Abstract
Honey bees are social insects that exhibit striking caste-specific differences in longevity. Queen honey bees live on average 1–2 years whereas workers live 2 to 6 weeks in the summer and about 20 weeks in the winter. It is not clear whether queen-worker differences in longevity are due to intrinsic physiological differences in the rate of senescence, to differential exposure to extrinsic factors such as predation and adverse environmental conditions, or both. To determine if the relatively short life span of worker bees involves senescence, we measured age-specific resistance to three different physiological stressors (starvation, thermal, and oxidative stress) while eliminating age-related differences in foraging activity and minimizing age-related differences in energy expenditure. Despite these manipulations, older worker bees were still significantly less resistant to all three stressors than were younger bees. These results indicate that the regulation of worker bee lifespan involves senescence, in addition to extrinsic factors.
Keywords: aging, eusociality, stress-resistance, evolution, physiology
Introduction
Senescence is defined as an age-related decline in physiological function, performance, survival, or reproduction (Finch, 1990). Senescence (often referred to simply as “aging”) is a nearly-universal feature of multicellular organisms, and appears to occur even in unicellular yeast and bacteria (Lithgow, 1996; Kirkwood, 2000). Understanding the biological processes that lead to senescence, and why different organisms senesce at dramatically different rates, is a long-standing problem in both molecular and evolutionary biology (Lithgow, 1996; Finch, 1990; Rose, 1991; Partridge, 1993; Kirkwood, 2000).
In some eusocial insects (ants, bees, wasps, and termites), queens and workers of the same species sometimes show a 100-fold difference in lifespan, with reproductive queens having longer lifespans than the non-reproductive workers (Winston, 1987; Keller and Genoud, 1997; Page and Peng, 2001). Strikingly, the long life of social insect queens does not come at the cost of low reproduction: queens of many social insects lay hundreds or thousands of eggs per day throughout their adult life. Their ability to sustain both high reproductive effort and long life makes social insects particularly promising model systems for studies of aging (Parker et al, 2004; Seehus et al, 2006; Corona et al., 2005; Corona et al., 2007).
In the honey bee, Apis mellifera, queens have an average lifespan of 1–2 years and workers have an average lifespan of 15–38 days in the summer and 140 days in the winter (Winston, 1987). Queens and workers are not genetically distinct, so biological differences between castes are due to gene expression differences that depend on social and dietary cues that individuals experience during development (Corona et al., 2005). Therefore studying the comparative physiology and molecular biology of queens and workers is an attractive paradigm for investigating proximate mechanisms of lifespan differences (Parker et al., 2004; Corona et al., 2005). However, there is a potential serious flaw in this paradigm: it is currently not known whether caste-specific lifespan differences result from inherent physiological differences in the rate of senescence or, alternately, from caste-related differences in exposure to risk.
In nature, queen bees leave the protected environment of the hive only to take mating flights at 1 to 2 weeks of age, and possibly once more later in their life, during colony fission. In contrast, workers spend the first 2–3 weeks of adult life mostly in the hive performing tasks such as brood care (“nursing”) before shifting to foraging outside the hive for nectar and pollen, making over ten trips a day, sometimes at distances of up to 2 km (Winston, 1987). Foragers thus experience risks from predation, thermal stress, and physical exhaustion; risks that queens (and pre-foragers, such as nurse bees) do not experience to the same extent. Thus, a plausible hypothesis for the difference in queen and worker lifespan is that workers, once they become foragers, experience high extrinsic mortality and therefore have a much shorter mean lifespan than queens.
Only a few studies have addressed the question of whether worker bee lifespan is determined by senescence or exposure to extrinsic risk. Neukirch (1982) compared lifespans of foragers with different amounts of flight experience and found that lifespan was inversely related to daily flight experience. She argued that foragers have fixed energy reserves, and, once the reserve is depleted, foragers cannot fly and fail to return to the hive. This idea does not require physiological senescence. In contrast, later studies found patterns consistent with senescence. Schmid-Hempel and Wolf (1988) found that workers had fixed lifespans regardless of energy expenditure, and Visscher and Dukas (1997) found that behavioral and foraging performance declined after 10 days of foraging. A limitation of all these studies is that age-specific survival data were collected on foragers and so were possibly confounded by the cumulative effects of energy expenditure and foraging activity. Because of the lifestyle of the forager, age-related increases in mortality rates could be due to accumulation of injuries or exhaustion of energy reserves, which are not necessarily due to intrinsic physiological deterioration.
We exploited the honey bee’s strong plasticity for division of labor (Robinson, 1992) to remove the confounding effects of energy expenditure and risks associated with foraging. Worker bees respond to changing social conditions by accelerating, delaying, or reversing their typical pattern of behavioral maturation. For example, if there is a shortage of foragers or large numbers of young larvae in the hive, some bees delay their transition to foraging and become “overage” nurses (Robinson et al., 1989). We studied age-specific stress resistance in overage nurses that did not did not experience the extrinsic risk factors associated with foraging. We predicted that if there is worker senescence, then older bees should have lower survival under each stress treatment than younger bees.
Materials and Methods
Experimental colonies
We set up five single-cohort colonies (Robinson et al., 1989), each initially composed of ca. 10,000 one-day-old bees. We obtained one-day old worker bees by removing frames of pupae from typical field colonies (headed by naturally mated queens) and placing them in an incubator (34°C and 80% relative humidity). The bees were marked with a paint dot on the dorsal thorax, color coded according to day of emergence and source colony. This process was continued over a 5- day period for each colony to obtain the 10,000 bees. Each single-cohort colony was then given a (naturally-mated) queen, 4 frames of honey and pollen, and 2 frames for the queen to lay eggs in. We encouraged the development of overage nurses by removing frames of brood prior to the emergence of new adult bees and replacing them with frames of younger brood.
Collections of bees
At each collection date, we collected 300 bees from each age class (10, 30, and 50 days old) that was available at that date. We collected bees that were displaying typical nursing behavior (head in cell containing a larva; see Huang and Robinson (1996). Collections were made when foragers were out of the hive during times of active foraging to minimize the chances of misidentification. The five single-cohort colonies were set up in a time-staggered design, so that bees of different age classes were available on the same day (Figure 1). We were thus able to evaluate the effects of age on stress resistance, and decouple these effects from effects of source colony and date of collection (seasonality). Bees were held individually in cages within a plexiglass tray, provided with 50% sucrose solution ad lib, and kept at constant temperature (34°C) for 24 h prior to the start of the stress tests. After 24 h, the surviving bees were randomly assigned to the three treatment groups. Total sample sizes for each age class and treatment group are given in Table 1.
Figure 1.

Schematic of time-staggered experimental design, so that nurse bees of different age classes were available for treatment on the same day. Rows indicate each experimental (single-cohort) colony and columns indicate the collection dates for bees of the different age classes used in the three stress resistance tests. This experimental design enabled us to evaluate the effects of age on stress resistance, taking into account influences of both source colony and date of collection (seasonality). We collected 10, 30, and 50 day old bees from all colonies with the exception of Colony 4 (10- and 30-day-old bees only) and Colony 5 (10-day old bees).
Table 1.
Effects of starvation, heat, and hydrogen peroxide (oxidative stress) on lifespan for nurse honey bees 10, 30, and 50 days of age. Mean and median lifespan (h).
| Treatment | Age class | Mean (S.E) | Median | Sample Size |
|---|---|---|---|---|
| Starvation | 10 | 91 (5.0) | 42 | 478 |
| 30 | 46 (1.4) | 36 | 375 | |
| 50 | 31 (0.9) | 24 | 287 | |
| Heat | 10 | 120 (2.2) | 120 | 484 |
| 30 | 107 (2.5) | 102 | 371 | |
| 50 | 106 (2.6) | 102 | 288 | |
| Hydrogen Peroxide | 10 | 82 (2.5) | 75 | 479 |
| 30 | 81 (2.8) | 66 | 371 | |
| 50 | 75 (2.5) | 66 | 287 |
Stress tests
To detect senescence, we measured the effects of oxidative stress, heat stress, and starvation on bees from each age class. Resistance to these stressors typically declines in senescing insects, causing increased mortality (Luckingbill et al., 1984; Rose, 1984; Nghiem, 2000). After the collections were made, during the next 24 hours, bees were housed in an incubator at 34 °C and were provided a 50% sucrose solution so that they could feed freely. After that, bees of the same age class that were still alive were randomly assigned to three different trays and one of the three trays was assigned to each one of the treatments (Starvation, heat stress, or hydrogen peroxide). Treatment details are as follows.
Hydrogen peroxide
Bees were given a 50% sucrose solution that contained 20% hydrogen peroxide. This dose was based on results from Drosophila melanogaster that showed that a dose of 5% hydrogen peroxide produced high mortality (Sun and Tower, 1999), adjusting for differences in body mass between honey bees and fruit flies.
Heat stress
We exposed bees to 42°C in an incubator; colonies typically maintain their hives at approximately 34°C by behavioral thermoregulation, and it has been reported (Mardan and Kevan, 2002) that bees kept at 42°C showed decreased longevity. Bees were kept at 42°C until they died.
Starvation
Bees were maintained in an incubator without any food at 34°C, and were provided with water to prevent desiccation. In all treatments, bees were housed in individual cages within a plexiglass tray. With the exception of the starvation treatment, food was provided in the tray, and bees were allowed to feed freely. Food in the trays was replaced every 6 hours, and water was replenished for the bees in the starvation treatment. Food replacement was of special importance for the hydrogen peroxide treatment, since hydrogen peroxide degrades in water. Bees in all incubators were maintained in a 24-hour dark cycle; the hive is naturally dark, except for whatever light penetrates from the hive entrance.
Censusing mortality
Bees were censused at 0.00, 6.00, 12.00 and 18.00 hours until all were dead. Information on age and source colony was obtained from the thorax markings. Six bees escaped during the experiment (3 in the heat stress and 3 in the starvation treatment) the escape time of these bees was treated as a right-censored observation in the data analysis.
Lipid analysis
Because the most striking differences in age-specific stress resistance were observed in the starvation test (see Results), we explored whether the results could be explained by differences in lipid reserves. We measured the abdominal lipid levels of young and overage nurses, using foragers as a comparison group, since foragers have the lowest lipid levels among worker bees (Toth and Robinson, 2005). We used young nurses less than 7 days of age (n=23), 50-day old nurses (n=23) and 50-day old foragers (n=22). Each abdomen was dissected and the digestive tract and sting apparatus removed; abdomens were then freeze-dried, homogenized in a 2:1 chloroform:methanol solution, and dried down to a constant volume of 2 ml. The lipid assay was performed using 100 μl of each sample, following the procedures in Toth et al (2005). We measured the absorbance of each sample using a SpectraMax 190 spectrophotometer (Molecular Devices, CA), with readings at 525 nm. Absorbance readings were converted to milligrams of lipid using a cholesterol standard. The lipid assay was performed twice on each sample.
Data Analysis
We calculated Kaplan-Meier (product-limit) survival estimates for the 10, 30-, and 50-day old workers for each stress treatment. We tested for differences in survival among age classes within a treatment using the log-rank and Wilcoxon tests produced by SAS Proc Lifetest (SAS System v.9.1). Wilcoxon tests are more sensitive to differences in survival occurring earlier in the trials, while log-rank tests are more sensitive to differences that occur later (Allison, 1995). Results of both tests were consistent in every case, so we report only the log-rank test results. We also tested for significant differences between age classes using Cox proportional hazards models as implemented in SAS Proc Phreg. This test allowed direct comparison of the hazard rate (risk of death per unit time) for each age class within a treatment group, and formal statistical tests for pairwise differences in hazard rates between age classes (Allison, 1995). In this analysis, a hazard ratio >1 indicates a higher hazard for the older bees, and a value <1 indicates a lower hazard for the older bees. We repeated the pairwise contrast analysis after removing data for colonies 4 and 5; because these colonies are represented by two (or one) age classes there is a possibility of confounding age and colony effects. For the analysis of lipid data, we treated the replicate measures for each sample as repeated measures in a general linear model (repeated-measures ANOVA) using SAS Proc Mixed (Littell, 2002).
Results
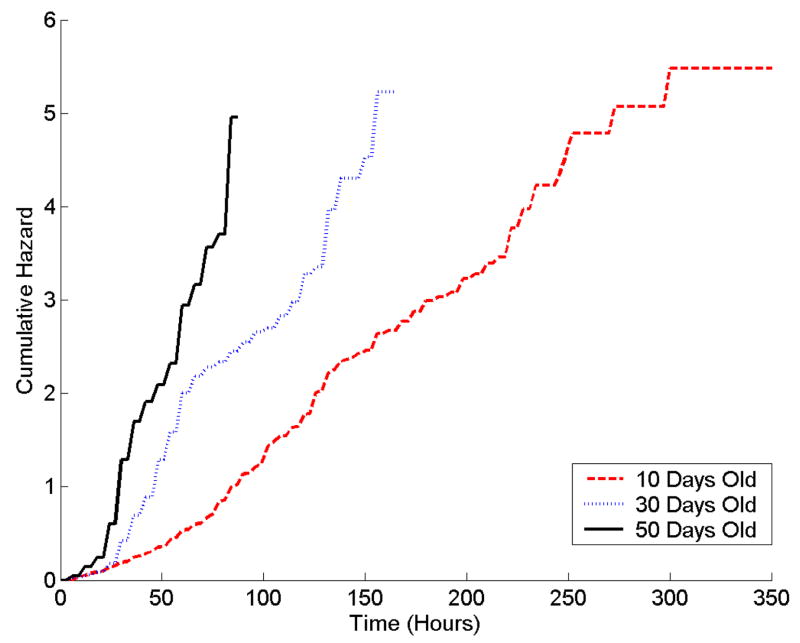
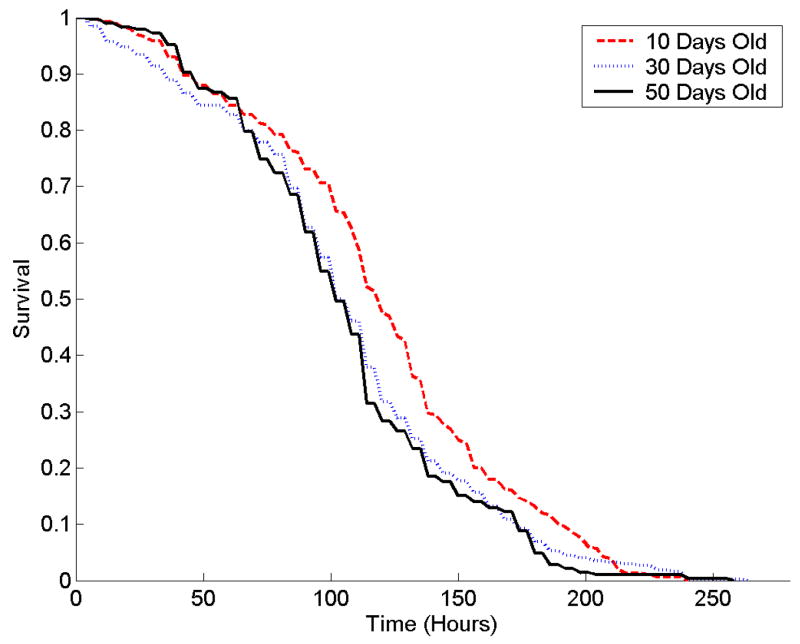
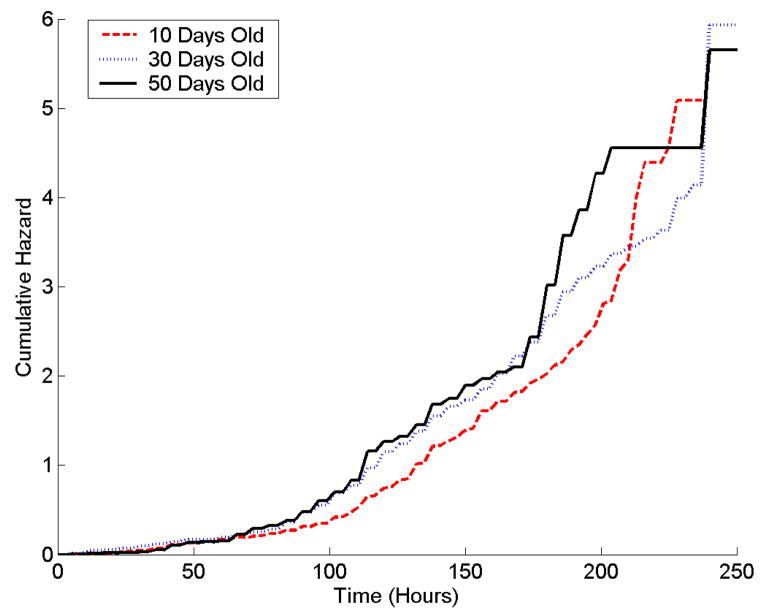
Mean survival times for 10-day old bees were longer than for older bees in all three stress tests (Table 1). Survival curves show that 10-day old bees had higher survival at each time point than did 50-day old bees (Figure 2).
Figure 2.
Age-related differences in resistance to: A) starvation; B) heat, and C) hydrogen peroxide in nurse honey bees. Survival distribution (top) function and cumulative hazard function (-Log (Survival), bottom) of 10- (red) 30- (blue) and 50-day-old (black) nurse bees. Bees were censused every 6 h. Note difference in scale for treatments. Eighteen 10-day old bees were alive in the starvation treatment; one 10-day old bee and one 30-day old bee were alive in the hydrogen peroxide treatment after 350 hours of exposure to stress.
Log-rank tests of survival times indicated that age classes differed significantly for the starvation (χ2= 202.6, p<.0001) and heat stress treatments (χ2= 20.9, p<.0001), but not for the hydrogen peroxide treatment (χ2= 2.6, p=0.27). However, the semi-parametric tests of the proportional hazards model indicated that differences in hazard rates between age classes were significant for all three treatments: starvation (χ2= 102.2, p<.0001), heat stress (χ2= 50.82, p<.0001), and hydrogen peroxide (χ2=7.8, p= 0.0205).
Similarly, pairwise contrasts of the hazard rates within treatments indicated that ten-day old bees had significantly lower mortality per unit time than did 50-day olds in each treatment (Table 2). All hazard ratio estimates were >1, indicating higher mortality rates for older bees in each comparison; comparisons were significant in 6 out of 9 pairwise tests, and marginally non-significant at P=0.05 in one additional comparison (Table 2). Limiting the analysis to colonies 1 through 3 produced qualitatively identical results. In this analysis, 10-day old bees had significantly lower mortality than 50-day old bees in all three stress treatments (Appendix 1).
Table 2.
Effects of starvation, heat, and hydrogen peroxide (peroxide) on lifespan for nurse honey bees 10, 30, and 50 days of age. Hazard ratios for each pairwise comparison between age classes (ratio of older to younger bees); degrees of freedom = 1 in every case. Results in bold indicate significant differences.
| Treatment | Contrast | Hazard ratio | Confidence | limits | χ2 | P |
|---|---|---|---|---|---|---|
| Starvation | 10 vs. 30 | 1.514 | 1.280 | 1.791 | 23.4 | <.0001 |
| Starvation | 10 vs. 50 | 2.637 | 2.183 | 3.184 | 101.4 | <.0001 |
| Starvation | 30 vs. 50 | 1.741 | 1.469 | 2.064 | 40.9 | <.0001 |
| Heat | 10 vs. 30 | 1.506 | 1.3 | 1.744 | 29.7 | <.0001 |
| Heat | 10 vs. 50 | 1.739 | 1.475 | 2.049 | 43.5 | <.0001 |
| Heat | 30 vs. 50 | 1.155 | 0.982 | 1.358 | 3.0 | 0.08 |
| Peroxide | 10 vs. 30 | 1.073 | 0.927 | 1.241 | 0.9 | 0.34 |
| Peroxide | 10 vs. 50 | 1.257 | 1.069 | 1.478 | 7.6 | 0.006 |
Appendix 1.
Effects of starvation, heat, and hydrogen peroxide (peroxide) on lifespan for nurse Money bees 10, 30, and 50 days of age (restricted to colonies 1, 2, and 3). Hazard ratios for each pairwise comparison between age classes (ratio of older to younger bees), degrees of freedom = 1 in every case. Results in bold indicate significant differences.
| Treatment | Contrast | Hazard ratio | Confidence | limits | χ2 | P |
|---|---|---|---|---|---|---|
| Starvation | 10 vs. 30 | 1.109 | 1.821 | 2.651 | 67.4 | 0.27 |
| Starvation | 10 vs. 50 | 2.197 | 0.921 | 1.334 | 1.2 | <.0001 |
| Starvation | 30 vs. 50 | 1.982 | 1.658 | 2.368 | 56.5 | <.0001 |
| Heat | 10 vs. 30 | 1.524 | 1.286 | 1.806 | 23.6 | <.0001 |
| Heat | 10 vs. 50 | 1.702 | 1.434 | 2.020 | 37.1 | <.0001 |
| Heat | 30 vs. 50 | 1.117 | 0.945 | 1.321 | 1.68 | 0.19 |
| Peroxide | 10 vs. 30 | 1.025 | 0.869 | 1.209 | 0.0839 | 0.7721 |
| Peroxide | 10 vs. 50 | 1.213 | 1.027 | 1.433 | 5.1783 | 0.0229 |
| Peroxide | 30 vs. 50 | 1.184 | 1.002 | 1.399 | 3.9418 | 0.0471 |
There were no significant differences in stored lipid in young and old nurses (F[1,64]= 2.6, P=0.12, Figure 3). Both young and old nurses had significantly higher lipid content than foragers (young nurses vs. foragers, F[1,64]=52.3, P<0.0001; old nurses vs. foragers, F[1,64]=31.9, P<0.0001). These results indicate that results of the starvation test are not attributable to differences in stored lipids between young and old nurse bees; overage nurses have lipid levels characteristic of nurses, and not of foragers. These results are consistent with findings from Toth et al. (2005).
Figure 3.
Abdominal lipid content for 7-day-old nurses, 50-day old nurses, and 50-day old foragers. Letters indicate groups that differ significantly in mean lipid content by pair-wise contrasts. Numbers at bottom of bars indicate sample size
Discussion
Our results provide the first clear demonstration of worker honey bee senescence. In our experiments this physiological decline began between 10 and 30 days of age and continued through 50 days of age. These results indicate that honey bee workers experience an intrinsic physiological decline at an age that is consistent with their observed maximal lifespan in the summer and their longevity does not depend solely on extrinsic mortality factors.
Our results are unlikely to be due to differences in physical activity because we used overage nurses rather than foragers. It is unlikely that our results, especially for the starvation treatment, can be attributed to older nurses having lower nutritional reserves than younger nurses. Our lipid analysis showed no difference between lipid stores in young and overage nurses, but other nutritional indicators such as glycogen content were not measured. We conclude that the marked decline in stress resistance in 30- and 50-day old bees strongly suggests physiological senescence.
Results from the heat stress assay indicated that 30- and 50-day old bees were more likely to die than 10-day old bees. Although the differences were highly significant, they were less extreme than in the starvation assay. Perhaps this is because the treatment was relatively less extreme. Honey bees can tolerate temperatures up to 45 °C for at least 2 hours, and humidity is an important factor in their ability to tolerate high temperatures (Free and Spencer-Booth, 1962). Perhaps our treatment was not as stressful as it could have been because bees were provided with an unlimited source of sugar syrup and full water containers were kept in the incubator at all times.
Differences between age classes in the hydrogen peroxide treatment were relatively small (though statistically significant) compared to the starvation and heat treatments. It seems unlikely that bees in the hydrogen peroxide treatment were not feeding since the median lifespan of bees of all age classes surpassed that of bees in the starvation treatment. It is possible that the concentration of hydrogen peroxide we used was too weak to induce much oxidative stress or stress-related mortality in our bees. This speculation is supported by the observation that paraquat (another free radical inducing agent) caused greater mortality in a comparable experiment (Corona et al., 2007). In that experiment, the median lifespan for worker bees 30 days of age was 33 h, compared to 66 h in our experiment. This observation is further supported by another experiment comparing paraquat-induced oxidative stress resistance in worker bees where complete mortality was reached within 60 hours of paraquat injection (Seehuus et al, 2006).
Hydrogen peroxide is an oxidizing agent that slowly decomposes into water and oxygen at room temperature. The decomposition of hydrogen peroxide can be accelerated in the presence of light and at high temperatures, increasing by a factor of 2.2 for every 10 °C rise in temperature. Such decomposition is also catalyzed by dissolved ions of metals, and suspended oxides and hydroxides (Goor et al, 1992). Even though we replaced the hydrogen peroxide and sugar solution in the trays every 6 hours, there is a possibility that the decomposition of hydrogen peroxide into water and oxygen may have caused failure to induce mortality in our bees.
Ruepell et al. (2005) found that age-specific mortality increased exponentially in drones after about the 10th day of flying activity, consistent with either senescence, non-replenishment of resources or ‘wear and tear’. They also reported that lifespan after the initiation of flying activity was negatively correlated with age at first flight, and suggested that this pattern was due to the onset of senescence even before the initiation of flight. This suggestion is consistent with our experimental results for workers.
Senescence of honey bee hemocytic cells has been reported by Amdam et al. (2004 and 2005). Amdam et al (2004) found that foragers had low zinc concentrations compared to nurses, which in turn resulted in decreased hemocyte counts in the hemolymph; foragers also possessed a higher number of pycnotic cells than nurses. Working with reverted nurses, Amdam et al (2005) showed that these changes were related to both age and behavioral role; reverted nurses had a higher hemocyte count relative to similarly aged bees that continued to forage, but reverted nurses had lower counts relative to normal-age (young) nurses. The authors assumed that hemocyte count and cell pycnosis are measures of senescence at the cellular level. However, there is no data on the relationship between hemocyte count and immune response or mortality rate, so it is not clear in this case that cellular senescence leads to organismal senescence.
Our results show that worker bees show senescence. In contrast, in a recent study Ruepell et al (2007) assessed age-dependent behavioral performance of foragers using a battery of behavioral tests that included light sensitivity, sucrose responsiveness, learning of olfactory cues, and walking velocity. In that study, the authors conclude that worker bees do not exhibit an age-dependent decline in performance but show an increase in mortality with chronological age. The discrepancies between our results and those of Ruepell et al. may be attributed to the nature of the behavioral tests employed. Although the behavioral tests employed are related to foraging activity they may not prove demanding to the bees and thus not allow the possibility for a decline to be manifest. Previous studies in D. melanogaster show that age-related declines in behavior differ depending on the nature of the behavior being tested, the genotype, and the gender of the flies (Fernandez et al, 1999; Martin and Grotewiel, 2006; Simon et al, 2006).
We have shown here that limited worker lifespan is due at least in part to intrinsic senescence and not solely to extrinsic mortality factors. Of interest would be to determine if honey bee queens also show senescence. Studying senescence in queens is a more difficult question to address than in workers, given their extended lifespan. In addition, conducting such tests in a eusocial species presents special challenges since queens are fed and groomed by workers. Although we did not directly study queen senescence, queens are known to lay up to 2000 eggs per day and the laying rate does not appear to decline at least through the first year of life (Winston, 1987), suggesting negligible senescence during this period. In contrast, we have shown that senescence in workers begins before 50 days of age. This comparison suggests that the extended lifespan of queens is due to slower senescence and not just to lower extrinsic mortality.
Acknowledgments
We thank Karen Pruiett for field assistance; Charles Nye, Sara Kantarovich, Spencer Beard, Gabriel Fuenzalida, Aaron Bergman, and Adam Escalante for assistance with nurse bee collections; Amy Toth and James Bilof for assistance with lipid assays, and members of the Hughes and Robinson laboratories for comments that improved the manuscript. Supported by NIH-NIA grant AG022824 (GER and KAH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison PD. Survival Analysis Using SAS: A Practical Guide. SAS Institute, Inc; Cary, N.C: 1995. [Google Scholar]
- Amdam GV, Simões ZLP, Hagen A, Norberg K, Schrøder K, Mikkelsen O, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Experimental Gerontology. 2004;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Aase ALTO, Seehuus SC, Fondrk MK, Norberg K, Hartfelder K. Social reversal of immunosenescence in honey bee workers. Experimental Gerontology. 2005;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mechanisms of Ageing and Development. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Corona M, Velarde RV, Remolina S, Moran-Lauter A, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling and queen honey bee longevity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez JR, Grant NM, Tulli LM, McClearn GE. Differences in locomotor activity across the lifespan of Drosophila melanogaster. Experimental Gerontology. 1999;34:621–631. doi: 10.1016/s0531-5565(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. Chicago: The University of Chicago Press; 1990. [Google Scholar]
- Free JB, Spencer-Booth Y. The upper lethal temperatures of honeybees. Entomologia Experimentalis et Applicata. 1962;5:249–254. [Google Scholar]
- Goor G, Glenneberg J, Jacobi S. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VHC; 1992. Hydrogen Peroxide. [Google Scholar]
- Huang ZY, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behavioral Ecology and Sociobiology. 1996;39:147–158. [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Kirkwood TBL, Austad S. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, Kirkwood TBL. Mechanisms and evolution of aging. Science. 1996;273:5271–5280. doi: 10.1126/science.273.5271.80. [DOI] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Freund RJ. SAS for Linear Models. SAS Institute, Inc; Cary, N.C: 2002. [Google Scholar]
- Luckinbill LS, Arking R, Clare MJ, Cirocco WC, Buck SA. Selection for delayed senescence in Drosophila melanogaster. Evolution. 1984;38:996–1003. doi: 10.1111/j.1558-5646.1984.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Mardan M, Kevan PG. Critical temperatures for survival of brood and adult workers of the giant honeybee, Apis dorsata (Hymenoptera: Apidae) Apidologie. 2002;33:295–301. [Google Scholar]
- Matrin I, Grotewiel MS. Distinct genetic influences on locomotor senescence in Drosophila revealed by a series of metrical analyses. Experimental Gerontology. 2006;41:877–881. doi: 10.1016/j.exger.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Neukirch A. Dependence of the lifespan of the Honeybee (Apis Mellifica) upon flight performance and energy consumption. Journal of Comparative Physiology B. 1982;146:35–40. [Google Scholar]
- Nghiem D, Gibbs AG, Rose MR, Bradley TJ. Postponed aging and desiccation resistance in Drosophila melanogaster. Experimental Gerontology. 2000;35:957 –969. doi: 10.1016/s0531-5565(00)00163-7. [DOI] [PubMed] [Google Scholar]
- Page RE, Peng CYS. Aging and development in social insects with emphasis on the honey bee, Apis Mellifera L. Experimental Gerontology. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Parker JD, Parker KM, Sohal BH, Sohal RS, Keller L. Decreased expression of Cu-Zn superoxide dismutase 1 in ants with extreme life-span. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3486–3489. doi: 10.1073/pnas.0400222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Evolution of aging: Testing the theory using Drosophila. Genetica. 1993;91:89–98. doi: 10.1007/BF01435990. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Page RE, Strambi C, Strambi A. Hormonal and genetic control of behavioral integration in honeybee colonies. Science. 1989;246:109–112. doi: 10.1126/science.246.4926.109. [DOI] [PubMed] [Google Scholar]
- Robinson GE. Regulation of division of labor in insect societies. Annual Review of Entomology. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Rose M. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Rose M. Evolutionary Biology of Aging. Oxford University Press; New York: 1991. [Google Scholar]
- Rueppell O, Fondrk K, Page R. Biodemographic analysis of male honey bee mortality. Aging Cell. 2005;4:13–19. doi: 10.1111/j.1474-9728.2004.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Christine S, Mulcrone C, Groves L. Aging without functional senescence in honey bee workers. Current Biology. 2007;17:R274–R275. doi: 10.1016/j.cub.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P, Wolf T. Foraging effort and lifespan of workers in a social insect. The Journal of Animal Ecology. 1988;57:509–521. [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Liang DT, Krantz DE. Differential decline in behavioral performance of Drosophila melanogaster with age. Mechanisms of Ageing and Development. 2006;127:647–651. doi: 10.1016/j.mad.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Molecular and Cellular Biology. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AL, Robinson GE. Worker nutrition and division of labour in honeybees. Animal Behaviour. 2005;69:427–435. [Google Scholar]
- Toth AL, Kantarovich S, Meisel AF, Robinson GE. Nutritional status influences socially regulated foraging ontogeny in honey bees. The Journal of Experimental Biology. 2005;208:4641–4649. doi: 10.1242/jeb.01956. [DOI] [PubMed] [Google Scholar]
- Visscher PK, Dukas R. Survivorship of foraging honeybees. Insectes Sociaux. 1997;44:1–5. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Cambridge, Massachusetts: Harvard University Press; 1987. [Google Scholar]