. In: Chemical Signals in Vertebrates VI. Doty R L, Muller-Schwarze D, editors. New York: Plenum; 1992. pp. 175–181. [Google Scholar]
- 32.Leon M. Physiol Behav. 1974;13:441–453. doi: 10.1016/0031-9384(74)90098-5. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki K, Beauchamp G K, Kupniewski D, Bard J, Thomas L, Boyse E A. Science. 1988;240:1331–1332. doi: 10.1126/science.3375818. [DOI] [PubMed] [Google Scholar]
- 34.Porter R H, Cernoch J M, McLaughlin F J. Physiol Behav. 1983;30:151–154. doi: 10.1016/0031-9384(83)90051-3. [DOI] [PubMed] [Google Scholar]
- 35.Schaal B, Marlier L. Biol Neonate. 1998;74:267–273. doi: 10.1159/000014033. [DOI] [PubMed] [Google Scholar]