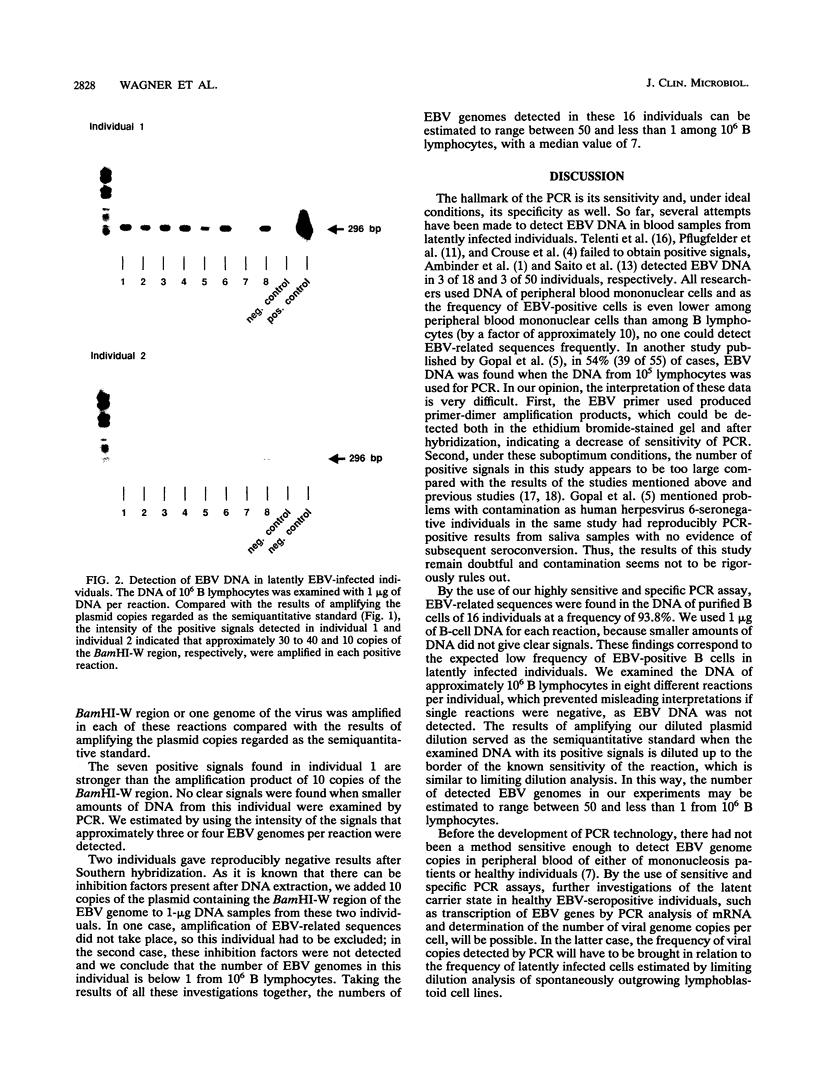
Abstract
We designed a highly sensitive and specific polymerase chain reaction assay for the detection of Epstein-Barr virus (EBV)-related sequences in B-cell DNA of EBV-seropositive healthy individuals. By using this assay, we were able to amplify at least 10 copies of a plasmid containing the BamHI-W region, which is repeated up to 11 times within the EBV genome, in the presence of 1 microgram of EBV-negative DNA, indicating that one virus genome was detectable from 150,000 cells. In 15 of 16 tested individuals, EBV-related sequences were found frequently when the DNA from 10(6) B lymphocytes was examined and 1 microgram of DNA was used in each polymerase chain reaction. When the results of amplifying the diluted plasmid were used as a semiquantitative standard, the number of EBV genomes detected could be estimated to range between 50 and less than 1 from 10(6) B lymphocytes. The results of our study will provide the basis for further investigations of the characteristics of the latent carrier state in healthy EBV-seropositive individuals, such as the determination of the number of virus copies per cell and expression of antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambinder R. F., Lambe B. C., Mann R. B., Hayward S. D., Zehnbauer B. A., Burns W. S., Charache P. Oligonucleotides for polymerase chain reaction amplification and hybridization detection of Epstein-Barr virus DNA in clinical specimens. Mol Cell Probes. 1990 Oct;4(5):397–407. doi: 10.1016/0890-8508(90)90030-4. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Cheung A., Kieff E. Long internal direct repeat in Epstein-Barr virus DNA. J Virol. 1982 Oct;44(1):286–294. doi: 10.1128/jvi.44.1.286-294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse C. A., Pflugfelder S. C., Cleary T., Demick S. M., Atherton S. S. Detection of Epstein-Barr virus genomes in normal human lacrimal glands. J Clin Microbiol. 1990 May;28(5):1026–1032. doi: 10.1128/jcm.28.5.1026-1032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal M. R., Thomson B. J., Fox J., Tedder R. S., Honess R. W. Detection by PCR of HHV-6 and EBV DNA in blood and oropharynx of healthy adults and HIV-seropositives. Lancet. 1990 Jun 30;335(8705):1598–1599. doi: 10.1016/0140-6736(90)91433-b. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. Epstein-Barr virus and blood transfusions. Prog Clin Biol Res. 1985;182:201–209. [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R. Recommended procedures for the diagnosis of persistent active herpesvirus infections. J Virol Methods. 1988 Sep;21(1-4):291–300. doi: 10.1016/0166-0934(88)90074-2. [DOI] [PubMed] [Google Scholar]
- Pflugfelder S. C., Crouse C., Pereira I., Atherton S. Amplification of Epstein-Barr virus genomic sequences in blood cells, lacrimal glands, and tears from primary Sjögren's syndrome patients. Ophthalmology. 1990 Aug;97(8):976–984. doi: 10.1016/s0161-6420(90)32476-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M., Boos H., Hirsch F., Mueller-Lantzsch N. Characterization of a latent protein encoded by the large internal repeats and the BamHI Y fragment of the Epstein-Barr virus (EBV) genome. Virology. 1988 Oct;166(2):586–590. doi: 10.1016/0042-6822(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Seibl R., Höltke H. J., Rüger R., Meindl A., Zachau H. G., Rasshofer R., Roggendorf M., Wolf H., Arnold N., Wienberg J. Non-radioactive labeling and detection of nucleic acids. III. Applications of the digoxigenin system. Biol Chem Hoppe Seyler. 1990 Oct;371(10):939–951. doi: 10.1515/bchm3.1990.371.2.939. [DOI] [PubMed] [Google Scholar]
- Telenti A., Marshall W. F., Smith T. F. Detection of Epstein-Barr virus by polymerase chain reaction. J Clin Microbiol. 1990 Oct;28(10):2187–2190. doi: 10.1128/jcm.28.10.2187-2190.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Blaese R. M. Epstein-Barr virus infection and immunoregulation in man. Adv Immunol. 1985;37:99–149. doi: 10.1016/s0065-2776(08)60339-9. [DOI] [PubMed] [Google Scholar]
- Yao Q. Y., Rickinson A. B., Epstein M. A. A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int J Cancer. 1985 Jan 15;35(1):35–42. doi: 10.1002/ijc.2910350107. [DOI] [PubMed] [Google Scholar]