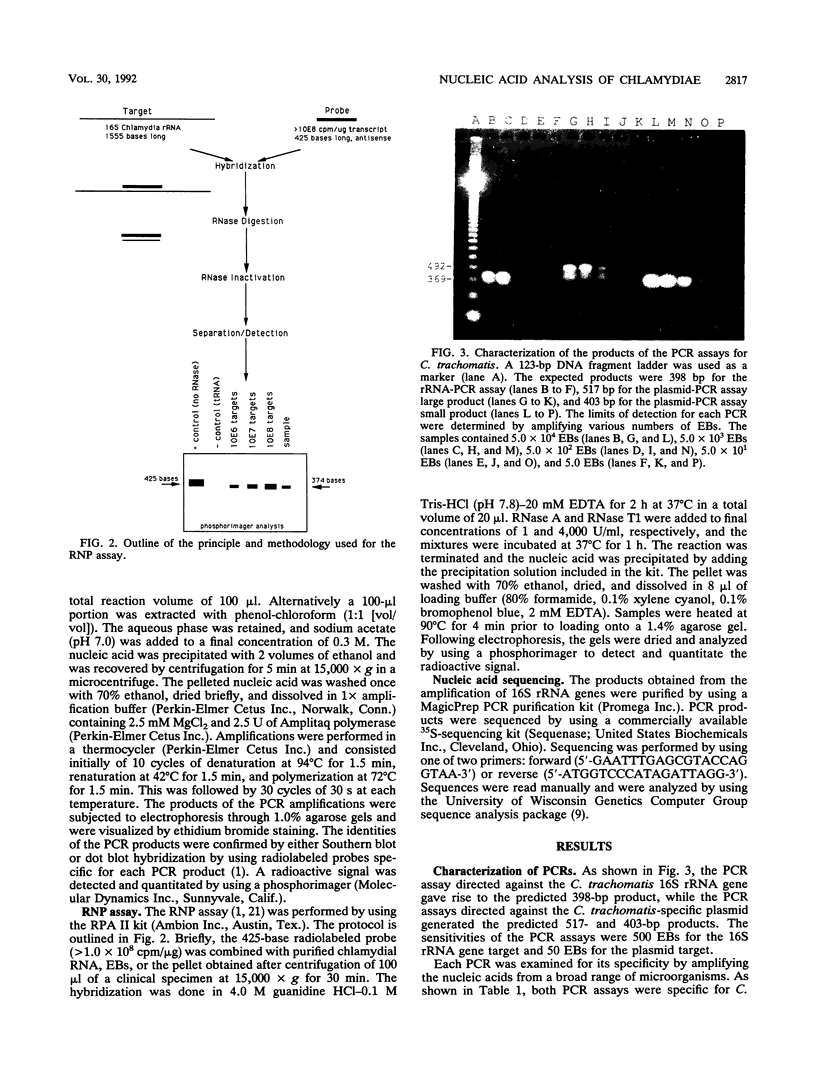
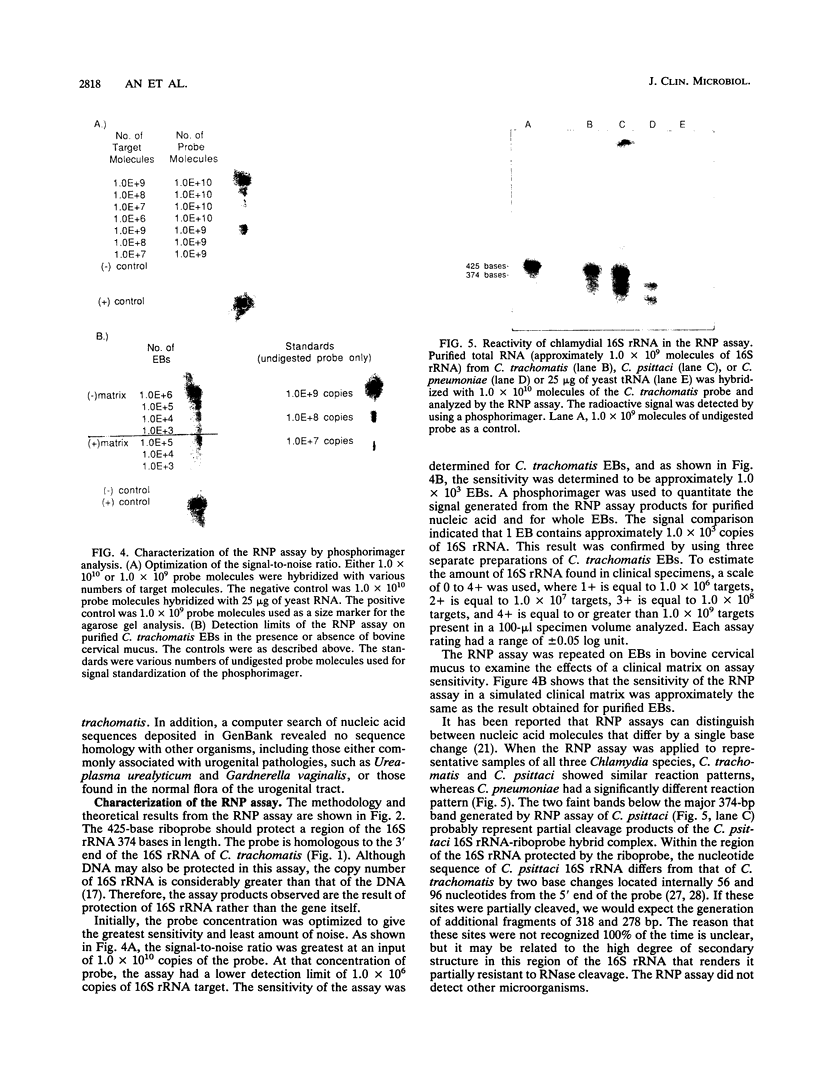
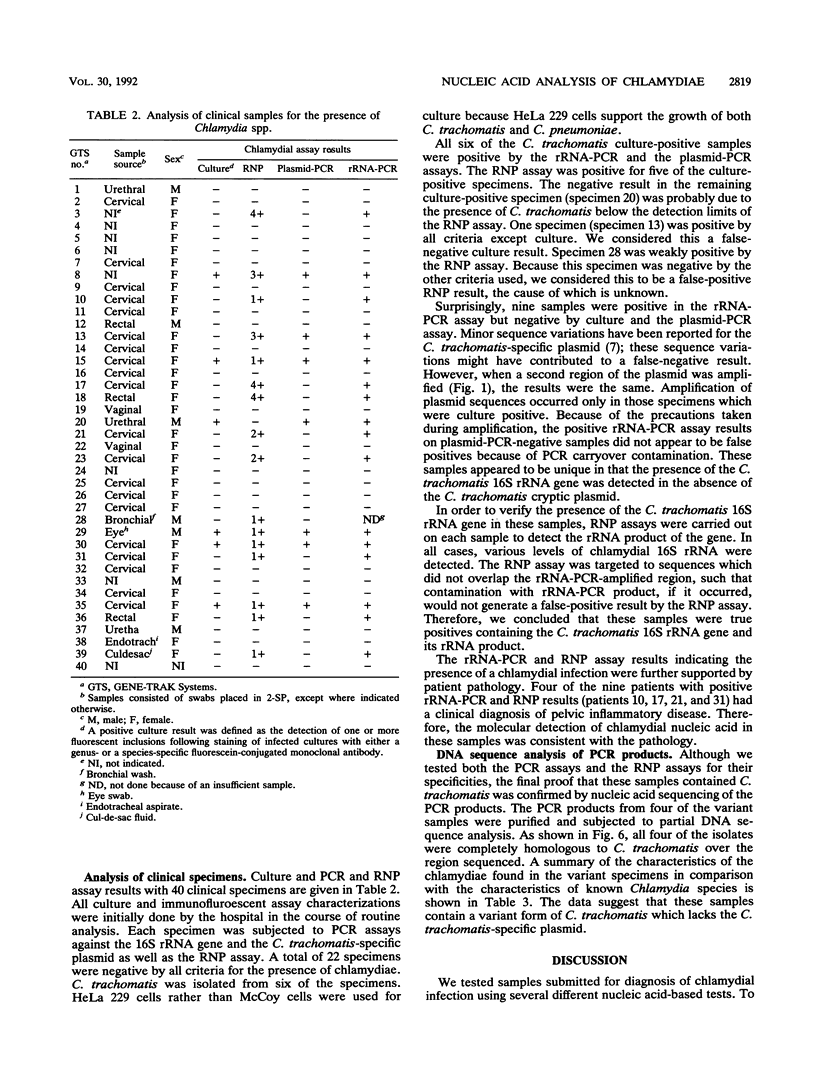
Abstract
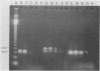
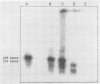
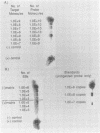
Clinical samples in transport media from 40 patients exhibiting pathologies potentially caused by Chlamydia trachomatis infection were analyzed for chlamydial nucleic acid, and the results were compared with those of culture. Chlamydial culture was performed by a shell vial centrifugation method with HeLa 229 host cells. Polymerase chain reaction (PCR) assays were used to detect either regions on a 7.5-kb plasmid characteristic of C. trachomatis (plasmid-PCR) or a segment of the 16S rRNA genes (rRNA-PCR). All PCR results were confirmed by hybridization with probes for the specific amplified products in either a Southern or a dot blot format. An RNase protection (RNP) assay was used to detect genus-specific chlamydial 16S rRNA directly from the clinical samples. The PCR assays detected C. trachomatis but not other bacteria, including Chlamydia spp. C. trachomatis was isolated from six samples which were positive by the rDNA-PCR and plasmid-PCR assays. Five of the culture-positive specimens were positive by the RNP assay. Twenty-two samples were negative by all criteria. Surprisingly, nine samples were positive by rRNA-PCR and RNP assays only. Nucleic acid sequencing of the rRNA-PCR-amplified products indicated a close relationship between the variants and C. trachomatis. The data may indicate an unrecognized process in C. trachomatis infection or that these patients were infected by a variant strain of C. trachomatis which lacks the C. trachomatis-specific plasmid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R. C. Laboratory diagnosis of human chlamydial infections. Clin Microbiol Rev. 1989 Apr;2(2):119–136. doi: 10.1128/cmr.2.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barns S. M., Lane D. J., Sogin M. L., Bibeau C., Weisburg W. G. Evolutionary relationships among pathogenic Candida species and relatives. J Bacteriol. 1991 Apr;173(7):2250–2255. doi: 10.1128/jb.173.7.2250-2255.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T. A., Grayston J. T. Centers for Disease Control guidelines for prevention and control of Chlamydia trachomatis infections. Summary and Commentary. Ann Intern Med. 1986 Apr;104(4):524–526. doi: 10.7326/0003-4819-104-4-524. [DOI] [PubMed] [Google Scholar]
- Cano R. J., Murrieta C. M., Spaulding D. C., Pascual A. Evaluation of a DNA probe of plasmid origin for the detection of Chlamydia trachomatis in cultures and clinical specimens. Mol Cell Probes. 1991 Dec;5(6):419–427. doi: 10.1016/s0890-8508(05)80013-1. [DOI] [PubMed] [Google Scholar]
- Comanducci M., Ricci S., Cevenini R., Ratti G. Diversity of the Chlamydia trachomatis common plasmid in biovars with different pathogenicity. Plasmid. 1990 Mar;23(2):149–154. doi: 10.1016/0147-619x(90)90034-a. [DOI] [PubMed] [Google Scholar]
- Comanducci M., Ricci S., Ratti G. The structure of a plasmid of Chlamydia trachomatis believed to be required for growth within mammalian cells. Mol Microbiol. 1988 Jul;2(4):531–538. doi: 10.1111/j.1365-2958.1988.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt C., Ward M. E., Clarke I. N. Analysis of the entire nucleotide sequence of the cryptic plasmid of Chlamydia trachomatis serovar L1. Evidence for involvement in DNA replication. Nucleic Acids Res. 1988 May 11;16(9):4053–4067. doi: 10.1093/nar/16.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. M., Gaydos C. A., Quinn T. C. Detection and differentiation of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae by DNA amplification. J Infect Dis. 1990 Oct;162(4):984–987. doi: 10.1093/infdis/162.4.984. [DOI] [PubMed] [Google Scholar]
- Iwen P. C., Blair T. M., Woods G. L. Comparison of the Gen-Probe PACE 2 system, direct fluorescent-antibody, and cell culture for detecting Chlamydia trachomatis in cervical specimens. Am J Clin Pathol. 1991 Apr;95(4):578–582. doi: 10.1093/ajcp/95.4.578. [DOI] [PubMed] [Google Scholar]
- Kellogg J. A., Seiple J. W., Murray C. L., Levisky J. S. Effect of endocervical specimen quality on detection of Chlamydia trachomatis and on the incidence of false-positive results with the Chlamydiazyme method. J Clin Microbiol. 1990 Jun;28(6):1108–1113. doi: 10.1128/jcm.28.6.1108-1113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans J. A., Niesters H. G., Mouton J. W., Quint W. G., Ijpelaar J. A., Van Rijsoort-Vos J. H., Habbema L., Stolz E., Michel M. F., Wagenvoort J. H. Performance of a nonisotopic DNA probe for detection of Chlamydia trachomatis in urogenital specimens. J Clin Microbiol. 1991 Dec;29(12):2685–2689. doi: 10.1128/jcm.29.12.2685-2689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- LeBar W., Herschman B., Jemal C., Pierzchala J. Comparison of DNA probe, monoclonal antibody enzyme immunoassay, and cell culture for the detection of Chlamydia trachomatis. J Clin Microbiol. 1989 May;27(5):826–828. doi: 10.1128/jcm.27.5.826-828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees M. I., Newnan D. M., Garland S. M. Comparison of a DNA probe assay with culture for the detection of Chlamydia trachomatis. J Med Microbiol. 1991 Sep;35(3):159–161. doi: 10.1099/00222615-35-3-159. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Palmer L., Falkow S. A common plasmid of Chlamydia trachomatis. Plasmid. 1986 Jul;16(1):52–62. doi: 10.1016/0147-619x(86)90079-x. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., Schachter J., de la Maza L. M. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid. 1990 Mar;23(2):144–148. doi: 10.1016/0147-619x(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Alexander R., Greenwood J. R., de la Maza L. M. Molecular techniques for the detection of Chlamydia trachomatis. J Clin Microbiol. 1989 Oct;27(10):2359–2363. doi: 10.1128/jcm.27.10.2359-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Stamm W. E. Diagnosis of Chlamydia trachomatis genitourinary infections. Ann Intern Med. 1988 May;108(5):710–717. doi: 10.7326/0003-4819-108-5-710. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D., Lee C. H., Larsen S. H. Detection of plasmid DNA from all Chlamydia trachomatis serovars with a two-step polymerase chain reaction. Appl Environ Microbiol. 1990 Aug;56(8):2494–2498. doi: 10.1128/aem.56.8.2494-2498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Young A., Scott J. C., Jr, Blair T. M., Johnson A. M. Evaluation of a nonisotopic probe for detection of Chlamydia trachomatis in endocervical specimens. J Clin Microbiol. 1990 Feb;28(2):370–372. doi: 10.1128/jcm.28.2.370-372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]