Abstract
Mammalian hibernation is a natural, fully reversible hypometabolic state characterized by a drastic reduction of body temperature and metabolic activity, which ensures survival to many species under adverse environmental conditions. During hibernation, many hibernators rely for energy supply almost exclusively on lipid reserves; the shift from carbohydrate to lipid metabolism implies profound rearrangement of the anabolic and catabolic pathways of energetic substrates. However, the structural counterpart of such adaptation is not known. In this study we investigated, by using immunoelectron microscopy, the fine intracellular distribution of two key enzymes involved in lipid metabolism, namely, the fatty acid synthase (FAS) and the long-chain fatty acyl-CoA synthetase (ACSL), in hepatocytes of euthermic, hibernating and arousing hazel dormice. Our results show that the two enzymes are differentially distributed in cellular compartments (cytoplasm, mitochondria and cell nuclei) of hepatocytes during euthermia. Quantitative redistribution of both enzymes among cellular compartments takes place during hibernation and arousal, in accordance with the physiological changes. Interestingly, this redistribution follows different seasonal patterns in cytoplasm, mitochondria and nuclei. In conclusion, our data represent the first quantitative morphological evidence of lipid enzyme distribution in a true hibernator throughout the year cycle, thus providing a structural framework to biochemical changes associated with the hypometabolism of hibernation.
Keywords: acyl-CoA synthetase, electron microscopy, fatty acid synthase, immunocytochemistry, liver
Introduction
Mammalian hibernation is a natural hypometabolic state characterized by a drastic reduction of body temperature, metabolic activity, heart rate and energy demand, which ensures survival to many species when the environmental conditions become adverse. This is a reversible state, as hibernators are able to leave the depressed metabolic state at any time using endogenously produced heat to rapidly restore all metabolic and physiological functions (recent reviews in Carey et al. 2003; Cannon & Nedergaard 2004; Geiser 2004).
During the hibernation season, which can last for several months and is composed of a sequence of long torpor bouts lasting for days or weeks interrupted by brief normothermic periods, many hibernators do not eat or drink, relying for energy supply almost exclusively on lipid reserves accumulated during summer and fall months, while carbohydrate utilization is drastically reduced (review in Carey et al. 2003; Cannon & Nedergaard 2004; Geiser 2004). This shift from carbohydrate to lipid metabolism implies profound rearrangements of the anabolic and catabolic pathways of these energetic substrates, such as hormone-mediated mobilization of fatty acids from the accumulation sites, increased expression of enzymes responsible for fatty acid utilization, and suppression of glycolytic activity (recent review in Dark 2005).
Most of the information about the above adaptive changes related to the preferential use of fatty acids during hibernation originated from biochemical studies, while morpho-functional data are scarce (e.g. Malatesta et al. 2001, 2002). In particular, direct quantification of the immunoreactivity for the enzymes in tissues is lacking, thereby hampering identification of possible changes in the amount/distribution of enzymes during the active and resting phases. In this study, the fine intracellular distribution of two key enzymes involved in lipid metabolism, namely, the fatty acid synthase (FAS) and the long-chain fatty acyl-CoA synthetase (ACSL), was investigated in the liver of a true hibernator, the hazel dormouse Muscardinus avellanarius.
Animal FAS is a complex multifunctional enzyme that catalyzes the synthesis of long-chain fatty acids – especially palmitate – by using acetyl-CoA as a primer, malonyl-CoA as a two-carbon donor, and NADPH as a reductant (Wakil 1989). Long-chain fatty acids are essential constituents of membrane lipids and are also important substrates for cellular energy metabolism. FAS expression has been demonstrated to be controlled by both hormones (insulin, glucagon, glucocorticoids, and thyroid hormone) and nutrients (glucose and fatty acids) (Sul & Wang 1998).
In mammals, ACSL catalyzes the esterification or activation of long-chain fatty acids (10–20-carbon chain length, saturated or unsaturated): in this initial step, which is required for all cellular long-chain fatty acids utilization, a coenzyme A (CoA) moiety is linked in an ATP-dependent reaction to the 1-carbon of a long-chain fatty acid via a thioester linkage. The acyl-CoA esters are substrates for beta-oxidation, phospholipid and triglyceride biosynthesis, and protein acylation; moreover, they are involved in many physiological processes, such as enzyme activation, vesicular trafficking, cell signalling, and modulation of transcription factors (for a recent review see Soupene & Kuypers 2008). Both enzymes are expressed in large amounts in the liver, where most of the lipid metabolism takes place.
In our study, the distribution pattern of FAS and ACSL1 was analyzed using immunoelectron microscopy over the different cellular structural components in hepatocytes from euthermic, hibernating and arousing hazel dormice, to reveal possible modifications in distribution or amount throughout the seasonal cycle.
Materials and methods
Nine hazel dormice Muscardinus avellanarius (Gliridae) were used. These dormice are quite common in Italy and we were allowed to catch a limited number of individuals for the purpose of multiple investigations, with the permission of the appropriate authorities (Regione Marche, Decreto no. 308 SCP, 19/06/1996). Wild-living animals were trapped and then maintained in the countryside in an outdoor animal house supplied with food ad libitum (seeds, fruits, nuts) and bedding material. Under such conditions, they spontaneously began to hibernate in November and the final arousal and termination of hibernation was in March. As no manipulation of the animals was allowed by law, we could monitor the dormice activity only by the sawdust method. During the hibernating period (December–February) the ambient temperature varied from −6 °C to 10 °C but, in the nest, it never fell below 0 °C. Three animals were sacrificed during hibernation (January), three during the euthermic period (June–July) and three during arousal (March). Dormant dormice, i.e. animals resting for at least two consecutive days, were taken from the cage and immediately sacrificed. Arousing animals were allowed to awake undisturbed: the nest was exposed to daylight and the dormice allowed to arouse spontaneously; when evident arousal signals (e.g. shivering) became evident, a thermistor probe was put on the abdomen and the animals were sacrificed when the temperature reached 26 °C, i.e. before the arousal process came to completion. Euthermic animals were anaesthetized with ether before being sacrificed. Immediately after sacrifice, samples of liver were fixed by immersion in 4% paraformaldehyde in 0.1 M Sörensen phosphate buffer at 4 °C for 2 h. After washing in Sörensen buffer and in phosphate-buffered saline (PBS), free aldehydes were blocked in 0.5 M NH4Cl in PBS at 4 °C for 45 min. Following washing in PBS, the specimens were dehydrated through graded concentrations of ethanol and embedded in LRWhite resin polymerized under UV light. Ultrathin sections were placed on grids coated with a Formvar-carbon layer and then processed for immunocytochemistry.
To investigate the fine distribution of two enzymes involved in lipid metabolism, liver samples were treated with a mouse monoclonal anti-FAS (BD Bioscience, San Jose, CA) or a rabbit polyclonal anti-long chain fatty acid CoA ligase 1 (one member of the ACSL family) antibody (Aviva System Biology, San Diego, CA). Sections were floated for 3 min at room temperature on normal goat serum (NGS) diluted 1 : 100 in PBS and then incubated for 17 h at 4 °C with the primary antibodies diluted with PBS containing 0.1% bovine serum albumin (Fluka, Buchs, Switzerland) and 0.05% Tween 20. After rinsing at room temperature with PBS, sections were floated on NGS, and then reacted for 20 min at room temperature with secondary 12 nm gold-conjugated antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) diluted 1 : 10 in PBS. The sections were rinsed at room temperature with PBS and water, and finally air-dried. As controls, some sections were treated by omitting the primary antibody from the incubation mixture, and then processed as described above. All the immunolabelled sections were stained with uranyl acetate and observed in a Philips Morgagni transmission electron microscope equipped with a Megaview II camera for digital image acquisition.
It should be underlined that post-embedding ultrastructural immunocytochemistry allows only the detection of the molecular epitopes occurring on the section surface, thus leading to underestimation of the presence of the target molecules. As a further consequence, no absolute quantification can be performed by immunoelectron microscopy, only comparisons of different samples.
To get a quantitative estimate of the immunopositivity, the labelling density over some cellular compartments (cytoplasm, mitochondria and nuclei) was evaluated; only sections from the same immunolabelling experiment were scored. The surface area of 30–35 cytoplasmic regions (corresponding to 200 µm2), 20 nuclei and 50 mitochondria per animal was measured at a fixed magnification (×18 000) by using a computerized image analysis system (AnalySIS Image processing, Soft Imaging System GmbH, Muenster, Germany). Background evaluation was carried out on tissue of control samples. The gold grains present over each compartment were counted and the labelling density was expressed as the number of gold grains per square micrometer.
Statistical analysis was performed using the software statistica for Windows. For each analyzed variable, the Kolmogorov-Smirnov two-sample test was performed to verify the hypothesis of identical distribution of enzymes in the subcellular compartments among animals of each group. For each variable the data obtained in animals belonging to the same experimental group, i.e. euthermia, hibernation and arousal, were pooled and the mean ± SE values were calculated for each group. The normal distribution of the density data was verified by the F-test. Statistical analysis of the results was performed with the non-parametric two-way anovatest to evaluate the factor ‘cellular compartment’ (cytoplasm, mitochondria, nucleus) and ‘seasonal phase’ (euthermia, hibernation, arousal) as well as the interaction term between the two factors for each enzyme. Furthermore, to determine which pairs of samples tended to differ, Tukey's HSD test for multiple comparisons was used (post-hoc test). The Mann–Whitney U-test was used to compare background values to the weak labelling found in mitochondria and nuclei of samples treated with the anti-FAS antibody. Statistical significance was assumed at P ≤ 0.05.
Results
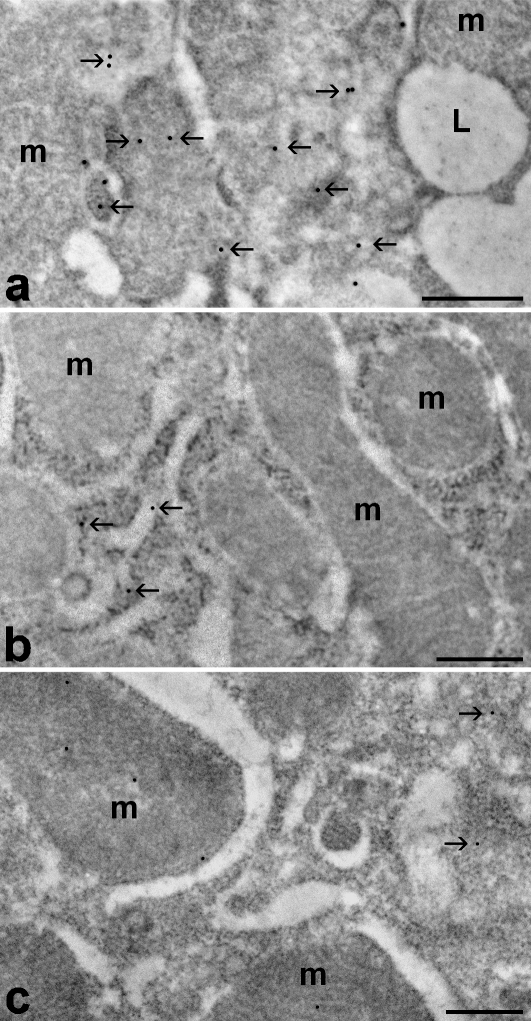
Electron microscopic analysis of immunolabelled hepatocytes revealed that the intracellular distribution of FAS underwent some modifications during the seasonal cycle (Fig. 1). During euthermia and hibernation the labelling was almost exclusively distributed in the cytoplasm, in particular in the cytosol, whereas mitochondria and nuclei were virtually devoid of signal. Upon arousal, the labelling persisted in the cytoplasm, but it appeared also inside mitochondria.
Fig. 1.
Hepatocytes from euthermic (a), hibernating (b) and arousing (c) hazel dormice; immunolabelling with anti-FAS antibody. The enzyme is distributed in the cytosol (arrows) in all three seasonal phases considered; however, in hibernation and arousal the signal is much lower than in euthermia. Mitochondria (m) are devoid of gold grains in euthermia and hibernation, whereas in arousing animals they show a weak but significant labelling. L, lipid droplet. The gold grain contrast was digitally enhanced by using Adobe photoshop. Bars: 0.5 µm.
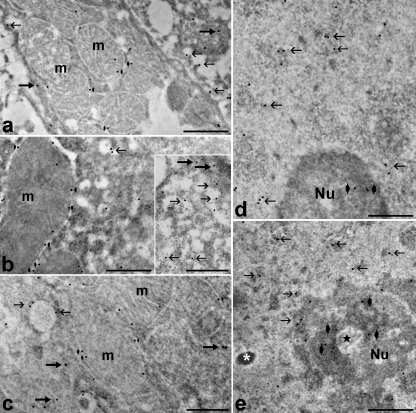
ACSL1 showed a similar intracellular distribution pattern in all the seasonal phases considered (Fig. 2). The labelling occurred in all compartments considered: in the cytoplasm, gold grains were preferentially associated with the smooth endoplasmic reticulum, although some signal was distributed also in the cytosolic regions; in the mitochondria the labelling mainly occurred close to the outer membrane; in the cell nuclei the labelling was mostly associated to perichromatin fibrils and, to a lesser extent, to the dense fibrillar component of the nucleolus.
Fig. 2.
Hepatocytes from euthermic (a,d), hibernating (b,e) and arousing (c) hazel dormice; immunolabelling with anti-ACSL1 antibody. In the cytoplasm (a–c) the labelling occurs in association with smooth endoplasmic reticulum (arrows) as well as free in the cytosol (thick arrows) in all animals; the mitochondria (m) show a labelling preferentially located at their periphery, probably in association with the outer membrane (small arrowheads). In the cell nuclei (d,e) gold grains are mainly associated with perichromatin fibrils (arrows) and, to a lesser amount, with the dense fibrillar component (arrowheads) of the nucleolus (Nu). Amorphous bodies (asterisk) and coiled bodies (star), nuclear structural constituents which accumulate during hibernation, are devoid of signal. The gold grain contrast was digitally enhanced by using Adobe photoshop. Bars: 0.5 µm.
Background values were negligible for both FAS (0.11 ± 0.03 gold grains/µm2) and ACSL1 (0.07 ± 0.02 gold grains/µm2).
The Kolmogorov–Smirnov test demonstrated identical distribution of enzymes in the subcellular compartments among animals of each group (P > 0.10 for each comparison). The F-test showed that the density data were not normally distributed (P < 0.05).
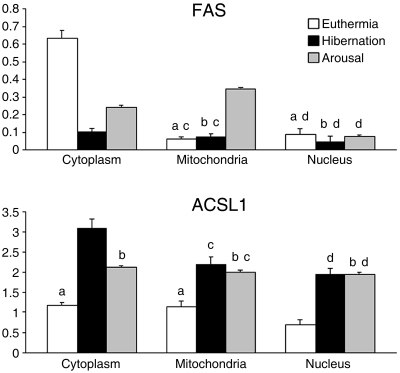
The mean ± SE values of the labelling density obtained on the different cellular compartments of hepatocytes from euthermic, hibernating and arousing dormice are shown in Fig. 3. The two-way anovatest revealed significant differences for both enzymes concerning the factors ‘cellular compartment’, ‘seasonal phase’ and the interaction term ‘cellular compartment-seasonal phase’ (Table 1).
Fig. 3.
Mean ± SE of labelling density (gold grains/µm2) on different subcellular compartments of hepatocytes from euthermic, hibernating and arousing hazel dormice with antibodies directed against FAS and ACSL1. The density values of anti-FAS labelling in mitochondria from euthermic and hibernating dormice were not significantly different from background (P = 0.702 and P = 0.779, respectively). Similarly, anti-FAS labelling in cell nuclei showed values similar to background in all seasonal phases (P = 0.861 in euthermia; P = 0.385 in hibernation; P = 0.231 upon arousal). Tukey's HSD test for multiple comparisons applied to the ‘cellular compartment’ (cytoplasm, mitochondria, nucleus) factor revealed significant differences for both FAS (euthermia: P < 0.001 for cytoplasm vs. mitochondria and cytoplasm vs. nucleus; hibernation: P = 0.045 for cytoplasm vs. mitochondria and P = 0.006 for cytoplasm vs. nucleus; arousal: P = 0.002 for cytoplasm vs. mitochondria, P = 0.016 for cytoplasm vs. nucleus and P < 0.001 for mitochondria vs. nucleus) and ACSL1 (euthermia: P = 0.041 for cytoplasm vs. nucleus and P = 0.036 for mitochondria vs. nucleus; hibernation: P < 0.001 for all comparisons). The values that do not differ significantly from each other are indicated by the same letter (a,b). Tukey's HSD test applied to the ‘seasonal phase’ (euthermia, hibernation, arousal) factor revealed significant differences for both FAS (cytoplasm: P < 0.001 for euthermia vs. hibernation and arousal, and P = 0.005 for hibernation vs. arousal; mitochondria:P < 0.001 for arousal vs. euthermia and hibernation) and ACSL1 (cytoplasm: P < 0.001 for euthermia vs. hibernation, P = 0.002 for euthermia vs. arousal and P = 0.008 for hibernation vs. arousal; mitochondria and nucleus: P < 0.001 for euthermia vs. hibernation and arousal). The values that do not differ significantly from each other are indicated by the same letter (c,d).
Table 1.
Two-way anovatest of the immunolabelling densities (gold grains/µm2) of FAS and ACSL1 in dormouse hepatocytes: cellular compartment (cytoplasm, mitochondria, nucleus), seasonal phase (euthermia, hibernation, arousal) and interaction term (cellular compartment – seasonal phase)
| FAS | |||
| Cellular compartment | df = 2 | F = 8.147 | P < 0.001 |
| Seasonal phase | df = 2 | F = 5.761 | P = 0.003 |
| Interaction term | df = 4 | F = 5.054 | P < 0.001 |
| Error | df = 225 | ||
| ACSL1 | |||
| Cellular compartment | df = 2 | F = 5.098 | P = 0.006 |
| Seasonal phase | df = 2 | F = 23.726 | P < 0.001 |
| Interaction term | df = 4 | F = 3.134 | P = 0.041 |
| Error | df = 252 | ||
The post-hoc test for FAS labelling in the cellular compartment (Fig. 3) demonstrated that, in euthermia and hibernation, the cytoplasm showed a labelling significantly higher than the other compartments (P < 0.001 for cytoplasm vs. mitochondria and cytoplasm vs. nucleus in euthermia; P = 0.045 for cytoplasm vs. mitochondria and P = 0.006 for cytoplasm vs. nucleus in hibernation), whereas no difference was found between mitochondria and nucleus (P = 0.304 in euthermia and P = 0.223 in hibernation). Upon arousal, the labelling distribution showed significant differences among all cellular compartments (P = 0.002 for cytoplasm vs. mitochondria, P = 0.016 for cytoplasm vs. nucleus, P < 0.001 for mitochondria vs. nucleus). In euthermia, anti-ACSL 1 labelling (Fig. 3) was similar in cytoplasm and mitochondria (P = 0.520), but significantly lower in nucleus (P = 0.041 for cytoplasm vs. nucleus and P = 0.036 for mitochondria vs. nucleus); in hibernation, significant differences were found among all compartments (P < 0.001 for all comparisons), whereas, upon arousal, no significant difference was found (P = 0.897 for cytoplasm vs. mitochondria, P = 0.614 for cytoplasm vs. nucleus, P = 0.386 for mitochondria vs. nucleus).
The post-hoc test for FAS labelling in seasonal phase (Fig. 3) showed that in the cytoplasm the anti-FAS labelling density significantly changed among euthermia, hibernation and arousal (P < 0.001 for euthermia vs. hibernation and arousal, P = 0.005 for hibernation vs. arousal). In the mitochondria, the labelling density showed similar values in euthermia and hibernation (P = 0.814) but significantly increased upon arousal (P < 0.001 for arousal vs. euthermia and hibernation). In the nucleus no difference was found among seasonal phases (P = 0.081 for euthermia vs. hibernation, P = 0.164 for euthermia vs. arousal, P = 0.541 for hibernation vs. arousal). As to the anti-ACSL1 antibody (Fig. 3), the cytoplasmic labelling showed significant differences in seasonal phases (P < 0.001 for euthermia vs. hibernation, P = 0.002 for euthermia vs. arousal, P = 0.008 for hibernation vs. arousal); the mitochondria and nucleus showed similar values in hibernation and arousal (P = 0.655 and P = 0.552, respectively), whereas euthermic values were significantly lower (P < 0.001 for euthermia vs. hibernation and arousal in both mitochondria and nucleus).
Discussion
Our study shows that the fine subcellular distribution of two key enzymes involved in lipid biosynthesis (FAS) and catabolism (ACSL1) changes with season and physiological state in dormice.
The intracellular distribution pattern of FAS in euthermia is characterized by accumulation of most enzyme in the cytoplasm and shows some modifications in hibernation, i.e. a drastic decrease in cytoplasmic density. However, the most striking change takes place upon arousal, with many FAS molecules appearing inside mitochondria (the labelling density becomes higher in mitochondria than in cytoplasm). In euthermic summer dormice the anti-FAS labelling is almost exclusively located in the cytoplasm, in particular in the cytosol, according to previous biochemical data (Bhaumik et al. 2005). This finding is also supported by previous light microscopy analyses demonstrating the cytoplasmic distribution of this enzyme in several tissues, including the liver (Kusakabe et al. 2000). During hibernation, the intracellular distribution of FAS molecules is retained, although the cytoplasmic FAS significantly decreases, probably due to prevalence of the fatty acid degradation processes over the synthetic ones in this physiological phase (Dark 2005). This finding confirms and extends previous biochemical studies showing a marked reduction of FAS activity during hibernation (Mostafa et al. 1993; Wang et al. 1997). Moreover, it is known that FAS expression is transcriptionally regulated by several factors such as hormones and nutrients (Sul & Wang 1998). In particular, in the liver, FAS is negatively regulated by polyunsaturated fatty acids (review in Duplus & Forest 2002); accordingly, during hibernation, polyunsaturated fatty acids are preferentially retained in the organism as they positively affect the depth and duration of torpor (Geiser et al. 1994). Moreover, food intake as well as insulin generally stimulates FAS expression, and the prolonged starvation together with the low levels of this hormone during hibernation (Castex & Hoo-Paris 1987) probably contributes to the decrease of this enzyme in the hepatocytes. Upon arousal, the amount of cytoplasmic FAS increases significantly, but still remains below the euthermic values. Arousals are metabolically expensive events requiring the rapid and massive mobilization of many substrates (glucose, ketones and fatty acids) for energy production; therefore the catabolic activity remains prevalent on the anabolic one. It is likely that it is only after complete re-warming and resumption of feeding that new FAS molecules are synthesized to fully restore lipid biosynthesis. Interestingly, in arousing dormice the density of FAS molecules found in mitochondria is higher than in cytoplasm. The occurrence of enzymes responsible for the lipid biosynthesis is common in both prokaryote and plant mitochondria, whereas only two components of the FAS complex have been identified so far in animal mitochondria (Zhang et al. 2003). Although the significance of the labelling found in the mitochondria of arousing dormice remains unclear, it can not be excluded that some components of the enzymatic complex FAS, able to react with the antibody used in this study, would be overexpressed upon arousal.
ACSL1 appeared to be evenly distributed in cellular compartments in euthermia undergoing marked redistribution in hibernation, with most enzyme accumulating in the cytoplasm; on the other hand, such changes disappeared upon arousal, mainly due to enzyme depletion from cytoplasm and mitochondria, whereas no change occurred in nucleus.
During euthermia, many ACSL1 molecules occur in the cytoplasm, both in the cytosol and in association with the smooth endoplasmic reticulum; similar amounts of anti-ACSL1 labelling have been detected on mitochondria, especially close to the outer membranes, whereas cell nuclei show significant but weaker density signal. These quantitative ultrastructural data are in agreement with recent biochemical studies showing the presence of the enzyme in the cytosol as well as in the endoplasmic reticulum, mitochondria and cell nuclei of hepatocytes (Soupene & Kuypers 2008). The localization of ACSL1 on cell membranes would allow the enzyme to act on non-polar hydrophobic substrates (fatty acids) and to generate water-soluble products (acyl-CoAs) at the water–lipid interface. Moreover, ACSL1 in the outer membrane of mitochondria would play a basic role in fatty acid import (review in Kerner & Hoppel 2000; Soupene & Kuypers 2008), necessary to their beta-oxidation for ATP production. Finally, nuclear ACSL1, which has been already detected biochemically in rat liver (Ves-Losada & Brenner 1996), would be involved in the regulation of RNA transcription, as acyl-CoA esters are implicated as ligands for transcription factors (Raman et al. 1997; Hertz et al. 1998). Nuclear ACSL1 is mostly distributed on perichromatin fibrils, the structural counterpart of pre-mRNA transcription and early splicing (Fakan 2004), and in the nucleolar dense fibrillar component, where pre-rRNA is synthesized and processed (Biggiogera et al. 2001). During hibernation, a marked increase in ACSL1 occurs in all the cellular compartments considered, the cytoplasm showing the highest immunopositivity; this is probably related to the increased utilization of lipids as energy source. Accordingly, during hibernation the smooth endoplasmic reticulum increases (Zancanaro et al. 1997, 1999; Malatesta et al. 1998, 2002) and mitochondria undergo structural modifications related to the preferential utilization of fatty acids as energetic substrate (Malatesta et al. 2001). As for the nuclear ACSL1, it is possible that during hibernation higher amounts of this enzyme would be required for the regulation of gene expression (Black et al. 2000); alternatively, the enzyme could accumulate in cell nuclei to be used during arousal to efficiently restore the functional activities, in the frame of a far-reaching program for the modulation of RNA transcription involving several nuclear factors (Malatesta et al. 2008). Upon arousal, ACSL1 levels become similar in the three cellular compartments considered: they significantly decrease in the cytoplasm and mitochondria, while remaining higher than in euthermia; conversely, in the nucleus the amount of ACSL1 maintains the hibernation values. It is known that, during early arousal, carbohydrates represent the main energy source; nevertheless, the bulk of arousal remains dependent upon fatty acids oxidation (Dark 2005), thus explaining the persistence of high ACSL1 levels. Moreover, fatty acids, especially the long-chain ones, play an important role as substrates for thermogenesis upon arousal (Carneheim et al. 1989; Brustovetsky et al. 1992), acting as natural uncouplers of oxidative phosphorylation in mitochondria (Wojtczak & Schonfeld 1993). Interestingly, while the amount of cytoplasmic and mitochondrial ACSL1 rapidly changes following modifications in metabolic substrate utilization through the seasonal cycle, in the nucleus large amounts of ACSL 1 molecules are retained upon arousal, supporting the hypothesis of an involvement in the restoration of nuclear activity.
In conclusion, this study provides the first quantitative morphological evidence that two key enzymes of lipid metabolism are differentially distributed in cellular compartments of hepatocytes. Moreover, it has been shown that quantitative redistribution of both enzymes takes place among cellular compartments throughout with euthermia/hibernation cycle, following different seasonal patterns in cytoplasm, mitochondria and nucleus. These data thereby provide a structural framework for the biochemical changes associated with the hypometabolism of hibernation. The interaction of the two factors (cellular compartment and metabolic state) is significant for both enzymes, indicating that a shift in enzyme location is instrumental for the physiological effect. Further, the amounts of FAS and ACSL1 in the cytoplasm are inversely regulated during the year cycle, being higher for FAS and lower for ACSL1 in euthermia.
Acknowledgments
A.S. participated in sample processing and carried out electron microscopy analyses and morphometrical evaluations. M.M. conceived the study, participated in sample processing and drafted the manuscript. C.Z. participated in the design and coordination of the study and helped to draft the manuscript.
References
- Bhaumik P, Koski MK, Glumoff T, Hiltunen JK, Wierenga RK. Structural biology of the thioester-dependent degradation and synthesis of fatty acids. Curr Opin Struct Biol. 2005;15:621–628. doi: 10.1016/j.sbi.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Biggiogera M, Malatesta M, Abolhassani-Dadras S, Amalric F, Rothblum LI, Fakan S. Revealing the unseen: the organizer region of the nucleolus. J Cell Sci. 2001;114:3199–3205. doi: 10.1242/jcs.114.17.3199. [DOI] [PubMed] [Google Scholar]
- Black PN, Faergeman NJ, DiRusso CC. Long-chain acyl-CoA-dependent regulation of gene expression in bacteria, yeast and mammals. J Nutr. 2000;130:305S–309S. doi: 10.1093/jn/130.2.305S. [DOI] [PubMed] [Google Scholar]
- Brustovetsky NN, Egorova MV, Gnutov DYu, Gogvadze VC, Mokhova EN, Skulachev VP. Thermoregulatory, carboxyatractylate-sensitive uncoupling in heart and skeletal muscle mitochondria of the ground squirrel correlates with the level of free fatty acids. FEBS Lett. 1992;305:15–17. doi: 10.1016/0014-5793(92)80645-w. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Carneheim C, Cannon B, Nedergaard J. Rare fatty acids in brown fat are substrates for thermogenesis during arousal from hibernation. Am J Physiol. 1989;256:R146–154. doi: 10.1152/ajpregu.1989.256.1.R146. [DOI] [PubMed] [Google Scholar]
- Castex C, Hoo-Paris R. Regulation of endocrine pancreas secretions (insulin and glucagon) during the periodic lethargy-waking cycle of the hibernating mammal. Diabete Metab. 1987;13:176–181. [PubMed] [Google Scholar]
- Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–497. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- Duplus E, Forest C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem Pharmacol. 2002;64:893–901. doi: 10.1016/s0006-2952(02)01157-7. [DOI] [PubMed] [Google Scholar]
- Fakan S. Ultrastructural cytochemical analyses of nuclear functional architecture. Eur J Histochem. 2004;48:5–14. [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Geiser F, McAllan BM, Kenagy GJ. The degree of dietary fatty acid unsaturation affects torpor patterns and lipid composition of a hibernator. J Comp Physiol. 1994;164:299–305. doi: 10.1007/BF00346446. [DOI] [PubMed] [Google Scholar]
- Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4[alpha] Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486:1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Maeda M, Hoshi N, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48:613–622. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Zancanaro C, Marcheggiani F, et al. Ultrastructural, morphometrical and immunocytochemical analyses of the exocrine pancreas in a hibernating dormouse. Cell Tissue Res. 1998;292:531–541. doi: 10.1007/s004410051082. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Battistelli S, Rocchi MBL, Zancanaro C, Fakan S, Gazzanelli G. Fine structural modifications of liver, pancreas and brown adipose tissue mitochondria from hibernating, arousing and euthermic dormice. Cell Biol Int. 2001;25:131–138. doi: 10.1006/cbir.2000.0575. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Zancanaro C, Baldelli B, Gazzanelli G. Quantitative ultrastructural changes of hepatocyte constituents in euthermic, hibernating and arousing dormice (Muscardinus avellanarius) Tissue Cell. 2002;34:397–405. doi: 10.1016/s0040816602000745. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Biggiogera M, Baldelli B, Barabino SM, Martin TE, Zancanaro C. Hibernation as a far-reaching program for the modulation of RNA transcription. Microsc Res Tech. 2008;71:564–572. doi: 10.1002/jemt.20587. [DOI] [PubMed] [Google Scholar]
- Mostafa N, Everett DC, Chou SC, Kong PA, Florant GL, Coleman RA. Seasonal changes in critical enzymes of lipogenesis and triacylglycerol synthesis in the marmot (Marmota flaviventris) J Comp Physiol B. 1993;163:463–469. doi: 10.1007/BF00346930. [DOI] [PubMed] [Google Scholar]
- Raman N, Black PN, DiRusso C. Characterization of the fatty-acid responsive transcription factor FadR. J Biol Chem. 1997;272:30645–30650. doi: 10.1074/jbc.272.49.30645. [DOI] [PubMed] [Google Scholar]
- Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp Biol Med. 2008;233:507–521. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3 phosphate acyltransferase gene transcription. Annu Rev Nutr. 1998;18:331–351. doi: 10.1146/annurev.nutr.18.1.331. [DOI] [PubMed] [Google Scholar]
- Ves-Losada A, Brenner RR. Long-chain fatty acyl-CoA synthetase enzymatic activity in rat liver cell nuclei. Mol Cell Biochem. 1996;159:1–6. doi: 10.1007/BF00226056. [DOI] [PubMed] [Google Scholar]
- Wakil SJ. Fatty acid synthase, a proficient multifactorial enzyme. Am Chem Soc. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- Wang P, Walter RD, Bhat BG, Florant GL, Coleman RA. Seasonal changes in enzymes of lipogenesis and triacylglycerol synthesis in the golden-mantled ground squirrel (Spermophilus lateralis) Comp Biochem Physiol B. 1997;118:261–267. doi: 10.1016/s0305-0491(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta. 1993;1183:41–47. doi: 10.1016/0005-2728(93)90004-y. [DOI] [PubMed] [Google Scholar]
- Zancanaro C, Malatesta M, Vogel P, Osculati F, Fakan S. Ultrastructure of the adrenal cortex of hibernating, arousing and euthermic dormouse, Muscardinus avellanarius. Anat Rec. 1997;249:359–364. doi: 10.1002/(SICI)1097-0185(199711)249:3<359::AID-AR6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Zancanaro C, Malatesta M, Mannello F, Vogel P, Fakan S. The kidney during hibernation and arousal from hibernation. A natural model of organ preservation during cold ischaemia and reperfusion. Nephrol Dial Transplant. 1999;14:1982–1990. doi: 10.1093/ndt/14.8.1982. [DOI] [PubMed] [Google Scholar]
- Zhang L, Joshi AK, Smith S. Cloning, expression, characterization, and interaction of two components of a human mitochondrial fatty acid synthase: malonyltransferase and acyl carrier protein. J Biol Chem. 2003;278:40067–40074. doi: 10.1074/jbc.M306121200. [DOI] [PubMed] [Google Scholar]