Abstract
Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) egg development and its relation to environmental parameters is an understudied aspect of vector biology. Although several studies have illustrated the dramatic effects of temperature on egg development, egg hatching dynamics remain unclear. The objective of this study was to expose An. gambiae eggs to various temperatures for different lengths of time and determine the impact on egg development and hatching count. Batches of mosquito eggs (n = 30 eggs/replicate) were incubated under moist conditions at temperatures of 12, 22, 27, 33, and 42°C for intervals of 1, 3, 7, and 10 days. After that, they were flooded with distilled water at 27°C, and hatching counts were observed for up to 7 days. Mosquito eggs held at 22 and 27°C had the highest overall mean hatching count. During early incubation periods, eggs held at 33°C had hatching counts comparable to 22 and 27°C, but counts decreased drastically during later incubation periods. Temperatures of 12 and 42°C reduced mosquito egg viability, because few eggs hatched in these temperature regimes. Other experiments revealed that during early embryonic development, temperature had a major effect on the developing embryo, while later in embryonic development it had no dramatic effect. Microscopic observation of the An. gambiae embryo showed that extreme low and high temperatures affected the normal development of the embryo. A regression model was developed to describe the effect of incubation temperature and incubation period on egg hatching counts, which demonstrated that the optimum temperature for egg hatching ranges from 24 to 30°C, irrespective of incubation period. The interaction between temperature and time period may have implications for dry-season survival and climate-based models of malaria risk.
Keywords: Anopheles gambiae, eggs, hatching, temperature, time period
Introduction
Mosquito embryonic development is influenced by many environmental parameters, with temperature having the most profound impact on the growth and survival of the developing embryo. Much of our current knowledge of temperature effects, embryonic development, and hatching comes from aedine mosquitoes (Mortenson 1950, Judson 1960, Judson and Hokama 1965, Judson et al. 1966, Horsfall and Trpiš 1967, Trpiš et al. 1973, Kardatzke 1979, Shililu et al. 2004).
Relatively less is known about the developmental dynamics of anopheline eggs. Studies on the eggs of Anopheles maculipennis (Meigen), An. maculipennis labranchiae (Falleroni), and An. maculipennis melanoon (Hackett), identified different temperature optima for egg hatching success of these eggs (Kettle and Sellick 1947). Similar differences in temperature optima for egg hatching have been demonstrated between An. albitarsis (Lynch-Arribalzaga) and An. aquasalis (Curry) (de Carvalho et al. 2002). The effect of temperature on the egg stages of An. gambiae has received little attention relative to the ecology of this medically important African malaria vector. It has been demonstrated that An. gambiae eggs undergo morphological changes when the adult female is subjected to temperatures of 10–13°C immediately after a blood meal (Deane and Causey 1943). Armstrong and Bransby-Williams (1961) found that when eggs of An. gambiae mosquitoes were reared at 26.5°C, 74% of their eggs hatched, after flooding, during 4 days of monitoring. However, eggs reared at 21.2°C had only lower hatchability (66%), after flooding, during 4 days of monitoring. Other studies examining the duration of egg survival on various substrates have used temperatures ranging from 20 to 30°C (Muirhead-Thomson 1945, Holstein 1954, Beier et al. 1990, Shililu et al. 2004). The objective of this study was to expose An. gambiae eggs to various temperatures for different lengths of time and determine the impact on hatching counts.
Materials and Methods
Mosquitoes
Experiments were conducted with the use of laboratory-reared An. gambiae s.s. (MBITA strain) mosquitoes from a colony established with mosquitoes from Mbita, Kenya in February 2000. All mosquito life stages were maintained under laboratory conditions. Immature mosquitoes were reared under temperatures ranging between 30 and 35°C and humidity ranging between 80% and 90%, and were maintained on Tetramin® fish food. Adult mosquitoes were reared under temperatures of approximately 22-27°C, and were maintained on a 6% glucose solution, water, and routine human blood meals (3 times per week over 3 consecutive days). Ethical clearance was provided by Kenya National Ethical Review Committee, based at the Kenya Medical Research Institute. After the 3rd blood meal, mosquitoes were given 1 day to digest the blood meal, then a 75 × 25-mm oviposition cup was placed inside the cage. The oviposition cup contained Whatman® filter paper filled with distilled water, and this was put into the cage to allow mosquitoes to lay eggs.
Temperature-incubation bioassay
After mosquitoes were allowed to oviposit their eggs, oviposition cups were removed from the mosquito cages and 30 eggs were placed in 100 × 15-mm petri dishes with a dry filter paper. A small soft bristle brush wet with distilled water was used to spread the eggs onto the filter paper. Once eggs were spread on the filter paper they were moistened to prevent desiccation. Excess water was poured off to prevent premature hatching before eggs were flooded. Care was taken to avoid damaging the eggs. After eggs were put into the petri dishes, without flooding with water, they were placed at temperatures of 12, 22, 27, 33, or 42°C in Sanyo incubators, for time periods of 1, 3, 7, and 10 days. A total of 5 replicates (i.e., a petri dish with 30 eggs) per temperature per incubation period were performed. We made an arbitrary selection of time period to incubate the eggs. Selection of temperatures was based on a simple algorithm around 27°C, as this temperature is recognized as the optimum temperature for rearing An. gambiae under laboratory conditions (Armstrong and Bransby-Williams 1961). To maintain internal humidity, petri dishes were sealed with parafilm. Internal humidity was not recorded during the course of the study. Eggs were observed daily at each respective incubation temperature. Water was added to the filter paper in the petri dishes if eggs appeared to be drying after visual observation. At time points of 1, 3, 7, and 10 days postoviposition, the mosquito eggs were flooded with 30 ml of double-distilled water (ddH2O) at 27°C. Because it has been shown that 27°C is the optimal temperature for rearing An. gambiae (Armstrong and Bransby-Williams 1961), we continued to use this temperature for the flooding of the eggs in this bioassay. The hatching count (number of 1st instars per total number of eggs) was recorded daily for 7 days after flooding, upon which the hatched larvae for each day were removed with the use of a plastic transfer pipet. Care was taken not to disturb the other eggs when removing hatched larvae.
In a 2nd study, mosquito eggs were incubated at the same temperatures as mentioned above for 3 days. Following the 3-day incubation period, 3 replicates at each temperature were flooded and maintained at a temperature regime of 12, 22, 27, 33, and 42°C. The hatching count was recorded up to 7 days at each temperature.
Observation of embryological development
The development of An. gambiae eggs was observed under different temperature conditions. Briefly, 2 petri dishes of 30 eggs were incubated at constant temperatures of 12, 27, and 42°C, respectively (2 petri dishes per temperature) for 3 days. After the incubation, 3 petri dishes were flooded with room temperature water and the hatching counts recorded. The remaining 3 petri dishes were kept at −20°C for approximately 1 h to cool shock the eggs. Eggs were then bleached in 2.5% bleach solution for 3 h or until the chorion, the outer layer of the egg, became transparent (Mortenson 1950, Shililu et al. 2004). With the use of a dissecting microscope, morphological hallmarks of the developing embryo at the 3 temperatures were described and compared. We identified eggs that possessed major development hallmarks for late-stage mosquito embryo development, such as formation of the line of dehiscence and segmentation of abdomen and thorax. These events are crucial in the hatching process of a mosquito larva (Judson and Hokama 1965, Clements 1992). Pictures of the embryo were taken.
Statistical analysis
We performed an analysis of variance (ANOVA) using the general linear model procedure in SAS 8.0, with the dependent variable being the egg hatching count in a dish of 30 eggs. In our analysis for the temperature-incubation bioassay, we omitted the egg hatching count data collected from the entire temperature regime of 42°C and temperature regime of 22°C/incubation day 3 to equalize the variance. We also modeled egg-hatching counts of An. gambiae eggs with the use of the temperature-incubation bioassay by the stepwise regression procedure in SAS 8.0 and created a 3D graph of the estimated response surface with S-Plus®. In our analysis of the temperature response bioassay, we also omitted the entire 42°C incubation temperature regime to equalize the variance.
Results
Temperature incubation
Table 1 summarizes the mean hatching count of eggs held at various temperatures for different time periods. Eggs held at 12°C showed relatively high hatching counts at 1 and 3 days of incubation, but this reduced drastically at 7 and 10 days of incubation. Eggs held at 22 and 27°C showed a gradual decreasing trend in mean egg hatching count during 1-, 3-, and 10-day incubation periods; however, there was a slight increase in hatching counts during the 7-day incubation period, relative to 3-day incubation period. Of all temperatures, 22 and 27°C demonstrated relatively higher mean egg-hatching counts after 10 days of incubation. Eggs held at 33°C showed a similar trend in hatching count as eggs held at 12°C; there was high hatching at 1 and 3 days of incubation, but this reduced drastically at 7 and 10 days of incubation. The mean hatching count of eggs held at 42°C was drastically reduced relative to the other temperatures. A 1-day incubation period at 42°C yielded a low mean hatching count relative to the other temperatures. There was no hatching when eggs were held at 42°C for longer incubation periods of 3, 7, and 10 days. The GLM, excluding the group of eggs held at 42°C and a single observation at 22°C/incubation day 3, showed that temperature (F = 24.24, df = 3, P < 0.001, N = 79 eggs) and incubation period (F = 55.77, df = 3, P < 0.001, N = 79 eggs) were both significant factors affecting egg hatching. There was also a significant interaction between temperature and incubation period (F = 5.55, df = 9, P < 0.001, N = 79 eggs), suggesting the mean egg hatching depends on both the duration of incubation and temperature. Different hatching distribution of An. gambiae eggs was observed at the different temperatures and incubation periods. Eggs generally hatched within 4 days. However, a small portion of eggs was observed to hatch beyond 4 days in all temperatures except 42°C (Fig. 1).
Table 1.
Mean hatching count (±SE) of Anopheles gambiae eggs held at different temperatures and time periods, then flooded with 27°C double-distilled H2O.1
| Incubation temperature | |||||
|---|---|---|---|---|---|
| Incubation period (days) | 12°C | 22°C | 27°C | 33°C | 42°C |
| 1 | 16.8 ± 2.2 | 21.8 ± 4.6 | 23.2 ± 4.1 | 24.0 ± 2.4 | 1.8 ± 2.7 |
| 3 | 15.4 ± 3.3 | 16.8 ± 9.2 | 19.6 ± 3.3 | 20.2 ± 5.0 | 0.0 ± 0.0 |
| 7 | 5.4 ± 4.7 | 19.4 ± 3.2 | 19.6 ± 3.6 | 4.2 ± 3.0 | 0.0 ± 0.0 |
| 10 | 1.4 ± 1.5 | 11.6 ± 3.6 | 13.6 ± 7.5 | 2.6 ± 2.6 | 0.0 ± 0.0 |
A total of 5 experimental replicates/temperature/incubation period were conducted, n = 30 eggs/replicates.
Fig. 1.
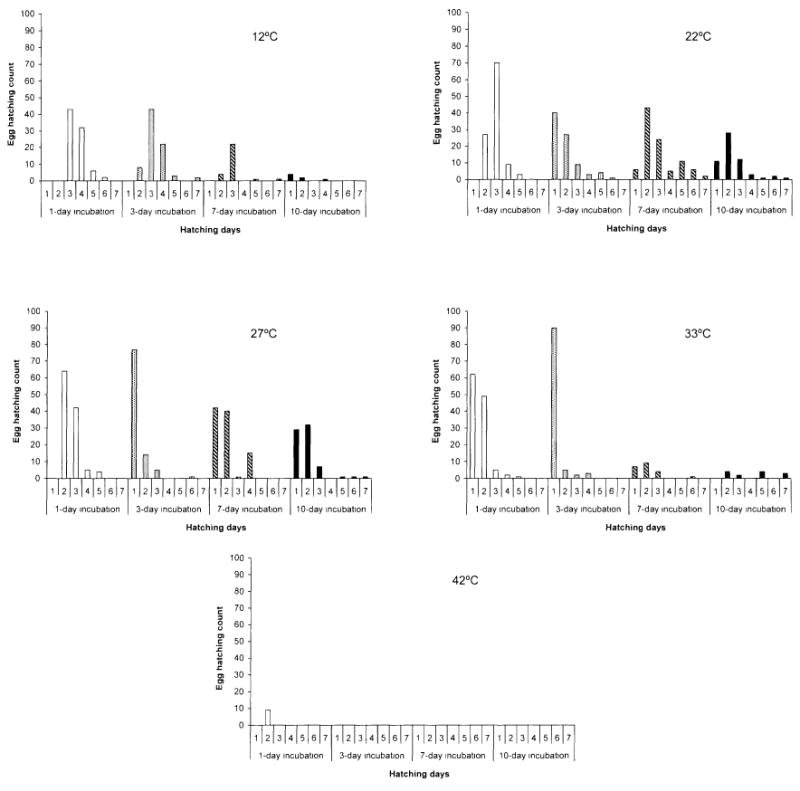
Egg hatching count of An. gambiae monitored for 7 days after being incubated at constant temperatures of 12, 22, 27, 33, and 42°C for incubation periods of 1, 3, 7, and 10 days, then being flooded and kept in 27°C double-distilled H2O.
The regression model showed the effect of temperature and incubation period on hatching count was best predicted by:
This estimated relationship between temperature, incubation period, and hatching count was displayed on a 3D surface (Fig. 2). This model revealed a decrease in egg hatching counts as the eggs were incubated for an increasing time period, and temperature caused hatching counts to takes on a quadratic function with the optimum temperature for hatching ranging from 24 to 30°C. Hatching counts are nonlinearly related to temperature but linearly related to incubation days.
Fig. 2.
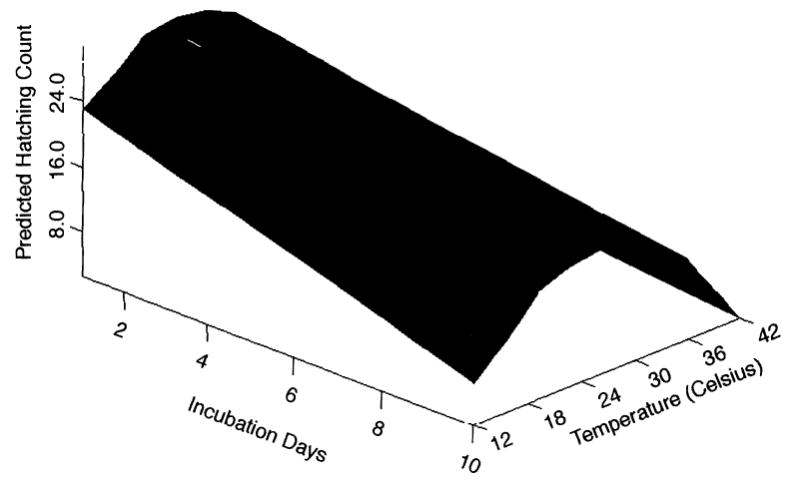
The predicted surface response of An. gambiae egg hatch counts to incubation days and incubation temperature.
Another set of experiments revealed that during early embryonic development, temperature (F = 13.26, df = 3, P < 0.001, N = 60 eggs) had a major effect on the developing embryo, whereas later in embryonic development temperature had no dramatic effect on their ability to hatch (F = 0.55, df = 4, P < 0.697, N = 60 eggs).
Observation of embryological development
After 6 petri dishes of 30 mosquito eggs were incubated at temperatures of 12, 27, and 42°C for 3 days (2 petri dishes per temperature), 1 dish from each temperature was removed and flooded with 30 ml of ddH2O at room temperature. Eleven of 30 eggs hatched at flooding in the 27°C group, and no larvae emerged in the 12 or 42°C group. After cool shocking and then bleaching the 2nd set of eggs, we observed that 23 out of 30 eggs held at 27°C were viable, showing classic hallmarks of late-stage embryos, such as segmentation and formation of the line of dehiscence. Five eggs did not have the classic hallmarks, and 2 eggs showed partial hatching in the bleach solution. All 30 eggs incubated at 42°C showed only a darkening of the serosal cuticle and all 30 eggs at 12°C showed neither formation of classic hallmarks nor darkening of serosal cuticle (Fig. 3).
Fig. 3.
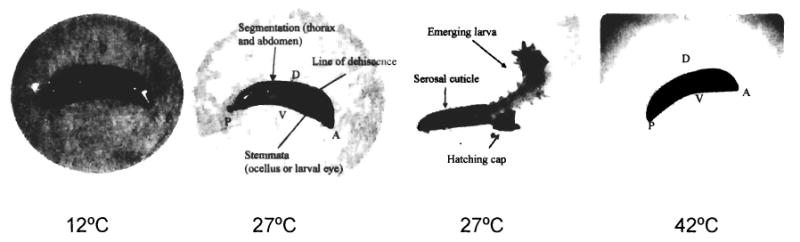
Eggs of An. gambiae held at 3 constant temperatures of 12, 27, and 42°C and flooded with room temperature double-distilled H2O to test for viability and to show presence or absence of major developmental hallmarks (V, ventral end; D, dorsal end; P, posterior pole; A, anterior pole).
Discussion
In this study, we show that different temperature conditions have a dramatic effect on the hatching counts of An. gambiae eggs, after incubation. When eggs were kept under different incubation periods, there was a tendency for relatively high and low temperatures to reduce hatching counts faster compared to eggs held at relatively moderate temperatures. Furthermore, early embryonic stages were most affected by temperature, and later embryonic stages seemed to be less affected. This observation suggests that once An. gambiae eggs develop at the optimum temperature, they will hatch despite the unsuitability of temperature in the hatching medium.
Eggs held at the optimum temperature of 27°C developed all characteristics of normal development, whereas eggs held at low temperatures seemed to have a stunted or delayed development. Eggs at high temperatures developed a darkened appearance, suggesting degradation of the embryo within the proteinacious coat. Incubating the eggs at high temperatures tended to decrease viability of the eggs at a relatively fast rate, whereas under cold conditions, eggs were able to hatch, but hatching counts were significantly reduced compared to temperatures of 22 and 27°C. This has been seen in studies with field populations of An. funestus (Giles) and An. gambiae. Mosquito eggs kept at 4°C were able to survive for up to 9 days, although the hatching rates were severely reduced (Beier et al. 1990).
We found the optimal temperature for An. gambiae eggs, for all incubation periods, to be within the range of 24–30°C. This is within the range of the optimum temperature for rearing mosquito colonies of An. gambiae (Armstrong and Bransby-Williams 1961). We also observed that temperatures moderately above the optimum range led to a high rate of hatching during early incubation periods. This is to be expected, as it is commonly accepted that warmer temperatures have a tendency to increase the rate of immature stage development (Bayoh et al. 2001). However, during incubation periods of 7 and 10 days, the hatching counts were drastically reduced, perhaps due to depletion of embryological resources during this quiescent period. Therefore prolonged exposure to temperatures above the optimum may adversely affect immature populations. Physiological studies looking at egg size and its relation to protein, glycogen, and lipid levels during various time intervals may be useful in understanding how the eggs are able to survive short periods of desiccation.
Our results may have implications for physiological aspects of dry-season survival of An. gambiae eggs. Short-term survival of tropical anopheline eggs on soil has been suggested as a mechanism for overcoming periods of drought (Stone and Reynolds 1939). The extent to which An. gambiae eggs can survive on surface soil is still an unresolved issue. This short-term survival may be a function of optimum environmental conditions needed for this phenomenon to occur. Though previous An. gambiae egg survival studies have only shown a 6–18 day survival rate (Deane and Causey 1943; Holstein 1954; Ramsdale and Fontaine 1970a, 1970b; Beier et al. 1990; Clements 1992; Shililu et al. 2004), the only abiotic variable that has been considered is moisture content under desiccating conditions. Our studies suggest the temperature may also have a regulating role on this phenomenon.
Afro-tropical malaria vector research has focused on climate and environmental models and entomological risk maps for the purpose of predicting outbreaks and mounting the appropriate vector control measures (Patz et al. 1998, Bayoh et al. 2001, Minakawa et al. 2002, Depinay et al. 2004). Our study may also have implications the development of climate–environmental models describing malaria outbreak risk. Taking into account the parameters of temperature and length of exposure, we present a regression model for egg hatching that has a 77% predictive power. Our result complements the vast information on adult and larval adaptation profiles to microclimates, and provides the contribution of egg stages to population size.
The effect of fluctuating temperature on anopheline mosquito may be more appropriate for determining the impact of temperature on anopheline egg development and each of the other respective stages. Studies in other insects show that fluctuating temperatures can have a profound impact on immature insects. Studies of Sigara alternata (Say) (Hemiptera: Corixidae) (the water boatman) showed that developmental rate was positively correlated with increased magnitude of diel temperature pulse (Sweeney and Schnack 1977). Further evaluation is needed to evaluate the impact of temperature on anopheline mosquitoes.
Acknowledgments
We thank Pauline N. Kungu, Milcah W. Gitau, and Jeremiah Ojude for their invaluable technical assistance and Maxwell Billah for his excellent photography. We thank Wesley B. Grueber for comments on manuscript and experimental design. We also thank Robert Duncan for statistical guidance. This research was supported by funds from the National Institute of Health (NIH) grants U19 AI45511 and D43 TTW01142.
References Cited
- Armstrong JA, Bransby-Williams WR. The maintenance of a colony of Anopheles gambiae with the observations on the effect of changes in temperature. Bull WHO. 1961;24:427–435. [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN, Thomas CJ, Lindsay SW. Mapping distributions of chromosomal forms of Anopheles gambiae in West Africa using climate data. Med Vet Entomol. 2001;15:267–274. doi: 10.1046/j.0269-283x.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- Beier JC, Copeland R, Oyaro C, Masinya A, Odago WO, Oduor S, Koech DK, Roberts CR. Anopheles gambiae complex egg-stage survival in dry soil from larval development sites in western Kenya. J Am Mosq Control Assoc. 1990;6:105–109. [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes. Cambridge: Chapman and Hall; 1992. [Google Scholar]
- de Carvalho SCG, de Jesus Martins AJ, Lima JBP, Valle D. Temperature influence on embryonic development of Anopheles albitarsis and Anopheles aquasalis. Mem Inst Oswaldo Cruz. 2002;97:1117–1120. doi: 10.1590/s0074-02762002000800009. [DOI] [PubMed] [Google Scholar]
- Deane MP, Causey OR. Viability of Anopheles gambiae eggs and morphology of unusual types found in Brazil. Am J Trop Med. 1943;23:95–102. [Google Scholar]
- Depinay JM, Mbogo CM, Killeen G, Knols B, Beier J, Carlson J, Dushoff J, Billingsley P, Mwambi H, Githure J, Toure AM, McKenzie FE. A simulation model of African Anopheles ecology and population dynamics for the analysis of malaria transmission. Malaria J. 2004;3:29. doi: 10.1186/1475-2875-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein MH. Biology of Anopheles gambiae. WHO Monograph. Geneva, Switzerland: World Health Organization; 1954. (9). [Google Scholar]
- Horsfall WR, Trpiš M. Eggs of floodwater mosquitoes. X. Conditioning and hatching of winterized eggs of Aedes strictus (Diptera: Culicidae) Ann Entomol Soc Am. 1967;60:1021–1025. doi: 10.1093/aesa/60.5.1021. [DOI] [PubMed] [Google Scholar]
- Judson CL. The physiology of hatching aedine mosquito eggs: hatching stimulus. Ann Entomol Soc Am. 1960;53:688–691. [Google Scholar]
- Judson CL, Hokama Y. Formation of the line of dehiscence in aedine mosquito eggs. J Insect Physiol. 1965;11:337–345. doi: 10.1016/0022-1910(65)90081-8. [DOI] [PubMed] [Google Scholar]
- Judson CL, Hokama Y, Kliewer JW. Embrogeny and hatching of Aedes sierrensis eggs (Diptera: Culicidae) Ann Entomol Soc Am. 1966;53:1181–1184. [Google Scholar]
- Kardatzke JT. Hatching of eggs of snow-melt Aedes (Diptera: Culicidae) Ann Entomol Soc Am. 1979;72:559–562. [Google Scholar]
- Kettle DS, Sellick G. The duration of the egg stage in the races of Anopheles maculipennis Meigen (Diptera, Culicidae) J Anim Ecol. 1947;16:38–43. [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Githeko A, Yan G. The effect of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002;39:833–841. doi: 10.1603/0022-2585-39.6.833. [DOI] [PubMed] [Google Scholar]
- Mortenson EW. The use of sodium hypochlorite to study Aedes nigromaculis embryos. Mosq News. 1950;10:211–212. [Google Scholar]
- Muirhead-Thomson RC. Studies on the breeding places and control of Anopheles gambiae and A. gambiae var. melas in costal districts of Sierra Leone. Bull Entomol Res. 1945;36:185–252. [Google Scholar]
- Patz JA, Strzepek K, Lele S, Hedden M, Greene S, Noden B, Hay SI, Kalkstein L, Beier JC. Predicting key malaria transmission factors biting and entomological inoculation rates using modelled soil moisture in Kenya. Trop Med Int Health. 1998;3:818–827. doi: 10.1046/j.1365-3156.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- Ramsdale CD, Fontaine RE. Ecological investigations of Anopheles gambiae and Anopheles funestus. I. Dry season studies in villages near Kaduna, Nigera—1970. WHO document. 1970a WHO/MAL/70.735. [Google Scholar]
- Ramsdale CD, Fontaine RE. Ecological investigations of Anopheles gambiae and Anopheles funestus. II. Dry season studies with colony-reared A. gambiae species B, Kaduna, Nigeria—1970. WHO document. 1970b WHO/MAL/70.736. [Google Scholar]
- Shililu JI, Grueber WB, Mbogo CM, Githure JI, Riddiford LM, Beier JC. Development and survival of Anopheles gambiae eggs in drying soil: influence of the rate of drying, egg age, and soil type. J Am Mosq Control Assoc. 2004;20:243–247. [PubMed] [Google Scholar]
- Stone WS, Reynolds FHK. Hibernation of anopheline eggs in the tropics. Science. 1939;90:371–372. doi: 10.1126/science.90.2338.371. [DOI] [PubMed] [Google Scholar]
- Sweeney BW, Schnack J. Egg development, growth, and metabolism of Sigara alternata (Say) (Hemiptera: Corixidae) in fluctuating thermal environments. Ecology. 1977;58:265–277. [Google Scholar]
- Trpiš M, Haufe WO, Shemanchuk JA. Embryonic development of Aedes (O.) strictus (Diptera: Culicidae) in relation to different constant temperatures. Can Entomol. 1973;105:43–50. [Google Scholar]


