Abstract
Mark-release-recapture (MRR) experiments were conducted with emerging Anopheles gambiae s.l. and Anopheles funestus Giles at Jaribuni and Mtepeni in Kilifi, along the Kenyan Coast. Of 739 and 1,246 Anopheles released at Jaribuni and Mtepeni, 24.6 and 4.33% were recaptured, respectively. The daily survival probability was 0.96 for An. funestus and 0.95 for An. gambiae in Jaribuni and 0.83 and 0.95, respectively, in Mtepeni. The maximum flight distance recorded was 661 m. The high survival probability of An. gambiae and An. funestus estimated accounts for the continuous transmission of malaria along the Kenyan coast. This study also shows that the release of young, emergent female Anopheles improves the recapture rates and may be a better approach to MRR studies.
Keywords: Anopheles gambiae, Anopheles funestus, mark-release-recapture, dispersal, survival probability
The planning of future malaria vector control interventions requires information on the vector population, such as vector dispersal and survival. This information is important not only as determinants of the epidemiology of malaria but also for operational malaria vector control activities (Killeen et al. 2003). The dispersal of mosquito vectors—to find mates, nectar sources, resting sites, oviposition sites, and bloodmeals-underlies the spatial distribution of vectors, and it plays a major role in shaping population structure (Service 1997). Mosquito survivorship and dispersal ability are also critical for understanding malaria transmission risk (Carter et al. 2000). However, little is known about these important life history traits of Anopheles mosquitoes in nature.
One of the methods most commonly used to obtain information on mosquito populations is the mark-release-recapture (MRR) technique, which has been conducted widely with populations of Anopheles and Aedes mosquitoes. Service, (1993) lists 150 such studies. The majority of MRR data on Anopheles gambiae s.l. is from studies conducted in West Africa (Thomson et al. 1995, Constantini et al. 1996, Toure et al. 1997) and from some studies conducted in eastern Africa (Charlwood et al. 1997, Takken et al. 1998). Previous MRR studies focused on the dispersal of wild females from a central sentinel house. Most of these studies recorded low recovery rates, the average being 4.2%. In a study comparing the dispersal rates of two cohorts of Aedes aegytpi (L.) of different ages from a central release point, higher recapture and dispersal rates were observed in the younger cohort (Harrington et al. 2001). In this study, we conducted MRR experiments with emergent female Anopheles mosquitoes to determine the dispersal and survival probability of An. gambiae and Anopheles funestus Giles at two sites in an area of perennial malaria transmission on the coast of Kenya.
Materials and Methods
Study Areas
MRR experiments were conducted at Jaribuni and Mtepeni, two villages in Kilifi District, along the Kenyan coast. Jaribuni is located 03° 37.3′ S and 039° 44.6′ E. The Jaribuni River runs across the site flowing year-round. During the rainy season, water levels rise and temporary larval habitats are formed at the edge of the river. These habitats expand and contract with the rise and fall of water and may disappear when the water levels reduce to extremely low levels. Mtepeni is located 03° 54.5″ S and 039° 43.6″ E. A seasonal stream passes through the area, which has a more hilly terrain, compared with the Jaribuni site.
Seasonality of rainfall is marked along the coast. The rains are generally bimodal with long rains falling from April to June (with peak in May) and short rains from October to December. Average annual rainfall varies from 400 to 1,200 mm. Mean daily minimum temperature averages 22°C and the maximum temperature averages 30°C, with an average relative humidity of 70% (Mtwapa meteorological Station, Kilifi).
Houses are mainly of one type, square or rectangular shaped, mud walled with makuti (palm leaves) thatched roofs and an open space between the walls and the roof, leaving ample space for mosquito entry. During the study period, none of the houses had screens on the windows or doors. Residents are mainly subsistence farmers growing cassava, Manihot esculenta Crantz; cashews, Anacardium occidentale L.; coconuts, Cocos nucifera L.; mangoes, Mangifera indica L.; and maize, Zea mays L.
Mapping of Houses and Larval Habitats
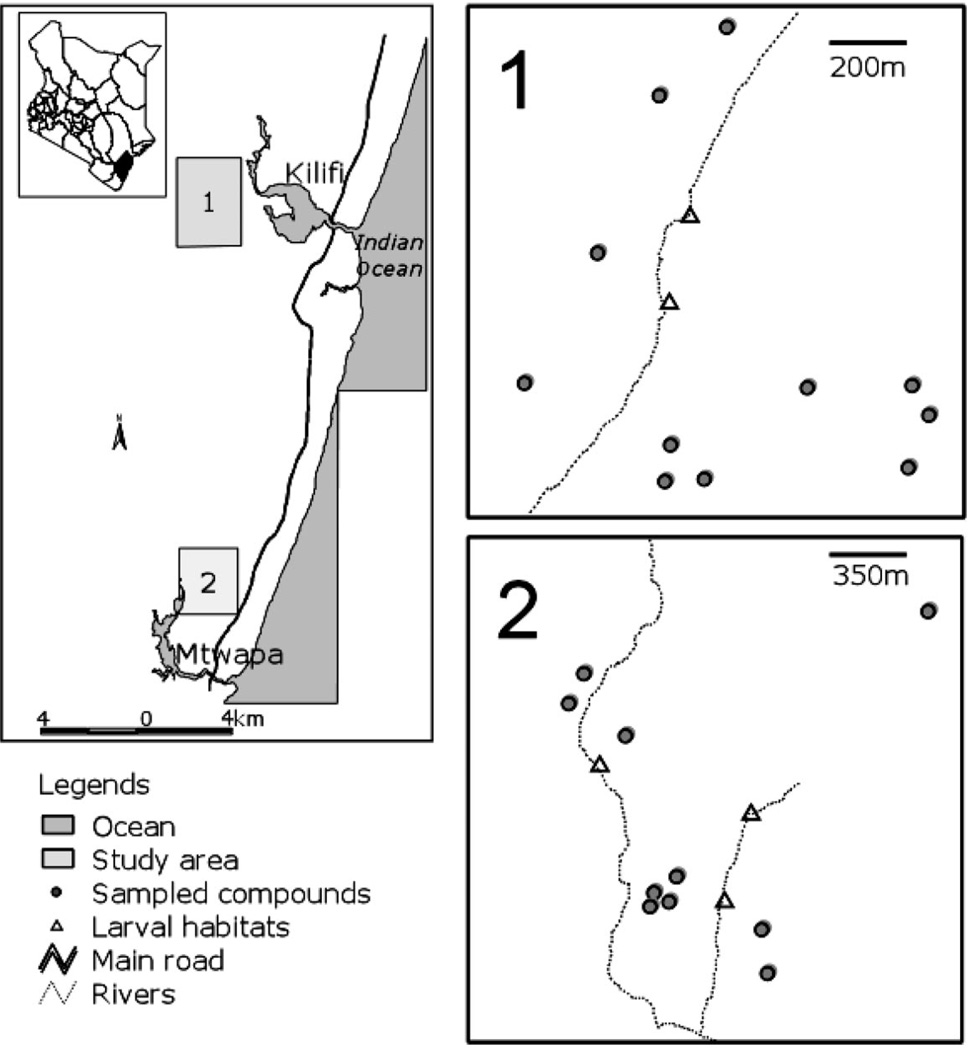
In December 2001, the study sites were surveyed to identify potential larval habitats. The latitude and longitude data of the productive larval habitats and households in the study area were recorded using a hand-held global positioning system (GPS) (Garman International Inc., Olathe, KS). Georeferenced layers of roads and major landmarks were overlaid onto the coverage to depict the distributions of larval habitats and house-holds on a base map in ArcView 3.2a (ESRI, Redlands, CA). The point distance command in ArcInfo was used to generate distances between larval habitats and the nearest neighbor households. Figure 1 illustrates the location of Kilifi District and the two study areas along the Kenyan coast.
Fig. 1.
Map of the study area, showing Kilifi District and the two study sites along the Kenyan Coast. Site 1, Jaribuni; Site 2, Mtepeni. Boxes 1 and 2 on the right show the spatial location of the larval habitats where larvae were collected and of compounds where adult r ecapture collections were made.
Mosquito Rearing and Mark–Release–Recapture Experiments
The study was conducted from February to April 2002 at Jaribuni and from April to June 2002 at Mtepeni. During that year, Kilifi District experienced a prolonged dry season. Only two productive larval habitats were available along the Jaribuni River and in Mtepeni, three larval habitats were available. These habitats were swampy areas that never dried up after the previous rainy season. Larvae collected from these habitats were reared to adults under semifield conditions in temporary field insectaries constructed at the two sites. Larvae were fed on Tetramin baby fish food (TetraWerke, Melle, Germany). Every batch of emergent adults was held for 3 d before release, and while awaiting release, adults were fed on 6% sugar solution. Before release, 0.5-liter paper cups were dusted with fluorescent powder. The adults were then manually aspirated and counted into the paper cups. The powders for dusting were Day-Glo fluorescent pigments (Day-Glo Color Co., Cleveland, OH). A different color of dye was used for each release. All the marked mosquitoes were then returned to a point adjacent to the original larval habitat and the lid of the paper cup was removed to allow the marked mosquitoes to fly out freely. The mosquitoes that seemed exhausted (moribund) and did not fly out of the cups were counted, and their numbers were subtracted from the total marked. All the releases were made from 1500 to 1600 hours to minimize the effects of high daytime temperatures on the released mosquitoes. A total of nine separate releases were made at Jaribuni, four releases from the first habitat and five from the second habitat. At Mtepeni, five releases were made, two releases from the first two habitats and one release from the third habitat. Each of the releases consisted of different numbers of females. At both sites, there was an interval of at least 8 d between releases.
Recapture
Mosquito recaptures from houses in selected compounds within the study villages began 1 d after the day of release and continued for 14 consecutive days. Maximum distance covered during the recapture efforts was up to 1 km. Two methods were used: daytime catches by manual aspiration of indoor resting mosquitoes (DRI) and human landing catches both indoors and outdoors (HBI and HBO). DRI collections were done daily in all the houses from 8 a.m. to 11 a.m., whereas HBO and HBI was conducted from 7:00 p.m. to 12:00 a.m. It was not possible to conduct HBO and HBI in all the houses each night; so, a sequential sampling scheme was generated for use during the night collections, whereby a total of eight compounds selected on either side of the river were sampled each night. To determine which compounds to sample daily, compounds located on both sides of the river were randomly selected and then assigned a night of collection. Collections of mosquitoes were placed in coolers and transported to the laboratory.
In the laboratory, all the mosquitoes were counted and examined at 40× by using a fluorescent compound microscope (Olympus B201, Olympus, Tokyo, Japan) to detect color-marked individuals. Mosquitoes were classified to gonotrophic state based on abdominal appearance (unfed, bloodfed, gravid, and half-gravid). This procedure was followed by morphological identification (Gillies and De Meillon 1968An. gambiae complex was done by polymerase chain reaction (Scott et al. 1993). Sporozoite enzyme-linked immunosorbent assay (ELISA) tests were conducted on the blood-fed mosquitoes recaptured 10 d after release to determine infection.
Data Analysis
Recapture data from nine releases at two habitats in Jaribuni and data from the five releases at three habitats in Mtepeni were combined during analysis to estimate the daily survival probability of An. gambiae and An. funestus mosquitoes. Recapture rates were calculated as a proportion of the total number of marked mosquitoes recaptured over the total number originally marked and released. Recapture probability was estimated using the linear corrected estimate approach described by Buonaccorsi et al. (2003). Chisquare tests were conducted to determine significant differences between number of An. gambiae s.s. and An. funestus recaptured.
We used the exponential model developed by Gillies (1961) to estimate the daily survival probability of An. gambiae and An. funestus mosquitoes. In this model, the loss of marked recaptures is described by the function A = Napn, where A is number of marked females recaptured, N is total numbers marked and released, a is recapture probability, p is survival rate, and n is days after release. Using this model, a plot of the logarithm of the number of recaptures (logA) over days after release (n), allows the estimation of p as the antilogarithm of the slope of the fitted regression line.
We considered the dispersal of An. gambiae and An. funestus as the total distance traveled from the habitat of release to the recapture compound. The distances between the habitats and each compound were calculated by the point distance command in ArcInfo by using the longitude and latitude records for all compounds and habitats obtained using a GPS. We plotted the distances covered against the number of days between release and recapture to determine whether over time, more mosquitoes would be recaptured in houses closer or farther away from the release habitat.
Ethical Clearance and Informed Consent
This MRR project was reviewed and approved by the Institutional Review Board of the Kenya Medical Research Institute, (Nairobi, Kenya). Written consent was obtained from the household heads to permit mosquito collection from their houses and from individuals who conducted the human landing catches after the study was explained in the local language.
Results
Recapture Rates
At Jaribuni, 182 Anopheles mosquitoes were recaptured from a total of 739 released, corresponding to a recapture rate of 24.6% (95% CI, 21.6–27.9) (Table 1). Of the total recaptured, 74% were An. funestus and 26% An. gambiae s.s. (χ2 = 7.16; df = 1, P = 0.007). At Mtepeni, 1,246 Anopheles in total were released and of this number, 54 mosquitoes were recaptured (Table 2), corresponding to a recapture rate of 4.33% (95% CI, 3.27–5.62) (Table 2). Of these mosquitoes, 3.7% were An. funestus and 96.3% were An. gambiae (χ2 = 8.32, df = 1, P = 0.004). The adjusted probability of recapture was estimated as 0.015 for releases made at Jaribuni and as 0.007 for releases made at Mtepeni.
Table 1.
Numbers of Anopheles mosquitoes released and recaptured at Jaribuni
| Release site |
Release no. |
No. released (a) |
No. captured (n) |
No. marked and recaptured (r) |
% recaptured |
|---|---|---|---|---|---|
| A | 1 | 88 | 1,260 | 1 | 1.1 |
| 2 | 184 | 2,540 | 60 | 32.6 | |
| 3 | 52 | 2,348 | 19 | 36.5 | |
| 4 | 75 | 1,029 | 4 | 5.3 | |
| B | 1 | 97 | 726 | 37 | 38.1 |
| 2 | 80 | 2,384 | 17 | 21.3 | |
| 3 | 96 | 1,059 | 35 | 36.5 | |
| 4 | 47 | 1,029 | 0 | 0 | |
| 5 | 20 | 1,546 | 9 | 45 |
Table 2.
Numbers of Anopheles mosquitoes released and recaptured at Mtepeni
| Release site |
Release no. |
No. released (a) |
No. captured (n) |
No. marked and recaptured (r) |
% recaptured |
|---|---|---|---|---|---|
| C | 1 | 94 | 13 | 3 | 3.2 |
| 2 | 398 | 40 | 11 | 2.8 | |
| D | 1 | 234 | 45 | 8 | 3.4 |
| 2 | 185 | 67 | 12 | 6.5 | |
| E | 3 | 335 | 25 | 20 | 6.0 |
Sporozoite ELISA analysis revealed a 4.40% (8/182) Plasmodium falciparum sporozoite infection rate for the recaptured anophelines. Of these, 2.75% were An. gambiae and 1.65% were An. funestus.
Survival
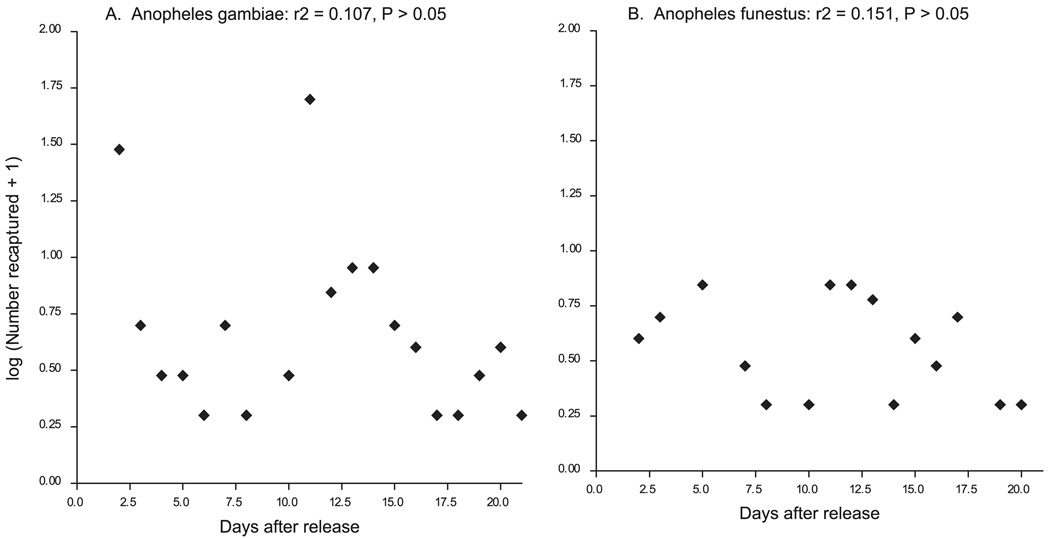
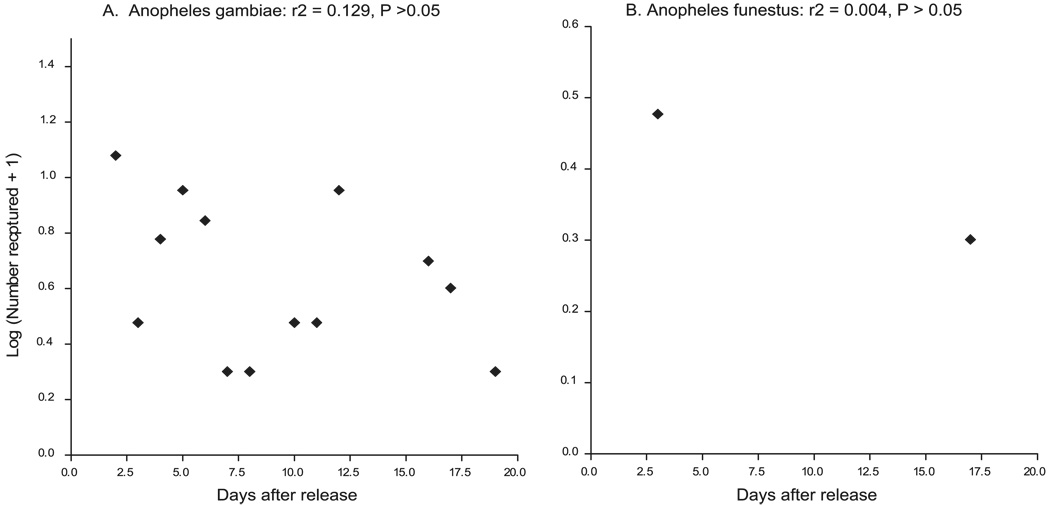
Survival probability was estimated for all the recaptured mosquitoes, mainly An. gambiae and An. funestus. At the Jaribuni site, estimated daily survival probability was 0.95 (95% CI = 0.88–1.00) for An. gambiae and 0.96 (95% CI, 0.89–1.00) for An. funestus. Figure 2A and B shows the scatter plots of the logarithm of the number of marked recaptures of An. gambiae and An. funestus, respectively, plotted against the days after release at Jaribuni. At Mtepeni, estimates of daily survival probability by using the same method were 0.95 (95% CI, 0.8718–1.033) for An. gambiae and 0.83 (95% CI, 0.7956–0.8656) for An. funestus. Figure 3A and B shows the scatter plots of the logarithm of the number of marked recaptures of An. gambiae and An. funestus, respectively, over the days after release. The longest period between release and recapture was 25 d in Jaribuni and 19 d in Mtepeni.
Fig. 2.
Regression of the daily number (Log n+1) of marked An. gambiae (A) and An. funestus (B) females recaptured after release at Jaribuni.
Fig. 3.
Regression of the daily number (Log n+1) of marked An. funestus (A) and An. gambiae (B) females recaptured after release at Mtepeni.
Dispersal
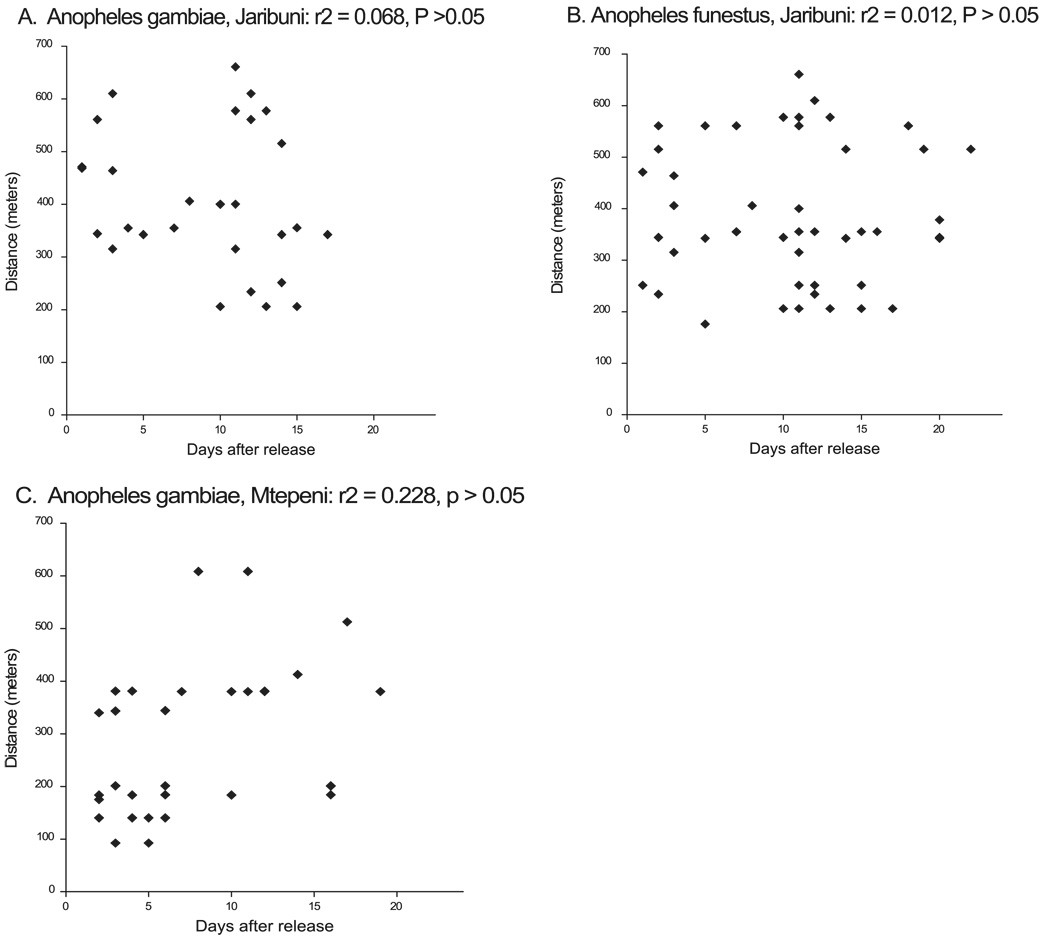
At both study sites, mosquitoes released from different habitats were recaptured in the same house; and on some occasions, recaptures were made from houses located further from the release habitat, although there were houses nearer to the release site. We observed that there was no direct relationship between distance traveled and the number of days after release (Fig. 4). But a cluster of recaptures was observed between days 1 and 5 and between days 10 and 15 for both An. gambiae and An. funestus. This number decreases at day 20 (Fig. 4). On average, both An. gambiae and An. funestus from both sites were recaptured 11 ± 6 d after the day of release. Maximum recoveries of marked An. gambiae and An. funestus were made from compounds located between 200 and 400 m from the release habitats. Although some mosquitoes were recaptured further from the release habitat, these numbers dropped with an increase in distance. Between 650 and 750 m, <5 An. gambiae and An. funestus were recaptured. The maximum distance recorded was 661 m.
Fig. 4.
Distance moved as a function of days after release. (A) An.gambiae, and (B) An. funestus at Jaribuni; (C) An. gambiae at Mtepeni.
Discussion
In this study along the Kenya Coast, we estimated the survival probability and dispersal ability of An. gambiae and An. funestus. The release of young, adult female mosquitoes in these MRR experiments resulted in an improvement in the recapture rates. We obtained recapture rates of 24.6% at the Jaribuni study area and 4.33% at the Mtepeni study area. The difference in recapture rates seen at the two sites may be due to local differences in topographical and ecological features at the sites, because experimental procedures and recovery efforts were similar at both sites. It is, however, expected that rate of mosquito movement was different in the two study areas, and this might influence the outcome of recaptures.
This is the first study of this kind along the Kenyan coast, and it is also the first MRR study with Anopheles mosquitoes in which young mosquitoes were released at the larval habitat as opposed to the release of females of unknown age, which have completed part of their gonotropic cycle. The results obtained in this study suggest that the release of young mosquitoes might be a much better approach to improving the success of MRR experiments, because young females must disperse to find food and bloodmeals, and they are most likely to survive longer. The possibility that laboratory-reared mosquitoes disperse more than wild-caught adults has been found for An. culicifacies (Rawlings et al. 1981). In experiments in Puerto Rico and Thailand, two cohorts of young 3-d-old and 13-d-old adult Ae. aegypti females were released and results indicated a higher recapture rate for the younger cohort (35%) compared with the older cohort (16%) (Harrington et al. 2001).
The daily survival probability recorded for both An. gambiae and An. funestus in this study was high, up to 95%. Survival probabilities were not significantly different between the two species. Additional evidence on the high survival of the population comes from the malaria infection seen in some of the mosquitoes, which were recaptured 12 d after the day of release and tested by sporozoite ELISA.
If the daily mortality of an Anopheles population averages 50%, then <1% of the females are likely to survive to the minimum of 10 d necessary for the extrinsic cycle of P. falciparum (White 1982). For female Anopheles to have vector potential, their daily survival probability must be at least 60%, usually 80–90%. One mosquito was recaptured 25 d after release, and this observation would be highly unlikely if survivorship was low in this population. The high survival probabilities of both An. gambiae s.s. and An. funestus reported here, coupled with their high preference to feed on humans (Mwangangi et al. 2003), are factors to consider as important in the continuous transmission of malaria especially in Kilifi where studies have shown that at some sites, malaria prevalence rates of 50% occur even in the presence of very few mosquitoes (Mbogo et al. 2003).
The dispersal of An. gambiae and An. funestus was determined by estimating the distance traveled over time. Our observations indicated that dispersal was random for both species and within the first 5 d after release, the mosquitoes dispersed variably, with some covering distances as low as 167 m, and others dispersing to houses located further away (661 m). A similar pattern of movement also was observed between 10 and 15 d after release, but this pattern is not similar at the two sites. It is possible that mosquitoes recaptured farther from the release habitat within 10–15 d might have been seeking their second blood-meal. At Jaribuni, the numbers recaptured at days 2–5 are as high as those recaptured at days 10–15. This is not the case at Mtepeni, where the numbers recaptured at days 10–15 are much lower. The difference in observations at the two study areas indicates that the factors influencing mosquito dispersal at the two sites might be different. No difference in dispersal was observed between An. gambiae and An. funestus. These results suggest that the choice of destination by mosquitoes might be determined by other factors and that the location or distance of the house relative to the habitat might not be the only factor determining the destination of dispersers.
In conclusion, we have estimated the dispersal and survival of An. gambaie and An. funestus by MRR experiments, in which 3-d-old Anopheles females were released at their larval habitat. From our results, we suggest that there is a need to conduct more MRR experiments by using this method and to compare results with the approach where naturally caught females are released, to provide a better comparison of the two methods. Information on the dispersal and survival of mosquitoes is important especially where malaria control by reducing human-vector contact is a priority. This information is also necessary for malaria vector control programs focusing on integrated vector management methods where dispersal data are important for determining the range of barrier zones around management areas.
Acknowledgments
We thank the Jaribuni and Mtepeni community members for cooperation during this study. We are also grateful to members of the entomology team, KEMRI-CGMRC, Kilifi for very active participation during the fieldwork. This paper is published with the permission of the director of KEMRI. This study received funding from the National Institutes of Health grants U19 A145511, D43 TW01142, D43 TW00920, and D43 TW01505. J.T.M. received support from the German Academic Exchange Programme (DAAD).
References Cited
- Buonaccorsi JP, Harrignton L, Edman JD. Estimation and comparison of mosquito survival rates with release recapture removal data. J. Med. Entomol. 2003;40:6–17. doi: 10.1603/0022-2585-40.1.6. [DOI] [PubMed] [Google Scholar]
- Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull W.H.O. 2000;78:1404–1411. [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOK, Meuwissen JMET. Survival and Infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull. Entomol. Res. 1997;87:453–455. [Google Scholar]
- Costantini C, Li SG, Della Torre A, Sagnon N, Coluzzi M, Taylor C. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan Savanna village. Med. Vet. Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Gillies MT. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bull. Entomol. Res. 1961;52:99–127. [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara. 2nd ed. Publication of the South Africa Institute of Medical Research No. 54; 1968. [Google Scholar]
- Harrington LC, Bounaccorsi JP, Edman JD, Costero A, Kittayapong P, Clarke GG, Scott TW. Analysis of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. J. Med. Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Knols BGJ, Gu W. Taking malaria transmission out of the bottle: implications of mosquito dispersal for vector control interventions. Lancet Infect Dis. 2003;3:297–302. doi: 10.1016/s1473-3099(03)00611-x. [DOI] [PubMed] [Google Scholar]
- Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter J, Swalm C, Keating J, Regens JL, Shililu JI, I J, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am. J. Trop Med. Hyg. 2003;68:734–742. [PubMed] [Google Scholar]
- Mwangangi JM, Mbogo CM, Nzovu JG, Githure JI, Yan G, Beier JC. Blood meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J. Am. Mosq. Control Assoc. 2003;19:371–375. [PubMed] [Google Scholar]
- Rawlings P, Curtis CF, Wickramasinghe MB, Lines J. The influence of age and season on dispersal and recapture of Anopheles culicifacies in Sri Lanka. Ecol. Entomol. 1981;6:307–319. [Google Scholar]
- Scott JA, Brodgon WG, Collins FH. Identification of single specimens of Anopheles gambiae complex by polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. 2nd ed. London, United Kingdom: Elsevier Applied Science Publishers Ltd.; 1993. [Google Scholar]
- Service MW. Mosquito (Diptera: Culicidae) dispersal: the long and short of it. J. Med. Entomol. 1997;34:579–588. doi: 10.1093/jmedent/34.6.579. [DOI] [PubMed] [Google Scholar]
- Takken W, Charlwood JD, Billingsley PF, Gort G G. Dispersal and survival of Anopheles funestus and An. gambiae s.l. (Diptera: Culicidae) during the rainy season in Southeast Tanzania. Bull. Entomol. Res. 1998;88:561–566. [Google Scholar]
- Thomson MC, Connor SJ, Quinones ML, Jawara M, Todd J, Greenwood BM. Movement of Anopheles gambiae s.l. malaria vectors between villages in the Gambia. Med. Vet. Entomol. 1995;9:413–419. doi: 10.1111/j.1365-2915.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Toure YT, Guimogo D, Petrarca V, Traore SF, Bouare M, Dao A, Carnahan C, Taylor C. Mark Release recapture experiments with Anopheles gambiae s.l. in Banambani village, Mali to determine population size and structure. Med. Vet. Entomol. 1997;12:74–83. doi: 10.1046/j.1365-2915.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- White GB. Malaria vector ecology and genetics. Br. Med. Bull. 1982;38:207–212. doi: 10.1093/oxfordjournals.bmb.a071760. [DOI] [PubMed] [Google Scholar]