Abstract
Estrogen and the estrogen receptor (ER)α play an important role in the male reproductive tract and in fertility. Previous studies demonstrated that disruption of ERα function resulted in abnormal morphology of the testis and efferent ductules (EDs) of adult mice. However, the effect of a lack of a functional ERα during early postnatal development has not been determined. The present study is an evaluation of morphological changes effected by a lack of ERα in the male reproductive tract during the postnatal period. Age-equivalent wild-type and ERα knockout (αERKO) mice at 10, 18, 35 and 60 days of age after birth were used for morphological comparison of the testes and ED. Light microscopic examination of the testes of the αERKO mouse revealed a dramatic dilation of the rete testis as early as 10 days of age, premature lumen formation, reduced epithelial height and greatly dilated lumen of seminiferous tubules as early as 18 days of age. The proximal ED of the αERKO mouse showed lumen dilation, reduction of epithelial height and a decrease of nuclear height as early as 10 days of age. Similar, but somewhat less severe, morphological abnormalities were observed in the distal ED of the αERKO mouse. These results indicate that a lack of functional ERα leads to morphological changes of the testis and ED of the early postnatal developing mouse. Based on these observations, we conclude that ERα plays an important role in normal development of the testis and ED, not only during adulthood but also during the entire postnatal period and presumably during fetal development.
Keywords: efferent ductules, estrogen, estrogen receptor α, estrogen receptor α knockout mouse, morphology, rete testis, testis
Introduction
The male reproductive tract consists of the testis and excurrent duct system. The main parts of the excurrent duct are the efferent ductules (EDs) and epididymis. The EDs are a conduit that connects the testis and epididymis. The EDs are divided into three regions, i.e. proximal, conus and distal zones, based on histological and morphological characteristics (Ilio & Hess, 1994). The ED is composed of a columnar epithelium surrounded by a thin layer of smooth muscle and connective tissue (Ilio & Hess, 1994). The epithelial layer of the ED mainly possesses two cell types, ciliated and non-ciliated cells, with the latter being the major type (Robaire & Hermo, 1988; Ilio & Hess, 1994; Lee et al. 2000).
The ED is the major area where most of the testicular fluid is reabsorbed and thus spermatozoa become concentrated prior to entering the epididymis for maturation (Clulow et al. 1994; Ilio & Hess, 1994). The presence of relatively high concentrations of estrogen and the existence of the estrogen receptor (ER) in the male reproductive tract have been reported by a number of studies (Ganjam & Amann, 1976; Free & Jaffe, 1979; Fisher et al. 1997; Hess et al. 1997b). Our previous studies demonstrated that estrogen is involved in fluid reabsorption in the ED by regulating the expression of ion transporters and/or ion producers mainly via ERα and in part via ERβ (Hess et al. 1997a; Lee et al. 2001). In addition, the disruption of ERα function by genetic modification [ERα knockout (αERKO)] or chemical treatment results in morphological abnormalities in the ED and testis of adult mice (Eddy et al. 1996; Hess et al. 1997a; Lee et al. 2000). These abnormal features include a dilation of rete testis (RT) and seminiferous tubules (STs) in the testis and a dilation of the lumen, a thinning of the epithelial layer, and a shortening of microvilia and nuclear height in non-ciliated cells in the ED (Hess et al. 1997a; Lee et al. 2000). Together, these observations indicate that a functional ERα is necessary not only to sustain physiological functions but also to maintain normal morphology of the testis and ED in the adult mouse.
Few researchers have examined the role of ERα in the male reproductive tract during the postnatal developmental period. A significant increase in testis weight of the αERKO mouse has been seen between 30 and 80 days of age, followed by a transient decrease after 100 days of age (Hess et al. 1997a). Eddy et al. (1996) have reported that abnormal morphology, namely a dilation of STs, in the αERKO testis becomes apparent at 20 days of age after birth. Our recent research has revealed differential gene and protein expression of ion transporters in the ED of αERKO mice during postnatal development (Lee et al. 2008). Bartlett et al. (2001) also reported abnormal development of the gubernacula in the male reproductive tract of αERKO mice during early postnatal development. However, a detailed morphological examination of the testis and ED of the αERKO mouse during the postnatal period has not yet been conducted.
As seen in the adult αERKO mouse, abnormal morphologies of the testis and ED result in aberrant physiological functions or vice versa (Hess et al. 1997a; Lee et al. 2000, 2001). These findings suggest a strong correlation between morphology and the physiological roles of these tissues. Based on the findings from our previous and recent studies (Hess et al. 1997a; Lee et al. 2000, 2001, 2008), the present study was designed for morphological comparison of the testis and ED between wild-type (WT) and αERKO mice at various postnatal ages (10, 18, 35 and 60 days of age). Results from the current study indicate that a lack of functional ERα results in significant morphological abnormalities in the testis and ED of mouse during the postnatal period, earlier than previously reported (Eddy et al. 1996).
Materials and methods
Animals and tissue preparation
Homozygous αERKO and WT sibling (C57BL65/129SVJ) male mice were obtained from a resident breeding colony maintained at the University of Illinois. The animals were housed under controlled conditions and given ad libitum food (TekLad mouse chow; Harlan, Madison, WI, USA) and water. Four experimental groups consisting of both WT and αERKO mice were used at the following ages: (1) 10 days of age, WT (n = 6) and αERKO (n = 5); (2) 18 days of age, WT (n = 6) and αERKO (n = 6); and (3) 35 days of age, WT (n = 4) and αERKO (n = 5), and 60 days of age, WT (n = 5) and αERKO (n = 5). Mice were killed by cervical dislocation and the male reproductive tracts were rapidly isolated and fixed in 10% neutral-buffered formalin (pH 7.0) solution for 24 h. After fixation, the testes and ED were separated from the epididymis. The tissues were dehydrated, cleared and infiltrated with paraffin using a vacuum infiltration processor (Tissue-Tek VIP, Sakura Finetek USA Inc., Torrance, CA, USA). The tissues were then embedded in paraffin and sections were cut at 5 µm thickness. After rehydration in a series of ethanol, the sections were stained with Mayer's hematoxylin (Sigma-Aldrich Corp., St Louis, MO, USA) and Eosin Y (Sigma-Aldrich Corp.), followed by dehydration in ethanol and mounting. The histological analysis was evaluated with digitized images captured with an Olympus-MagnaFire camera (Olympus America, Melville, NY, USA) using MagnaFire Camera Imaging and Control version 1.1 software (Optronics, Goleta, CA, USA). The photographic images were processed in PhotoShop software (Adobe Systems, San Jose, CA, USA). This experiment was approved by the Institutional Animal Care and Use Committee of the University of Illinois.
Histological analysis
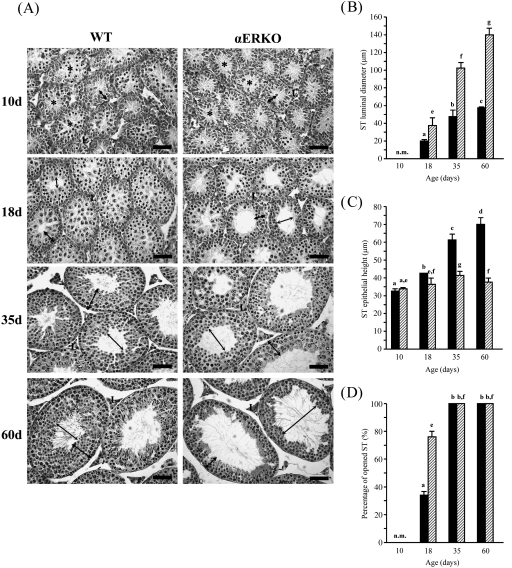
The digital images were analysed using NIH Image software, Image J (public domain). In the testes, each RT area was measured five times and averaged. For ST epithelial height, a total of 30 STs were randomly selected per testis and five regions per ST were selected to obtain a mean of epithelial height of the ST. Because all STs of WT and αERKO mice at 10 days of age and most STs of WT mice at 18 days of age had no apparent lumen formed (closed ST), the ST epithelial heights of these experimental groups were determined by measuring the distance from the center to the base of the ST (Fig. 2A). When there was an obvious lumen present in an ST (open ST, Fig. 2B), the distance from the tip to the basement membrane of the Sertoli cell was measured to obtain the ST epithelial height. The luminal diameter of an ST (if there was a lumen present) was determined by measuring and averaging three distinct distances from one side to the other at the tip of the Sertoli cell in the cross-sectional area. The luminal diameters of 20 randomly selected STs per animal were measured to obtain a mean. In addition, a total of 60 STs per mouse were counted to obtain a percentage of open STs in the testis.
Fig. 2.
Postnatal changes in the seminiferous tubules (STs) in the testes from wild-type (WT) and estrogen receptor α knockout (αERKO) mice. (A) Notable morphological differences appear at 18 days of age, characterized by premature lumen formation, thin epithelial layer and dilated lumen. These morphological abnormalities become more evident at 35 and 60 days of age. L, Leydig cell; thin arrow, luminal diameter; thick arrow, epithelial height of STs. Bar = 50 µm. (B) Changes in luminal diameter, (C) epithelial height of STs and (D) percentage of STs opened during postnatal development. Asterisks indicate signs of tubular lumen formation in the testes of WT and αERKO mice at 10 days of age. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
The proximal (close to the testis) and distal (close to the initial segment) EDs were recognized by their histological location and morphology in the male reproductive tract. The middle region (conus) of the ED was excluded from histological evaluation in the present study. The degree of dilation of the proximal ED lumen was determined by measuring the widest distance from one side to the other at the tip of the epithelia. The diameters of four to six ductules per mouse were measured three times and averaged to give a mean. Epithelial cell height was determined by measuring the distance from the basal membrane to the apex of non-ciliated cells. A total of 30 non-cilated cells per mouse from a representative tissue section were measured to obtain a mean. To determine nuclear height, linear measurements of non-ciliated cell nuclei were made from the base to the apex in cells at the center of the nucleus. A mean value of nuclear height per mouse was obtained from the measurements of 30 non-ciliated cells. Values of luminal diameter, epithelial cell height and nuclear height of the distal ED were achieved as described above.
Statistical analysis and data representation
Data are reported as means ± S.D. Comparison of means between WT and αERKO mice at the same age was made for each end-point. In addition, mean differences among WT or αERKO mice at different ages were evaluated. Differences among experimental groups for each end-point were analysed using two-way anova, followed by Tukey's HSD posthoc test. In all cases, results were considered significant if P < 0.05.
Results
Changes in rete testis areas of wild-type and estrogen receptor α knockout mice during postnatal development
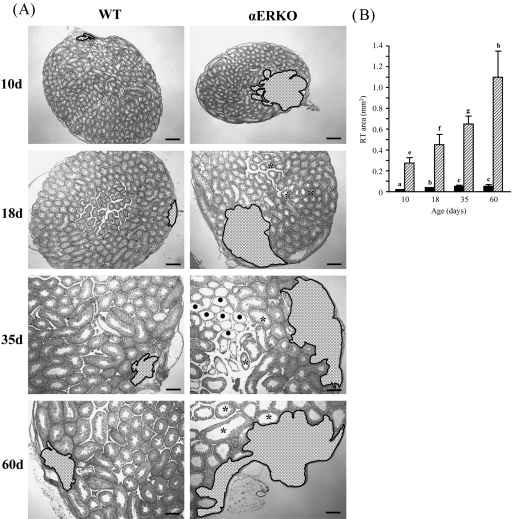
The changes in RT areas in the testes of postnatally developing WT and αERKO mice are shown in Figure 1. In WT mice, the RT area was relatively very small (about 0.0113 mm2) at 10 days of age (Fig. 1A,B). However, compared with 10 days of age, the RT areas were significantly increased at 18 and 35 days of age by two- and four-fold, respectively (Fig. 1A,B). No significant change of RT area was observed between 35 and 60 days of age (Fig. 1B). Interestingly, the RT of αERKO mice at 10 days of age were greatly dilated, about 23-fold greater than those of WT mice (Fig. 1A,B). At 18 days of age, the RT area in αERKO mice was significantly increased by 0.68-fold compared with that at 10 days of age (Fig. 1A,B). Further significant extensions of RT areas were found at 35 and 60 days of age (1.4- and 3-fold, respectively) compared with that at 10 days of age (Fig. 1A,B). Degenerative STs, characterized by the absence of an ST epithelial layer, were frequently observed in the testis of the αERKO mouse at 35 days of age (Fig. 1A). In addition, the RTs in αERKO mice were significantly dilated compared with the RTs in WT mice at all ages (Fig. 1B).
Fig. 1.
Representative light photographs of the testes from wild-type (WT) and estrogen receptor α knockout (αERKO) mice during postnatal development. (A) Note that the rete testis (shaded area,  ) of an αERKO mouse at 10 days of age is extensively dilated and abnormally dilated seminiferous tubules (asterisks) are first seen in the testis of αERKO mice at 18 days of age. Degenerative seminiferous tubules (
) of an αERKO mouse at 10 days of age is extensively dilated and abnormally dilated seminiferous tubules (asterisks) are first seen in the testis of αERKO mice at 18 days of age. Degenerative seminiferous tubules ( ) were frequently found in the testis of αERKO mice at 35 days of age. Bar = 100 µm. (B) Changes of rete testis (RT) area during postnatal development. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
) were frequently found in the testis of αERKO mice at 35 days of age. Bar = 100 µm. (B) Changes of rete testis (RT) area during postnatal development. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
Changes in luminal diameter and epithelial height of seminiferous tubules and percentage of opened seminiferous tubules in the testes
The morphological changes in the STs of WT and αERKO mice during postnatal development are shown in Figure 2. No visible morphological difference in the ST between the WT and αERKO mice was observed at 10 days of age (Fig. 2A). There was no significant difference in the luminal diameter and epithelial height of ST at this age (Fig. 2B,C). None of the STs had a clear lumen in the testes of both the WT and αERKO mice at 10 days of age, even though there was a sign of lumen formation (Fig. 2A,D, asterisks). However, morphological changes became visible in the testis of the αERKO mouse at 18 days of age (Fig. 2A).The luminal diameter of open STs in the αERKO mouse was significantly greater than that in the WT mouse (Fig. 2B), whereas the epithelial height in the αERKO mouse was significantly less than that in the WT mouse (Fig. 2C). In addition, a significant increase in frequency of open STs was found in the testis of the αERKO mouse at 18 days of age (Fig. 2D). At 35 days of age, the ST lumen was formed in all STs in WT and αERKO mice (Fig. 2A,D). The luminal diameter and epithelial height of STs in WT and αERKO mice at 35 days of age were significantly increased compared with those at 18 days of age (Fig. 2B,C). However, the degrees of these morphological changes were more extensive in the αERKO than in the WT mouse (Fig. 2B,C). At 60 days of age, the lumen of the ST was significantly extended compared with that of the WT and αERKO mice at 35 days of age (Fig. 2A,B). Interestingly, the epithelium of the ST in the αERKO mouse became significantly thinner, in contrast to the WT mouse that had thicker epithelia than at 35 days of age (Fig. 2C).
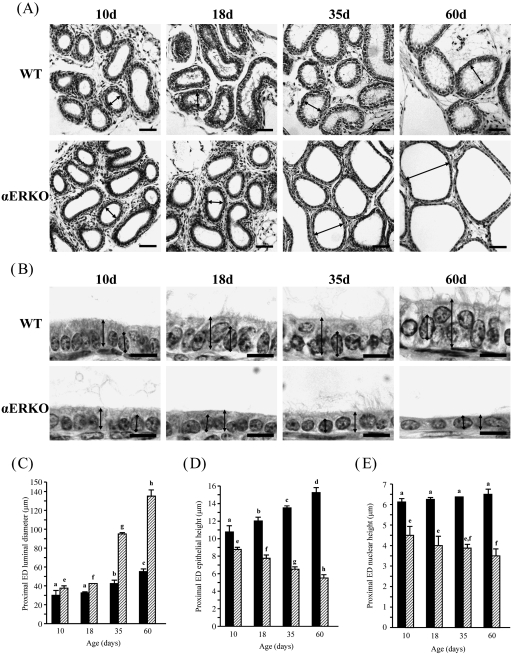
Changes in epithelial height, luminal diameter and nuclear height in the proximal efferent ductules
The proximal ED of the WT and αERKO mice underwent morphological changes for postnatal development (Fig. 3). The lumen of the ED was formed in the WT and αERKO mice at 10 days of age (Fig. 3A). In the WT mouse, the luminal diameter was not changed at 18 days of age, followed by significant increases at 35 and 60 days of age (Fig. 3A,C). However, a significant change in the luminal diameter of the ED in the αERKO mouse was detected at 18 days of age (Fig. 3A,C). A tremendous extension in the luminal diameter of the ED in the αERKO mouse was observed at 35 and 60 days of age (Fig. 3A,C). Compared with the WT mouse, the lumen of the ED in the αERKO mouse was dilated two- and three-fold at 35 and 60 days of age, respectively (Fig. 3A,C). The epithelial height of the αERKO mouse was less than that of the WT mouse at all ages (Fig. 3B,D). The epithelium of the proximal ED in the WT mouse became significantly thicker with age, whereas the epithelium in the αERKO mouse became thinner with age (Fig. 3B,D). There was no significant change in the nuclear height in the proximal ED of the WT mouse (Fig. 3B,E). However, a significant decrease in nuclear height was found in the αERKO mouse at 60 days of age compared with 10 days of age (Fig. 3B,E).
Fig. 3.
Morphological changes in the proximal efferent ductules (EDs) of wild-type (WT) and estrogen receptor α knockout (αERKO) mice during postnatal development. (A) At low magnification, dilation of the ED lumen (arrow) in αERKO is notable from 10 days of age and is greatly expanded with age. Bar = 50 µm. (B) At high magnification, note a significant increase of epithelial height ( ) in the WT mouse and significant decreases of epithelial (
) in the WT mouse and significant decreases of epithelial ( ) and nuclear (
) and nuclear ( ) heights in the αERKO mouse with age. Bar = 10 µm. (C) Change of luminal diameter, (D) change of epithelial height and (E) change of nuclear height. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
) heights in the αERKO mouse with age. Bar = 10 µm. (C) Change of luminal diameter, (D) change of epithelial height and (E) change of nuclear height. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
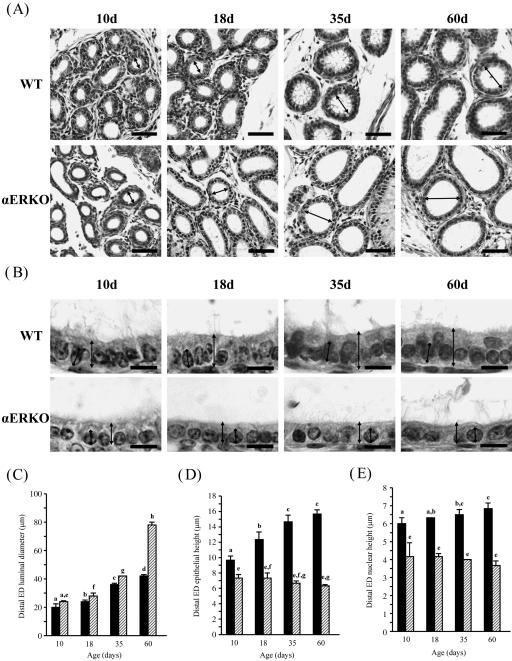
Changes in epithelial height, luminal diameter and nuclear height in the distal efferent ductules
Morphological change was also observed in the distal ED of WT and αERKO mice during postnatal development (Fig. 4). The luminal diameter of the distal ED in WT and αERKO mice was significantly increased with age (Fig. 4A,C). However, unlike in the proximal ED, a large increase of the luminal diameter of the αERKO mouse was found at 60 days of age, not at 35 days of age (Fig. 4C). The epithelial height was significantly greater in the WT mouse than in the αERKO mouse at all ages (Fig. 4B,D). In the WT mouse, the epithelial height was significantly increased until 35 days of age and then remained at a steady level at 60 days of age (Fig. 4B,D). In contrast, the epithelial height of the αERKO mouse remained unchanged at all experimental ages (Fig. 4B,D). Interestingly, compared with 10 days of age, the nuclear height in the distal ED of the WT mouse was significantly increased at 35 and 60 days of age, whereas there was no significant change in the nuclear height in the αERKO mouse throughout postnatal development (Fig. 4B,E).
Fig. 4.
Developmental changes in the distal efferent ductules (EDs) of wild-type (WT) and estrogen receptor α knockout (αERKO) mice. (A) At low magnification, dilation of the ED lumen ( ) in αERKO is notable from 18 days of age and is greatly expanded with age. Bar = 50 µm. (B) At high magnification, the epithelium (
) in αERKO is notable from 18 days of age and is greatly expanded with age. Bar = 50 µm. (B) At high magnification, the epithelium ( ) or nucleus (
) or nucleus ( ) in the WT mouse becomes thicker or heightened, respectively, with age, whereas no significant morphological change is observed in the αERKO mouse. Bar = 10 µm. (C) Change of luminal diameter, (D) change of epithelial height and (E) change of nuclear height. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
) in the WT mouse becomes thicker or heightened, respectively, with age, whereas no significant morphological change is observed in the αERKO mouse. Bar = 10 µm. (C) Change of luminal diameter, (D) change of epithelial height and (E) change of nuclear height. Values represent mean ± S.D. Different letters indicate significant differences among experimental groups (P < 0.05).
Discussion
The present study shows the developmental phenotypes of the testis and ED of the αERKO mouse compared with the age-matched WT mouse during the postnatal period. A lack of functional ERα results in a number of morphological abnormalities in these tissues from an early postnatal age, including the following: (a) premature dilation of the RT; (b) a reduction in epithelial height and an increased frequency in early lumen formation of STs in the testis; (c) an increase in luminal diameter of the proximal and distal ED; and (d) decreases in epithelial and nuclear heights of the ED.
It is technically difficult to directly collect and analyse the testicular fluid from the testis of mouse, especially at an early postnatal age. Thus, for the present study, we chose experimental age groups based on the feasible time at which the testicular fluid would be secreted from the testis. Formation of the tight junction between Sertoli cells and canalization of the STs are indicative of active testicular fluid secretion (Robaire & Hermo, 1988). The tight junction between Sertoli cells develops between 10 and 16 days of age in the mouse (Nagano & Suzuki, 1976; Gondos & Berndston, 1993). Thus, it is likely that active secretion of testicular fluid occurs after 16 days of age. In fact, others have shown the presence of Sertoli cell junctions and ST lumen at 18 days of age (Nagano & Suzuki, 1976; Gondos & Berndston, 1993). Puberty in the mouse starts at around 35 days of postnatal age. Thus, it is reasonable to consider that the testis actively secretes more testicular fluid at 35 days of age than at 18 days of age. We included an adult age group, 60 days of age, to compare the changes of developmental progression in the testes and ED between WT and αERKO mice.
A number of morphological abnormalities have been reported in the testis and ED of the adult αERKO mouse (Eddy et al. 1996; Hess et al. 1997a; Lee et al. 2000). In addition, Eddy et al. (1996) have provided a brief description of developmental defects in the male reproductive tract of αERKO. Even though a great dilation of the RT has been observed in the adult αERKO mouse (Eddy et al. 1996; Hess et al. 1997a; Lee et al. 2000), none of the studies have determined morphological alterations of the RT during the early postnatal period. The present study shows abnormally extended RT of the αERKO mouse at an early age and further expansion with age, indicating that a lack of functional ERα results in aberrant morphology of the mouse RT during postnatal development. This observation implies that ERα plays an important role in the development of the RT. In addition, it is notable that such abnormal dilation of the RT in the αERKO mouse occurs before active secretion of testicular fluid. Rivas et al. (2003) demonstrated a distension of the rat RT at prepubertal age by treatment with diethylstilbestrol in the early neonatal period. Together, these findings suggest that disruption of the ERα function leads to abnormal development of the RT after birth. However, we could not rule out the possibility that the great extension of the RT in the αERKO mouse would be a developmental consequence, due to a lack of functional ERα. Therefore, future studies might be directed to determine a role of ERα in the morphogenesis of the RT during the embryonic stage.
A previous study reported histological differences of the STs in the testes between WT and αERKO mice during the postnatal period (Eddy et al. 1996). This study reported that visible defects in STs of the αERKO mouse occurred as early as 20 days of age, followed by progressive disruption of the tubules throughout the postpubertal period (Eddy et al. 1996). Similar findings were also observed in the present study. There was no morphological difference in the STs of the αERKO mouse at 10 days of age. However, a number of morphological abnormalities in the STs of the αERKO mouse were noticed at 18 days of age, including a dilation of ST lumen, an increase of luminal diameter and premature formation of the lumen. Such abnormal morphology became more extensive with age, which is in agreement with a previous study (Eddy et al. 1996). It is also interesting to note that significant morphological differences in the STs between the WT and αERKO mice appear after 10 days of age, when the testis/blood barrier begins to form (Nagano & Suzuki, 1976; Gondos & Berndston, 1993). Unlike the RT, which showed a defective morphology at an early neonatal age, these findings clearly indicate that ERα has a role in maintaining the normal development of STs during postnatal development, probably relating to secretion of the testicular fluid. Detailed examination of the testis of the αERKO mouse at each postnatal age would provide clear evidence to explain a role of ERα in the development of STs during the neonatal period.
The present study shows defective morphology in the ED of the αERKO mouse during postnatal development, including dilation of lumen and thinning of epithelial and nuclear heights. These abnormalities in the ED were detected as early as 10 days of age and the degree of such morphological aberrants became more extensive with aging. In addition, the proximal part of the ED is somewhat more severely affected than the distal portion, especially the luminal diameter. Similar findings in the ED of the adult αERKO mouse were observed in our previous study (Lee et al. 2000). All previous studies on the ED of the αERKO mouse have focused on the consequences of a disruption of functional ERα in the adult (Hess et al. 1997a, 2000; Lee et al. 2000, 2001, Nakai et al. 2001). These earlier studies have demonstrated that treatment with a potent estrogen antagonist in adult WT animals could produce abnormal morphology and gene expression, comparable to those in the αERKO mouse. However, none of the studies could reproduce the same results as found in the αERKO mouse, suggesting the existence of a developmental effect due to the lack of ERα from conception (Hess et al. 2000; Lee et al. 2000, 2001; Nakai et al. 2001). In fact, our recent publication has demonstrated the differential expression of a number of genes in the ED of the αERKO mouse during the postnatal period (Lee et al. 2008). In addition, the present study shows a developmental morphological anomaly in the ED of the αERKO mouse, appearing even at an early postnatal age. Therefore, based on our recent and current findings, we believe that aberrant features and gene expression in the ED of the adult αERKO mouse are accumulated outcomes, due to the absence of functional ERα during development.
A number of studies have demonstrated that treatment with estrogenic compounds at a very early developmental age induces morphological abnormalities in the male reproductive tract of rodents, very similar to those detected in the present study. Exposure to diethylstilbestrol during the neonatal period results in permanent morphological alterations of many reproductive parameters in the rat testis and ED in adulthood, including decreases of tissue weight and sperm production, a dilation of RT area, increases of ED luminal and tubular diameters, a decrease of ED epithelial height, and a reduction of fertility (McKinnell et al. 2001; Williams et al. 2001; Rivas et al. 2002, 2003; Goyal et al. 2003). Furthermore, distension and thin epithelia of the STs in the testis have been observed in rodents neonatally treated with other potent estrogen agonists, i.e. ethinyl estradiol, octylphenol and estradiol benzonate (Aceitero et al. 1998; Atanassova et al. 1998; Aydoğan & Barlas 2006). In addition, Howdeshell et al. (2008) showed that ethinyl estradiol treatment during pregnancy to lactation led to permanent disruption of the male reproductive tract in the adult, including a reduction of testis and epididymal weights. Many of these studies have shown significant alterations of testosterone concentration in the serum and expression of androgen and estrogen receptors in the male reproductive tract by estrogenic compounds during the early developmental period (Atanassova et al. 1998; McKinnell et al. 2001; Williams et al. 2001; Rivas et al. 2002, 2003; Goyal et al. 2003; Howdeshell et al. 2008). Interestingly, most of these morphological abnormalities found in the testis and ED induced by diethylstilbestrol treatment were prevented by coadministration with testosterone (Rivas et al. 2003). Therefore, it is suggested that a balance between androgens and estrogens is important in the development of the male reproductive tract during the postnatal period, probably as well as during embryogenesis (McKinnell et al. 2001; Williams et al. 2001; Rivas et al. 2002, 2003). Indeed, it is known that the αERKO mouse has higher levels of serum testosterone than the WT mouse, probably due to a disruption of the negative feedback system as a consequence of a lack of functional ERα (Eddy et al. 1996; Weiss et al. 2008). Thus, we speculate that a high ratio of testosterone to estrogen in the αERKO mouse would be a factor causing morphological aberrant features in the testis and ED.
A number of recent publications have suggested the possibility that the development of the male reproductive tract may be influenced by various factors other than the classical estrogen-ERα genomic action. For example, morphological features of the testis and ED of the leucine-rich G protein-coupled receptor 4 (lgr4) gene knockout mouse resemble those of the αERKO mouse (Mendive et al. 2006). In addition, Weiss et al. (2008) have reported that the loss of the non-classical genomic action of the ERα causes morphological defects in the male reproductive tract. Unexpectedly, an inhibition of the endogenous production of estrogen in the P450 aromatase (cyp19) knockout mouse delayed the normal morphological appearance of the testis and ED until late adulthood (Robertson et al. 1999; Toda et al. 2008). Together, these observations suggest that normal development of the male reproductive tract is regulated by action of a complex of various factors, in addition to ERα.
In conclusion, we have demonstrated that a loss of functional ERα results in morphological abnormalities in the testis and ED during neonatal and prepubertal development, as well as in adulthood. However, a further examination is expected to determine if such morphological defects observed in the testis and ED of the αERKO mouse postpartum appear during embryogenesis and morphogenesis prepartum.
Acknowledgments
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST) (R01-2008-000-11920-0).
Authors’ contributions
K.-H.L. was the primary investigator who conducted the writing of the manuscript and collection of data. J.-H.P. had a major role in the statistical analysis and acquisition of data and provided advice on software-based measurement for morphological analysis. D.B. was involved in the establishment of the study concept and design and in drafting of the manuscript. D.B.L. provided experimental animals (WT and αERKO mice) for this study. J.M.B. had an important role in the guidance of the research and the critical revision of the manuscript.
References
- Aceitero J, Llanero M, Parrado R, Peña E, Lopez-Beltran A. Neonatal exposure of male rats to estradiol benzoate causes rete testis dilation and backflow impairment of spermatogenesis. Anat Rec. 1998;252:17–33. doi: 10.1002/(SICI)1097-0185(199809)252:1<17::AID-AR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Walker M, et al. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140:5364–5373. doi: 10.1210/endo.140.11.7108. [DOI] [PubMed] [Google Scholar]
- Aydoğan M, Barlas N. Effects of maternal 4-tert-octylphenol exposure on the reproductive tract of male rats at adulthood. Reprod Toxicol. 2006;22:455–460. doi: 10.1016/j.reprotox.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Bartlett JE, Washburn T, Eddy EM, Korach KS, Temelcos C, Hutson JM. Early development of the gubernaculum and cremaster sac in estrogen receptor knockout mice. Urol Res. 2001;29:163–167. doi: 10.1007/s002400100180. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones R, Hansen L. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994;79:915–928. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J Endocrinol. 1997;153:485–495. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- Free MJ, Jaffe RA. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod. 1979;20:269–278. doi: 10.1095/biolreprod20.2.269. [DOI] [PubMed] [Google Scholar]
- Ganjam VK, Amann RP. Steroid content of fluids and sperm entering and leaving the bovine epididymis, in epididymal tissue, and in accessory sex gland secretions. Endocrinology. 1976;99:1618–1630. doi: 10.1210/endo-99-6-1618. [DOI] [PubMed] [Google Scholar]
- Gondos B, Berndston WE. Postnatal and pubertal development. In. In: Russel LD, Griswold MD, editors. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993. pp. 115–154. [Google Scholar]
- Goyal HO, Robateau A, Braden TD, Williams CS, Srivastava KK, Ali K. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol Reprod. 2003;68:2081–2091. doi: 10.1095/biolreprod.102.010637. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee K-H, et al. A role for oestrogens in the male reproductive tract. Nature. 1997a;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, et al. Estrogen receptor (α & β) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997b;18:602–611. [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci. 2008;102:371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Ilio KY, Hess RA. Structure and function of the ductuli efferentes: A review. Microsc Res Tech. 1994;29:432–467. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor α has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Finnigan-Bunick C, Bahr J, Bunick D. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) α and ERβ. Biol Reprod. 2001;65:1534–1541. doi: 10.1095/biolreprod65.5.1534. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Bunick D, Lamprecht G, Choi I, Bahr JM. Differential expression of genes important to efferent ductules ion homeostasis across postnatal development in estrogen receptor-α knockout and wildtype mice. Asian-Aust J Anim Sci. 2008;21:510–522. [Google Scholar]
- McKinnell C, Atanassova N, Williams K, et al. Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. J Androl. 2001;22:323–338. [PubMed] [Google Scholar]
- Mendive F, Laurent P, Van Schoore G, Sharnes W, Pochet R, Vassart G. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol. 2006;290:421–434. doi: 10.1016/j.ydbio.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Nagano T, Suzuki F. The postnatal development of the junctional complexes of the mouse Sertoli cells as revealed by freeze-fracture. Anat Rec. 1976;185:403–418. doi: 10.1002/ar.1091850403. [DOI] [PubMed] [Google Scholar]
- Nakai M, Bouma J, Nie R, et al. Morphological analysis of endocytosis in efferent ductules of estrogen receptor-alpha knockout male mouse. Anat Rec. 2001;263:10–18. doi: 10.1002/ar.1071. [DOI] [PubMed] [Google Scholar]
- Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143:4797–4808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]
- Rivas A, McKinnell C, Fisher JS, Atanassova N, Williams K, Sharpe RM. Neonatal coadministration of testosterone with diethylstilbestrol prevents diethylstilbestrol induction of most reproductive tract abnormalities in male rats. J Androl. 2003;24:557–567. doi: 10.1002/j.1939-4640.2003.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: Structure, functions, and their regulation. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York, NY: Raven Press; 1988. pp. 999–1080. [Google Scholar]
- Robertson KM, O'Donnell L, Jones ME, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Okada T, Hayashi Y, Saibara T. Preserved tissue structure of efferent ductules in aromatase-deficient mice. J Endocrinol. 2008;199:137–146. doi: 10.1677/JOE-08-0257. [DOI] [PubMed] [Google Scholar]
- Weiss J, Bernhardt ML, Laronda MM, et al. Estrogen actions in the male reproductive system involve ERE-independent pathways. Endocrinology. 2008;149:6198–6206. doi: 10.1210/en.2008-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, McKinnell C, Saunders PT, et al. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Update. 2001;7:236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]