Abstract
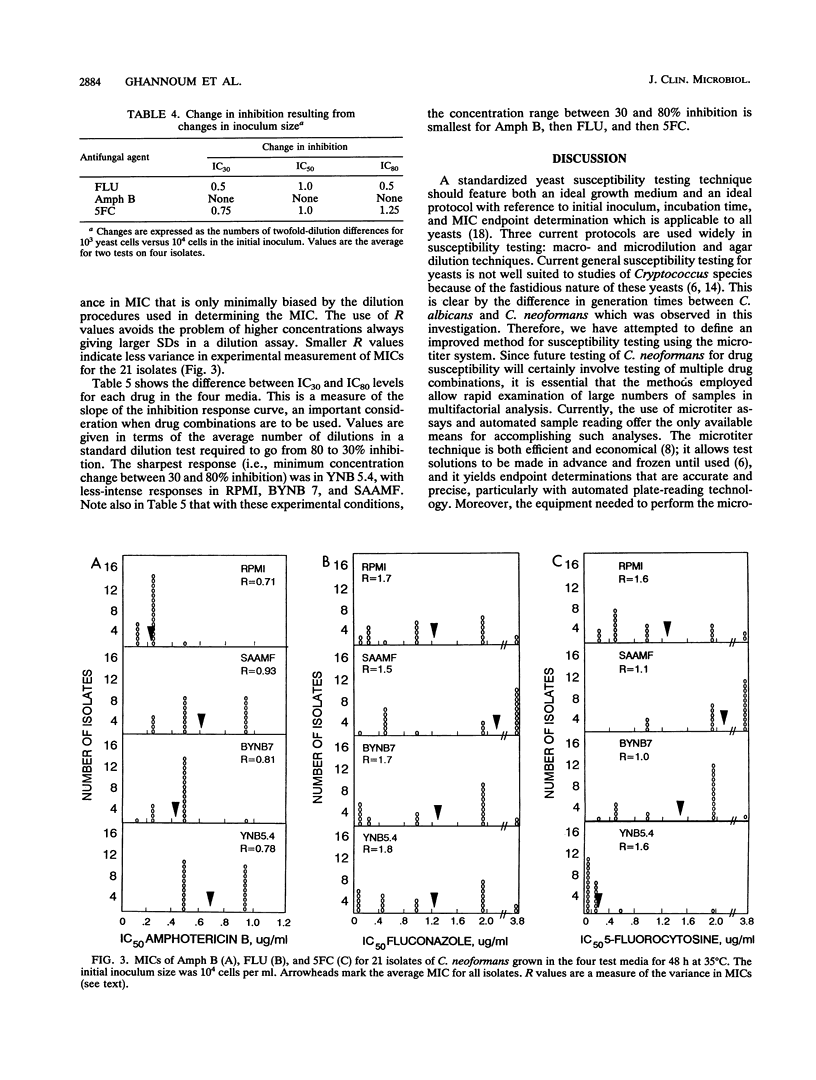
We studied a series of test conditions in a microtiter system to define the optimal method for determining the susceptibility of Cryptococcus neoformans to antifungal agents. Twenty-one isolates of C. neoformans were grown for 24 or 48 h in four chemically defined media: yeast nitrogen base (BYNB 7); RPMI 1640; synthetic amino acid medium--fungal (SAAMF), buffered at pH 7.0 to select the medium that best supported growth of this fastidious yeast; and yeast nitrogen base, pH 5.4 (YNB 5.4). Maximum growth of C. neoformans, at 35 degrees C, was obtained in YNB 5.4, with the next highest growth levels in BYNB 7, SAAMF, and RPMI. Growth at 24 h was uniformly poor in all media and lacked reproducibility. In contrast, incubation for 48 h gave adequate growth with low standard deviations, and 48 h was selected as the optimal incubation period for this study. Comparison of the relationship between growth kinetics and initial inoculum size for eight cryptococcal isolates showed that 10(4) cells per ml yielded optimal growth in BYNB 7 and YNB 5.4, whereas 10(5) cells per ml was optimal in RPMI and SAAMF. Furthermore, variation of inocula from 10(3) to 10(5) cells per ml showed small but significant inoculum effects in determining MICs of fluconazole, amphotericin B, and flucytosine for C. neoformans. Therefore, 10(4) cells per ml was chosen as the optimal inoculum for susceptibility testing in this study. Mean MICs of fluconazole, amphotericin B, and flucytosine for 21 crytococcal isolates in RPMI and BYNB 7 were low (for example, fluconazole had mean MICs of 1.2 and 1.3 micrograms/ml in RPMI and BYNB 7, respectively) and differed significantly from medium to medium. In contrast, the MICs obtained in SAAMF were significantly higher (e.g., fluconazole had a mean MIC of 2.2 micrograms/ml). Variance in MICs was large with fluconazole and flucytosine but small with amphotericin B, irrespective of the medium used. A microtiter system employing BYNB 7 as the medium, 48 h as the incubation period, and 10(4) cells per ml as the final inoculum is a simple, accurate, and reproducible method for the testing of C. neoformans susceptibility to fluconazole, amphotericin B, and flucytosine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block E. R., Jennings A. E., Bennett J. E. Variables influencing susceptibility testing of Cryptococcus neoformans to 5-fluorocytosine. Antimicrob Agents Chemother. 1973 Oct;4(4):392–395. doi: 10.1128/aac.4.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. A., McIntyre K. A., Galgiani J. N. Effects of incubation temperature, inoculum size, and medium on agreement of macro- and microdilution broth susceptibility test results for yeasts. Antimicrob Agents Chemother. 1990 Aug;34(8):1542–1545. doi: 10.1128/aac.34.8.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Dismukes W. E., Cloud G., Gallis H. A., Kerkering T. M., Medoff G., Craven P. C., Kaplowitz L. G., Fisher J. F., Gregg C. R., Bowles C. A. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med. 1987 Aug 6;317(6):334–341. doi: 10.1056/NEJM198708063170602. [DOI] [PubMed] [Google Scholar]
- Ellis N. S., Bartlett M. S., Smith J. W. Assay for yeast susceptibility to 5-fluorocytosine and amphotericin B in a frozen microtiter system. Am J Clin Pathol. 1979 Aug;72(2):194–198. doi: 10.1093/ajcp/72.2.194. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Bishburg E., Smith S. M., Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986 Jul;81(1):19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Kerkering T. M., Goldson P. R., Shadomy S. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1991 Jun;29(6):1089–1094. doi: 10.1128/jcm.29.6.1089-1094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiant J. N., Stevens D. A. Turbidimetric studies of growth inhibition of yeasts with three drugs: inquiry into inoculum-dependent susceptibility testing, time of onset of drug effect, and implications for current and newer methods. Antimicrob Agents Chemother. 1978 Feb;13(2):249–254. doi: 10.1128/aac.13.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum M. A. Effects of antineoplastic agents on growth, morphology and metabolism of Torulopsis glabrata. Mycopathologia. 1986 Sep;95(3):175–181. doi: 10.1007/BF00437124. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. The effect of pH and of temperature on the stability and bioactivity of nystatin and amphotericin B. J Pharm Pharmacol. 1973 May;25(5):401–407. doi: 10.1111/j.2042-7158.1973.tb10035.x. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Kovacs A. A., Polis M., Wright W. C., Gill V. J., Tuazon C. U., Gelmann E. P., Lane H. C., Longfield R., Overturf G. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Oct;103(4):533–538. doi: 10.7326/0003-4819-103-4-533. [DOI] [PubMed] [Google Scholar]
- Mazens M. F., Andrews G. P., Bartlett R. C. Comparison of microdilution and broth dilution techniques for the susceptibility testing of yeasts to 5-fluorocytosine and amphotericin B. Antimicrob Agents Chemother. 1979 Mar;15(3):475–477. doi: 10.1128/aac.15.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Quantitative microculture system with standardized inocula for strain typing, susceptibility testing, and other physiologic measurements with Candida albicans and other yeasts. J Clin Microbiol. 1991 Dec;29(12):2735–2740. doi: 10.1128/jcm.29.12.2735-2740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Burmeister L., Bartlett M. S., Rinaldi M. G. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988 Aug;26(8):1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radetsky M., Wheeler R. C., Roe M. H., Todd J. K. Microtiter broth dilution method for yeast susceptibility testing with validation by clinical outcome. J Clin Microbiol. 1986 Oct;24(4):600–606. doi: 10.1128/jcm.24.4.600-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuger A., Louie E., Holzman R. S., Simberkoff M. S., Rahal J. J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986 Feb;104(2):234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]
- Zuger A., Schuster M., Simberkoff M. S., Rahal J. J., Holzman R. S. Maintenance amphotericin B for cryptococcal meningitis in the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1988 Oct 1;109(7):592–593. doi: 10.7326/0003-4819-109-7-592. [DOI] [PubMed] [Google Scholar]


