Abstract
Clinical observations of patients with ventral frontal and anterior temporal cortical lesions reveal marked abnormalities in social attitudes. A previous study in seven patients with ventral prefrontal lesions provided the first direct experimental evidence for abnormalities in social attitudes using a well-established measure of gender stereotypes, the Implicit Association Test (IAT). Here, we were able to test whether these first findings could be reproduced in a larger sample of 154 patients with penetrating head injuries, and to determine the differential effects of ventromedial prefrontal (vmPFC) and ventrolateral prefrontal (vlPFC) cortical lesions on IAT performance. In addition, we investigated the role of the superior anterior temporal lobe (aTL), recently shown to represent conceptual social knowledge. First, we used a linear regression model to identify the role of each of the three regions, while controlling for the extent of damage to other regions. We found that larger lesions in either the vmPFC or the superior aTL were associated with increased stereotypical attitudes, whereas larger lesions in the vlPFC were associated with decreased stereotypical attitudes. Second, in a confirmatory analysis, we grouped patients by lesion location and compared their performance on the IAT with that of healthy volunteers. Compared to controls, patients with lesions in either the vmPFC or the superior aTL showed increased stereotypical attitudes, whereas patients with lesions in the vlPFC showed decreased stereotypical attitudes. The functional contributions of these regions in social attitudes are discussed.
Keywords: Ventromedial prefrontal cortex, Stereotypes, Implicit Association Test
1. Introduction
Stereotypes refer to “socially shared sets of beliefs about traits that are characteristic of members of a social category” (Greenwald & Banaji, 1995, p. 14). Although overgeneralized and resistant to new information, stereotypes represent an important, often implicit, component of adaptive behavior, serving as shortcuts that enable fast predictions of other people's behavior. Stereotypes may be considered a specialized instance of a social attitude and, as such, they influence our decision making and behavior in social situations (Wood, 2003). Using the Implicit Association Test (IAT; Greenwald, McGhee, & Schwartz, 1998), stereotypical attitudes have been extensively explored in personality and social psychology (e.g., Asendorpf, Banse, & Mucke, 2002; Rudman, Greenwald, & McGhee, 2001), yet their neural substrates are still unclear.
The first evidence on the neural basis of social attitudes came from clinical observations of marked changes in social, religious or moral attitudes after focal lesions of the ventral frontal lobes (Kleist, 1922). These changes in social attitudes have been observed in frontotemporal dementia patients with focal neurodegeneration of the frontal lobes (Miller et al., 2001) and patients with focal atrophy of the anterior temporal lobes (aTL; Edwards-Lee et al., 1997).
Experimental probes to directly investigate the neural basis of social attitudes have mostly relied on functional magnetic resonance imaging (fMRI) in healthy populations (Cunningham, Johnson, Gatenby, Gore, & Banaji, 2003; Cunningham & Zelazo, 2007; Knutson, Mah, Manly, & Grafman, 2007; Phelps et al., 2000). However, anterior temporal and ventral frontal activations in fMRI are difficult to detect without specific sequence optimization (Knutson et al., 2007). Furthermore, functional neuroimaging in healthy subjects cannot demonstrate whether activated brain regions are necessary for task performance (Price, Mummery, Moore, Frakowiak, & Friston, 1999). The study of patients with brain lesions is thus indispensable to elucidate the neural basis of social attitudes.
In a recent study using an experimental probe of social attitudes in patients with brain lesions, Milne and Grafman (2001) found that stereotypical attitudes about gender were diminished in seven patients with penetrating head injuries to the ventral prefrontal cortex (PFC) when compared with healthy volunteers and patients with damage to the dorsolateral PFC (dlPFC). The authors attributed the decrease in stereotypical attitudes to damage to the medial sector of the ventral PFC. In that study, however, the patients with ventral lesions had damage to lateral as well as medial sectors of the ventral PFC, and it is believed that these sectors may have different functional roles (Elliott, Dolan, & Frith, 2000; Kringelbach, 2005; Kringelbach & Rolls, 2004; Wood & Grafman, 2003). In addition, the number of subjects studied was small. Here, we tested whether decreased strength of stereotypical attitudes can be reproduced in a larger sample of patients, and whether this decrease is caused by damage to the ventromedial PFC (vmPFC) or the ventrolateral PFC (vlPFC).
Further, we investigated the contribution of the superior aTL to stereotypical gender attitudes. As mentioned above, neuropsychological studies have reported attitudinal changes and abnormalities of social behavior in frontotemporal dementia patients with aTL atrophy (Edwards-Lee et al., 1997; Liu et al., 2004). Two recent fMRI studies have shown that the superior aTL selectively represents abstract conceptual social knowledge (Zahn et al., 2007), and that conceptual social knowledge representations within the superior aTL are activated implicitly when evaluating social behavior (Zahn et al., 2009). Based on these studies, we suggest that the aTL may be important for stereotyping. In particular, we see two possible consequences of aTL damage. One possibility is that stereotyping depends critically on social knowledge representations within superior aTL. If this is the case, then damage to the superior aTL would decrease stereotypical attitudes. Alternatively, there might be an inverse relationship between the level of detail of representations in this region and stereotyping. That is, overgeneralized, stereotypical attitudes may be more prevalent in patients with degraded access to detailed and specific social conceptual knowledge. In this case, damage to the superior aTL would increase the strength of stereotypical attitudes.
2. Materials and methods
2.1. Participants
We selected subjects from the Vietnam Head Injury Study (VHIS) Phase 3, which was conducted between April 2003 and November 2006 at the Bethesda National Naval Medical Center. The VHIS (Phase 3) includes 199 Vietnam veterans with penetrating head injuries sustained during combat and 55 veterans with combat exposure, but no brain injury. Patients were evaluated using the Structured Clinical Interview for DSM-IV-TR Axis I disorders, nonpatient edition (SCID-I/NP; First, Spitzer, Gibbon, & Williams, 2001) and excluded from the present study if they had psychotic symptoms or met the criteria for bipolar disorder, major depression, alcohol/substance dependence or abuse. In addition, three patients were excluded because of physical inability to perform our test of interest and one patient was excluded because of technical problems, leaving a sample size of N = 154 patients (all males; mean age 58 years, SD = 2.4; mean education 14.8 years, SD = 2.5). Using the same exclusion criteria, we obtained a total of 43 controls (all males; mean age 58.7 years, SD = 2.1; mean education 15.3 years, SD = 2.5). Patients and comparison subjects did not differ in age (P = .10) or number of years of education (P = .24). All participants gave informed written consent, which was obtained according to the Declaration of Helsinki (BMJ 1991; 302: 1194). The work was approved by the Institutional Review Boards at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke/National Institutes of Health.
2.2. Lesion analysis
We acquired axial computed tomography (CT) scans (MRI scans could not be obtained in most patients because they still had metal fragments in the brain as a result of their injury) without contrast at the Bethesda Naval Hospital on a General Electric Medical Systems Light Speed Plus CT scanner in helical mode. We reconstructed the images with an in-plane voxel size of 0.4 mm × 0.4 mm, an overlapping slice thickness of 2.5 mm and a 1-mm slice interval. Brain lesions were evaluated using the Analysis of Brain Lesions (ABLe) software (Makale et al., 2002; Solomon, Raymont, Braun, Butman, & Grafman, 2007) contained in MEDx v3.44 (Medical Numerics, Germantown, MD). For each patient, the brain lesion was manually traced on each slice by V.R. (a psychiatrist with clinical experience of reading CT scans), and reviewed by J.G., who was blind to the results of the neuropsychological testing. The total volume of the lesion was calculated by summing the traced areas and multiplying by slice thickness. The skull and scalp components of the CT volume were then removed, a process known as de-skulling (Solomon et al., 2007). Each volume was spatially normalized to a de-skulled CT scan, which was previously spatially normalized to match the shape of the T1 MNI brain (standard of the International Consortium for Brain Mapping). The ABLe program that was used for lesion analysis has the option of excluding the manually delineated lesion from the spatial normalization process, thus improving registration accuracy. Spatial normalization was performed using the automated image registration (AIR) algorithm from Woods, Grafton, Watson, Sicotte, and Mazziotta (1998) using a 12-parameter affine model on de-skulled CT scans. Accuracy of registration was assessed qualitatively based on image fusion of the registration scan with the template image and quantitatively by computing an overlap of these two images. Based on these measures, all registrations were considered accurate. Finally, we defined regions of interest in terms of structures from the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and MNI coordinates (see Table 1) and quantified regional damage by analyzing the overlap of the spatially normalized lesion image with the AAL atlas image.
Table 1.
Description of the subregions.
| Regions of interest | AAL structures | MNI coordinates |
|---|---|---|
| vmPFC | Superior frontal gyrus, orbital | 0 ≤ x ≤ 20 (Right) |
| Middle frontal gyrus, orbital | −20 ≤ x < 0 (Left) | |
| Inferior frontal gyrus, orbital | z ≤ 1 | |
| Superior frontal gyrus, medial | ||
| Anterior cingulate and paracingulate gyri | ||
| Olfactory cortex | ||
| Gyrus rectus | ||
| vlPFC | Superior frontal gyrus, orbital | x > 20 (Right) |
| Middle frontal gyrus, orbital | x < −20 (Left) | |
| Inferior frontal gyrus, orbital | z ≤ 1 | |
| Olfactory cortex | ||
| dmPFC | Superior frontal gyrus, medial | 0 ≤ x ≤ 10 (Right) |
| Anterior cingulate and paracingulate gyri | −10 ≤ x < 0 (Left) | |
| Median cingulate and paracingulate gyri | z > 1 | |
| dlPFC | Superior frontal gyrus, dorsolateral | x > 10 (Right) |
| Middle frontal gyrus, lateral | x < −10 (Left) | |
| Inferior frontal gyrus, triangular part | z > 1 | |
| sup aTL | Superior temporal gyrus | y ≥ −10 |
| Temporal pole: superior temporal gyrus | ||
| middle/inf aTL | Middle temporal gyrus | y ≥ −10 |
| Inferior temporal gyrus | ||
| Temporal pole: middle temporal gyrus | ||
| inf pTL | Inferior temporal gyrus | y < −10 |
| medial TL | Hippocampus | |
| Parahippocampal gyrus | ||
| amygdala | Amygdala | |
| TPJ-pSTS | Inferior parietal | y < −10 |
| Supramarginal gyrus | ||
| Angular gyrus | ||
| Superior temporal gyrus Middle temporal gyrus |
We defined our regions of interest in terms of structures from the automated anatomical labeling (AAL) atlas and MNI coordinates. Abbreviations: vmPFC, ventro-medial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; sup aTL, superior anterior temporal lobe; middle/inf aTL, middle/inferior anterior temporal lobe; inf pTL, inferior posterior temporal lobe; medial TL, medial temporal lobe; and TPJ-pSTS, temporo-parietal junction/posterior superior temporal sulcus.
2.3. Neuropsychological assessment
As part of the VHIS (Phase 3), both patients and controls were administered an extensive clinical and experimental neuropsychological test battery. From that battery we used the following tests to measure neuropsychological functions that might influence performance on the IAT: the Wechsler Adult Intelligence Scale-III (Wechsler, 1997) to measure intellectual ability; the Token Test (McNeil & Prescott, 1994) to measure verbal comprehension; the Card-Sorting subtest from the Delis–Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001) to measure executive functions; and the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) to measure depression symptomatology. The neuropsychological test results are reported in Table 2.
Table 2.
Neuropsychological measures of patients and controls.
| Measure | Patients | Controls | Sig. |
|---|---|---|---|
| Verbal IQ (WAIS-III) | 104.3 ± 14.5 | 109.6 ± 13.0 | .032* |
| Performance IQ (WAIS-III) | 99.7 ± 14.7 | 108.4 ± 14.0 | .001* |
| Token Test | 97.7 ± 4.7 | 98.8 ± 1.6 | .013* |
| Card-Sortinga | 10.9 ± 3.1 | 11.4 ± 2.9 | .397 |
| BDI | 8.3 ± 8.4 | 11.7 ± 9.7 | .023* |
Mean ± SD values for neuropsychological tests with potential influence on IAT performance. Asterisks indicate significance <.05. Despite between-group differences in performance, the mean scores of both groups were within normal limits on these measures. Abbreviations: WAIS-III, Wechsler Adult Intelligence Scale-III; BDI, Beck Depression Inventory.
The Card-Sorting is a subtest from the Delis–Kaplan Executive Function System.
2.4. Implicit Association Test (IAT)
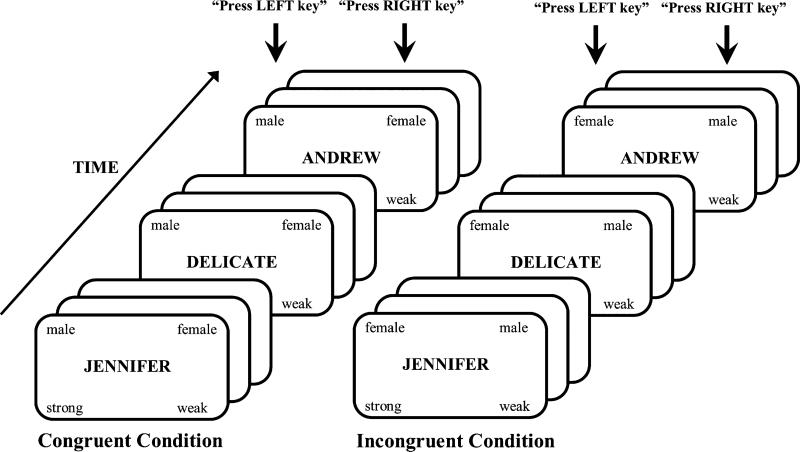
To measure the strength of stereotypical gender attitudes, we used the IAT, a computer-administered response time task. The IAT is a widely validated implicit measure of attitudes (Greenwald, Nosek, & Banaji, 2003; Hofmann, Gawronski, Gschwendner, Le, & Schmitt, 2005; Rudman et al., 2001) and has recently been applied in neuroimaging studies (e.g., Chee, Sriram, Soon, & Lee, 2000; Knutson, Wood, Spampinato, & Grafman, 2006; Luo et al., 2006). We chose this task because participants are generally unaware that their stereotypes are being assessed, hence their answers are free of social desirability concerns (Fazio & Olson, 2003). The IAT task design is depicted in Fig. 1. Stimuli were female names (e.g., “Jennifer”, “Mary”), male names (e.g., “Andrew”, “Paul”), words related to strength (e.g., “dominant”, “powerful”), and words related to weakness (e.g., “delicate”, “fragile”). The complete list of stimuli can be obtained from the authors. There were 20 stimuli for each category that were selected from previous gender IAT studies (Knutson et al., 2007; Rudman et al., 2001). Stimuli were presented in the centre of the visual field in random order using SuperLab Pro 1.75 (Cedrus Corporation, San Pedro, CA) on Macintosh G3 and G4 computers. Participants were asked to categorize names as female or male and words as strong or weak by pressing one of two keys on the computer keyboard. The categories for the classification were shown on the left and right sides of the screen. Each stimulus was shown on the screen until a response key was pressed, followed by a 500 ms blank screen. Participants were instructed to respond as quickly and as accurately as possible.
Fig. 1.
Design of the Implicit Association Test. Stimulus categories on the left or right side of the screen are mapped onto the corresponding response key. Stimuli are either male or female names alternating with strong or weak words. Congruent and incongruent conditions differ only by reversal of stimulus-response contingencies.
The IAT procedure consisted of five blocks. In Block 1 participants were asked to classify the stimuli as male or female names and in Block 2 as weak or strong words. In Block 3, the categorizations were combined such that participants had to press one response key for female names or strong words, and another response key for male names or weak words. This is known as the incongruent condition, since subjects are required to press the same response key for concepts that are not usually associated. In Block 4, participants were asked to categorize male and female names again, but this time the key assignment was switched as compared to Block 1. Finally, in Block 5, the categorizations were combined such that participants pressed one response key for male names or strong words and another response key for female names or weak words. This is known as the congruent condition, since subjects press the same response key for stereotypically associated concepts. The number of trials was 80 for each of the two critical blocks (Blocks 3 and 5), and 40 for each of the remaining blocks. All participants performed practice trials before each block. The stimuli used in the practice blocks were additional names and words that were not included in the test trials. The order of the blocks was counterbalanced across participants (the position of Blocks 1 and 3 were switched with those of Blocks 4 and 5, respectively).
The difference in response latencies between the congruent and incongruent conditions provides a measure of gender attitudes (“IAT effect”). If subjects associate women with weakness and men with strength, their response times will be faster in the congruent condition (for example, when they press the same response key for female names and weak words) than in the incongruent condition (when they press the same response key for female names and strong words). In the present study, this difference was computed as a D score in accordance with the recent scoring algorithm outlined by Greenwald et al. (2003, p. 214). Outliers (i.e., D scores exceeding ±2.5 standard deviations from the mean) were excluded from the analyses (N = 4). This was done to ensure that the results were not driven by outliers. The results did not differ when outliers were included.
2.5. Comparison with other measures
To test whether the observed IAT effects were specific for implicit gender stereotyping as compared to implicit associations in general, we asked participants to complete a second IAT which was identical in structure to the gender IAT except that positive or negative words were paired with words related to Vietnam or hockey. To test whether our findings were specific for implicit gender stereotyping as compared to explicit gender stereotyping, we asked participants to perform a short version of the Attitudes toward Women Scale (AWS; Spence & Helmreich, 1978). The AWS provides an explicit measurement of gender-related attitudes. Participants are shown 15 statements about traditional gender-roles beliefs (e.g., “The intellectual leadership of a community should be largely in the hands of men”). The degree of agreement to each statement is measured on a 5-point scale ranging from 1 (agree strongly)to 5(disagree strongly). A total score is derived by summing the responses after reverse scoring the egalitarian statements. High scores indicate a profeminist, egalitarian attitude while low scores indicate more traditional or sexist attitudes. The AWS is administered as a paper-and-pencil questionnaire.
2.6. Statistical analysis
To identify the effects of vmPFC, vlPFC and superior aTL lesions on stereotypical gender attitudes, we employed two analyses: (i) we entered percentages of regional damage into a multiple regression model and (ii) in a confirmatory analysis, we grouped patients by lesion location and compared their performance on the IAT with that of the controls.
We performed a multiple regression analysis to identify the independent contribution of each region of interest (vmPFC, vlPFC and superior aTL), while controlling for the other two and a number of confounding variables. We used the IAT D score as the dependent variable, and the percentages of lesion involvement in each of the regions as the independent variables. For each region, the percentage of lesion reported in the model was the average for the left and right hemisphere. To control for their potential confounding effects, we included in the model percentages of lesion involvement in the dorsomedial PFC (dmPFC) and dlPFC, total volume of the lesion, presence of seizures, and use of anticonvulsants. In addition, we checked whether the IAT effect correlated with demographic characteristics (age and education), mean response times on the IAT, and performance on the neuropsychological tests described earlier. Since two variables correlated with the IAT effect (mean response times on the IAT, r = −.260, P = .001, and performance on the Token Test, r = .229, P = .006), we included them in the model as well. All variables were assessed for multicollinearity (i.e., presence of high correlations between the variables in the regression equation), which may bias the estimates of the regression coefficients (Cohen, Cohen, West, & Aiken, 2003). Typically, variance inflation factors (VIFs) of 10 or more provide evidence of serious multicollinearity. In the present study, VIFs were never greater than 4.6 (see Table 3).
Table 3.
Results of the linear regression analysis.
| Independent variables | Unstandardized coefficients |
Standardized coefficients | t | Sig. | VIF | |
|---|---|---|---|---|---|---|
| B | Std. error | β | ||||
| vmPFC | .008 | .004 | .348 | 2.106 | .037* | 3.9 |
| vlPFC | −.008 | .004 | −.349 | −2.006 | .047* | 4.6 |
| dmPFC | −.014 | .008 | −.199 | −1.666 | .098 | 2.1 |
| dlPFC | .005 | .005 | .137 | .960 | .339 | 3.2 |
| sup aTL | .007 | .003 | .218 | 2.242 | .027* | 1.5 |
| Lesion volume | .002 | .001 | .278 | 2.210 | .029* | 2.4 |
| Token Test | .026 | .007 | .358 | 3.534 | .001* | 1.4 |
| Mean response time | −.0002 | .0001 | −.286 | −3.052 | .003* | 1.3 |
| Seizures | −.058 | .061 | −.098 | −.943 | .348 | 1.6 |
| Anticonvulsants | .11 | .067 | .170 | 1.645 | .103 | 1.6 |
The model (multiple linear regression model, listwise deletion of missing data) significantly predicted IAT performance [F(10, 115) = 4.02, P = .001, R2 = .26]. The table shows unstandardized regression coefficients (B and standard error), standardized regression coefficients (β), t values, significance, and variance inflation factors (VIFs) for each independent variable in the regression model. We used the IAT D score as the dependent variable, and 10 independent variables: percentages of lesion involvement for each region; lesion volume; total raw score on the Token Test; mean response time between congruent and incongruent conditions; presence/absence of seizures; and use/no use of anticonvulsants. Asterisks indicate significance <.05. β coefficients express the influence of a single predictor on the IAT effect, with the effects of the other independent variables being held constant. Since they are expressed in standardized form, β coefficients are comparable across independent variables and show the relative importance of significant predictors in the model. Abbreviations: vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; sup aTL, superior anterior temporal lobe.
In a confirmatory analysis, we grouped patients by lesion location and examined whether lesion groups performed significantly differently than controls in the directions predicted by the regression analysis. Lesion groups were defined for each region that significantly predicted IAT performance in the regression analysis. Based on previous studies (Koenigs et al., 2008; Tranel, Damasio, Denburg, & Bechara, 2005), a patient was assigned to a lesion group if his lesion occupied at least 15% of that region. Patients with damage to more than one region were classified based on the region with the greatest percentage of damage. This procedure was adopted to ensure that patients in a lesion group not only had damage to that region, but also had greater damage to that region than to the other regions under investigation (see Mah, Arnold, & Grafman, 2004 for a similar procedure). Patients whose damage was outside the regions of interest or <15% were not included in this confirmatory analysis. We performed planned comparisons between the control group and each lesion group using t tests for linear contrasts in a one-way analysis of variance (ANOVA). These planned comparisons were one tailed because we had a priori hypotheses on the direction of the effects that were derived from the regression analysis. Finally, data on the non-gender IAT and the AWS were analyzed using one-way ANOVAs to determine between-group differences. All statistical analyses were carried out using SPSS 15.0 (SPSS Inc., Chicago, IL). The significance level was set at P = .05.
3. Results
Table 3 shows the model derived from the multiple linear regression analysis, with standardized regression coefficients (β) and corresponding t values as well as significance for each independent variable. This model indicates that lesions in vmPFC, vlPFC, and superior aTL independently contributed to IAT performance, after controlling for each other and the rest of the confounding variables. Larger lesions in either the vmPFC or the superior aTL were associated with a higher IAT effect (i.e., stronger stereotypical associations). On the other hand, larger lesions in the vlPFC were associated with a lower IAT effect (i.e., weaker stereotypical associations). Lesions in the dmPFC and dlPFC did not significantly predict IAT performance. We computed additional multiple regression analyses that examined whether other temporal or parietal regions (not predicted a priori) significantly influenced IAT performance (including middle/inferior aTL, posterior inferior temporal lobe, medial temporal lobe, and temporo-parietal junction/posterior superior temporal sulcus). We found that only lesions in middle/inferior aTL (β = .206, P = .024), but not lesions in the other regions, significantly predicted IAT performance.
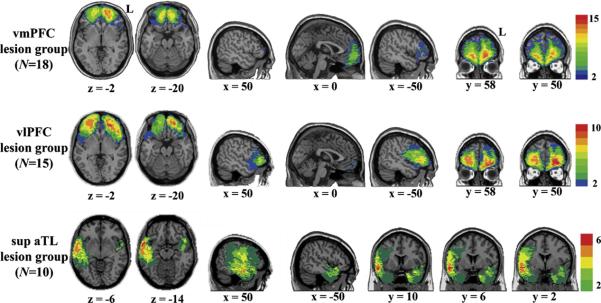
Fig. 2 shows the lesion overlaps for patients with damage to vmPFC (N = 18), vlPFC (N = 15), and superior aTL (N = 10). Table 4 provides information about lesion extent and neuropsychological performance for each patient subgroup. Table 5 reports performance on the gender and non-gender IATs and the AWS for the three patient subgroups and the control group. On the gender IAT, a one-way ANOVA of the mean IAT effect between the four groups showed a significant difference. Planned comparisons revealed that patients with damage to the vmPFC and superior aTL had a significantly higher IAT effect than controls, while patients with damage to the vlPFC had a significantly smaller IAT effect than the controls. In contrast to the group differences on the gender IAT, we found no significant differences between groups on the non-gender IAT and the explicit measure of gender stereotyping.
Fig. 2.
Lesion overlaps for the vmPFC, vlPFC and sup aTL groups. Color indicates the number of overlapping lesions at each voxel, with red denoting areas of greatest overlap of lesions among patients, and blue denoting areas of minimum overlap (we used a threshold of 2). There is some overlap between the vmPFC and vlPFC groups. However, lesions extended more laterally in the vlPFC group. It is important to note that this analysis was only confirmatory. The main analysis was the regression analysis, in which we were able to identify the role of each region, while controlling for the extent of damage to other regions. MNI coordinates are given. Abbreviations: vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; sup aTL, superior anterior temporal lobe; L, left. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
Table 4.
Lesion extent and neuropsychological performance for each patient subgroup.
| Measure | vmPFC lesion group | vlPFC lesion group | sup aTL lesion group | Sig. |
|---|---|---|---|---|
| Lesion volume (cc) | 47.0 ± 36.0 | 78.8 ± 61.3 | 76.5 ± 63.1 | .177 |
| Verbal IQ (WAIS-III) | 104.8 ± 13.1 | 100.2 ± 18.3 | 104.7 ± 13.0 | .652 |
| Performance IQ (WAIS-III) | 100.0 ± 14.5 | 100.4 ± 16.5 | 100.0 ± 17.2 | .998 |
| Token Test | 96.9 ± 3.4 | 96.4 ± 5.6 | 98.3 ± 2.1 | .551 |
| Card-Sorting | 10.3 ± 3.6 | 10.1 ± 4.00 | 11.7 ± 2.5 | .522 |
| BDI | 7.1 ± 6.5 | 9.7 ± 8.4 | 6.6 ± 6.6 | .487 |
Mean ± SD values for lesion volume as well as neuropsychological performance are reported separately for each patient subgroup. We did not find significant differences between the patient subgroups on any of these variables (P>.05). However, for some variables vlPFC patients had higher variability than the other groups of patients. It is particularly important to note the variability shown by the vlPFC patients on the Token Test, since performance on the Token Test, but not on the other neuropsychological tests, correlates with performance on the IAT (see Section 2.6 for further details). Although Token Test performance might have affected IAT performance in the group of vlPFC patients, our main analysis, the regression analysis, suggests that the vlPFC contribution to IAT performance is unlikely to be due to verbal comprehension as assessed by the Token Test. As a matter of fact, vlPFC damage significantly predicted IAT performance, after controlling for Token Test performance, as well as lesion volume (see Table 3).
Table 5.
Performance on the IATs and the AWS for the four groups of subjects.
| Task | Measure | Controls | vmPFC lesion group | vlPFC lesion group | sup aTL lesion group | Sign. |
|---|---|---|---|---|---|---|
| Gender IAT | Mean RTs (congr.) (ms) | 1073 ± 273 | 1251 ± 436 | 1361 ± 707 | 1088 ± 278 | .241 |
| Mean RTs (incongr.) (ms) | 1252 ± 352 | 1444 ± 507 | 1489 ± 728 | 1481 ± 530 | .096 | |
| Mean IAT effect (ms) | .37 ± .26 | .49 ± .21 | .25 ± .25 | .64 ± .22 | .001* | |
| Control IAT | Mean RTs (congr.) (ms) | 1199 ± 304 | 1199 ± 486 | 1171 ± 696 | 1062 ± 300 | .856 |
| Mean RTs (incongr.) (ms) | 1517 ± 379 | 1442 ± 467 | 1382 ± 606 | 1432 ± 435 | .798 | |
| Mean IAT effect (ms) | .45 ± .36 | .50 ± .41 | .39 ± 30 | .59 ± 37 | .649 | |
| AWS | Mean AWS score | 56.44 ± 7.21 | 52.89 ± 7.48 | 54.00 ± 8.64 | 55.20 ± 8.47 | .379 |
The table shows response times for congruent and incongruent conditions as well as IAT effect for both gender and non-gender IATs. The table also shows the mean score on the explicit measure (AWS). In the gender IAT, mean response times for the congruent and incongruent conditions were not significantly different between the four groups of subjects, while the mean IAT effect showed a significant difference. Planned comparisons revealed that patients with damage to the vmPFC and superior aTL had a significantly higher IAT effect than controls (P = .048 and P = .0015, respectively), while patients with damage to the vlPFC had a significantly smaller IAT effect than the controls (P = .047). Differences in the IAT effect can be observed even in the absence of differences in response times because the calculation of the IAT effect according to the most recently recommended algorithm (Greenwald et al., 2003) includes the use of practice-block data, use of error penalties (error trials are replaced with the mean of correct responses plus 600 ms) and use of subjects’ standard deviations. In contrast to the group difference on the gender IAT, there were no between-group differences on the control IAT and the explicit measure of gender stereotyping. On the control IAT, we found a positive IAT effect, with faster response times in the congruent condition (Vietnam/negative word pairs and hockey/positive word pairs) than in the incongruent condition (Vietnam/positive word pairs and hockey/negative word pairs), indicating that our subjects generally associated Vietnam with negative words and hockey with positive words. The scores on the AWS indicate that, in general, our subjects consciously expressed moderate profeminist, egalitarian attitudes. Asterisks indicate significance <.05. Abbreviations: RTs, response times; congr., congruent; incongr., incongruent; vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; sup aTL, superior anterior temporal lobe.
4. Discussion
This study examined dissociable contributions of prefrontal and temporal cortical subregions to stereotypical gender attitudes in 154 patients with penetrating head injuries. Detailed lesion analysis of brain images allowed us to quantify the extent of damage to specific subregions. To evaluate gender stereotypes, we used the IAT, an implicit task which reveals attitudes and beliefs even when they are not explicitly endorsed (Greenwald et al., 2002; Hofmann et al., 2005; Nosek, Banaji, & Greenwald, 2002). Using a multiple regression model, we found that larger lesions in either the vmPFC or the superior aTL were associated with increased stereotypical attitudes, whereas larger lesions in the vlPFC were associated with decreased stereotypical attitudes. In a confirmatory analysis, we divided the patients into lesion groups and compared their performance on the IAT with that of healthy volunteers. The lesion groups performed significantly differently than controls in the directions predicted by the regression analysis.
Our first main result was that lesions of the vmPFC and vlPFC had opposite effects on stereotypical attitudes about gender. Milne and Grafman (2001) found decreased stereotyping in patients with lesions in the ventral PFC. Here, by quantifying the extent of damage to each region and performing a multiple regression analysis, we were able to analyze subdivisions of the ventral PFC and detect functional differences between the medial and lateral sectors. This finding is in line with previous studies reporting a dissociation between medial and lateral aspects of the ventral PFC (e.g., Elliott et al., 2000; Kringelbach, 2005; Kringelbach & Rolls, 2004; O'Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; Wood & Grafman, 2003).
On the one hand, we found that vmPFC damage was associated with a stronger IAT effect, which indicates increased stereotyping. This finding is in line with a new study (Quadflieg et al., in press) that examined the neural correlates of gender stereotyping using fMRI. Consistent with the current neuropsychological data, Quadflieg et al. found activation within medial PFC for stereotypic versus nonstereotypic judgments. More research is needed to understand the nature of this finding. One potential explanation may be related to the role of the vmPFC in the modulation of the expression of social and emotional behavior (e.g., Grafman & Litvan, 1999; Pietrini, Guazzelli, Basso, Jaffe, & Grafman, 2000). It has been suggested that lesions of the vmPFC disrupt inhibitory and emotional mechanisms, leading to impulsive and socially inappropriate behavior and decision making (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Bechara, Damasio, Damasio, & Anderson, 1994). Indeed, lesions of the vmPFC have been associated with abnormal autonomic responses to socially meaningful stimuli (Damasio, Tranel, & Damasio, 1990), with “acquired sociopathy” (Eslinger & Damasio, 1985) and with the expression of aggressive and violent behavior (Blair & Cipolotti, 2000; Grafman et al., 1996). Notably, vmPFC patients may present inappropriate social behavior despite preserved capacities for general intelligence, logical reasoning, and declarative knowledge of social and moral norms (Eslinger & Damasio, 1985). These patients can access some types of social knowledge (Saver & Damasio, 1991) and describe social norms of behavior with a stranger (Beer, John, Scabini, & Knight, 2006). Based on this literature, we speculate that our finding of increased stereotyping associated with vmPFC damage may be related to the inability to suppress the expression of inappropriate social behavior, such as stereotyping. It is important to note that in this study we did not distinguish the contributions of orbital (Brodmann's Area [BA] 11), subgenual cingulate (BA 25, 32), and dorsal parts of the anterior vmPFC (BA 10, 32). From a phylogenetic and anatomical perspective, however, it is unlikely that these different areas are functionally heterogeneous (Kringelbach & Rolls, 2004).
On the other hand, we found that vlPFC damage was associated with a diminished IAT effect, which indicates decreased stereotyping. A recent fMRI study using a race IAT has suggested that the vlPFC might play a role in stereotyping (Beer et al., 2008). The involvement of the vlPFC in IAT performance has been also reported in two fMRI investigations, one assessing the association of visually depicted legal and illegal behaviors with positive animals (e.g., puppies) and negative animals (e.g., snake) (Luo et al., 2006), and the other assessing the association of flowers and insects with pleasant and unpleasant words (Chee et al., 2000). These previous imaging studies, coupled with the current results, suggest that the vlPFC may play a more general role in mediating implicit attitudes, rather than in gender stereotyping per se. Alternatively, the role of vlPFC in IAT performance may have less to do with attitudes than with the executive demands of the task, which include speeded responses and also, potentially, some degree of response inhibition. This interpretation is supported by previous studies demonstrating vlPFC involvement in go/no-go tasks, which require subjects to perform speeded responses on “go” trials and to inhibit their response on “no-go” trials (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Casey et al., 2001). The response variability in our vlPFC patients (see Table 5) may suggest that the vlPFC plays a role in the coordination of executive task demands unrelated to stereotype processing. Response variability in the vlPFC group may also originate from the fact that this group of patients was more heterogeneous than the other two (see Table 4). While the current results demonstrate the importance of vlPFC for normal performance on the IAT for gender stereotypes, future studies will be needed to clarify the role of vlPFC in the mediation of implicit (social or otherwise) attitudes versus general response selection processes. Another interesting question for future studies would be whether there is an effect of laterality in vlPFC function. Previous response inhibition findings primarily concerned the right vlPFC (Aron et al., 2003; Aron, Robbins, & Poldrack, 2004; Horn, Dolan, Elliott, Deakin, & Woodruff, 2003). In the present study we did not distinguish between left- and right-sided lesions.
In addition to the medial and lateral aspects of the ventral PFC, we also found a critical involvement of the superior aTL in IAT performance. The contribution of the aTL is unlikely to reflect general language-related difficulties. First, as reported in Table 4, performance on the verbal WAIS test and the Token Test was not different among the subgroups of patients. Second, performance on the verbal IQ did not correlate with performance on the IAT. Since performance on the Token Test correlated with performance on the IAT, this variable was entered in the regression model as a covariate. The regression analysis showed that aTL damage significantly predicted IAT performance, after controlling for Token Test performance (see Table 3). Instead, we suggest that the aTL may play a role in IAT performance by providing representations of conceptual social knowledge. As mentioned in Section 1, a recent neuroimaging study has shown that the superior aTL selectively represents conceptual social knowledge (Zahn et al., 2007). Selective activation in the superior aTL was found for social concepts (i.e., concepts describing social behavior: e.g., ‘polite’, ‘stingy’) as compared with concepts describing animal behavior (animal function concepts: e.g., ‘trainable’, ‘nutritious’). Remarkably, activity in the superior aTL increased with higher levels of social conceptual detail. Thus, representations within the superior aTL are recruited, particularly, when fine distinctions of the conceptual quality of social behaviors are required (e.g., whether somebody behaved “tactlessly” or “stingily”). Further it has been demonstrated that representations of conceptual qualities of social behavior in the superior aTL are independent of context of actions and emotions (Zahn et al., 2009). Based on these fMRI findings, we propose that damage to the superior aTL may lead to reduce social conceptual detail. This might in turn lead to greater reliance on stereotypes, which are characterized by a lack of conceptual detail. To our knowledge, this is the first study that suggests the potential importance of conceptual social knowledge impairments due to superior aTL damage as contributing to stereotypical social attitudes.
Because most superior aTL lesions extended into the middle/inferior aTL, we were unable to test whether the demonstrated effect was specifically due to superior or middle/inferior aTL damage. However, we excluded the contribution of adjacent regions (posterior inferior temporal lobe, medial temporal lobe, temporo-parietal junction/posterior superior temporal cortex, and vlPFC).
Given the proximity of superior aTL and amygdala, it is reasonable to ask whether amygdala damage could account for the observed effects in the superior aTL. Indeed, the amygdala is involved in the recognition of emotion in facial expressions (Adolphs, Tranel, Damasio, & Damasio, 1994) and in the perception of faces of a different race (Hart et al., 2000; Phelps et al., 2000). A neuropsychological study indicated that the amygdala is not critical for normal performance on the IAT (Phelps, Cannistraci, & Cunningham, 2003). Here, we were able to exclude a prominent role of the amygdala for the observed effects in the superior aTL. Only 2 subjects in the superior aTL group had significant damage to the amygdala, and their IAT effect was lower than the mean IAT effect of the aTL group. Further, since there are direct connections of the amygdala with the vmPFC via the medial forebrain bundle (Nieuwenhuys, 1982) which do not depend on connections with the lateral temporal pole, a disconnection syndrome of amygdalo-frontal fibers is unlikely to explain the impact of lateral aTL lesions on social attitudes.
The lack of group differences on the non-gender IAT supports the claim that the vlPFC, vmPFC, and aTL are critically involved in implicit gender stereotyping, rather than playing a more general role in implicit associations or IAT task performance. However, we realize that further studies will be required to more precisely characterize the specific roles of these brain areas in mediating social stereotypes. Previous lesion and neuroimaging studies suggest that superior aTL and ventral PFC may play a specific role in social cognition. As mentioned earlier, recent studies corroborate the hypothesis that the aTL selectively represents social concepts versus concepts describing animal behavior (Zahn et al., 2007). In addition, previous literature has confirmed the involvement of the ventral PFC in gender (Quadflieg et al., in press) as well as race (Beer et al., 2008) stereotyping. In this respect, it is important to note that much of what is currently known about the neural circuitry supporting stereotyping has been gathered from studies exploring racial attitudes (see Eberhardt, 2005 for a review of neuroimaging studies of race), and specifically the perception of outgroup faces (e.g., Cunningham et al., 2004; Golby, Gabrieli, Chiao, & Eberhardt, 2001; Hart et al., 2000; Phelps et al., 2000; Richeson et al., 2003).
To test whether our findings were specific for implicit gender stereotyping as compared to explicit gender stereotyping, we compared performance on the gender IAT with performance on an explicit measure of gender attitudes. The results showed no between-group differences on the explicit measure of gender stereotyping, supporting the conclusion that vmPFC, vlPFC, and aTL are critically involved in implicit but not explicit gender stereotyping. Our finding is in line with previous lesion and neuroimaging studies. For example, Milne and Grafman (2001) reported between-group differences on the IAT but not on the scales measuring explicit gender stereotyping. Phelps et al. (2000) found that brain activity during race stereotyping was correlated with the strength of participants’ implicit attitudes but not their explicit beliefs (although see Quadflieg et al., in press).
Our patients’ abnormalities on the IAT cannot be attributed to impairments in other neuropsychological domains. The patients’ performance on the IAT did not correlate with age, education, or performance on neuropsychological tests measuring intellectual ability, executive functions, and symptoms of depression (verbal IQ, performance IQ, performance on the Card-Sorting Test, and score on the BDI-II). Since the IAT D score correlated with mean response times on the IAT and performance on the Token Test, we controlled for the confounding effect of these variables by entering them as covariates into the multiple regression model. Additionally, our results resist general explanations related to lesion volume, because this variable was also entered in the regression model.
In conclusion, in this study we investigated the critical involvement of vmPFC, vlPFC and superior aTL in stereotypical attitudes about gender using the IAT. We found that damage to either the vmPFC or the superior aTL was associated with a greater IAT effect (i.e., increased stereotyping), whereas lesions of the vlPFC were associated with a diminished IAT effect (i.e., decreased stereotyping). These findings reveal the potential involvement of ventral frontal and anterior temporal brain regions in social attitudes.
Acknowledgments
We thank R. Zahn for insightful comments and fruitful discussions related to the data analysis and earlier versions of this manuscript, L. Mah for task programming, K. Reding for data management, and F. Krueger, J. Hassenplug, and N. Ruesch for their helpful comments on the manuscript. We thank the veterans for their participation in the study. This work was supported by the US National Institute of Neurological Disorders and Stroke intramural research program and a project grant form the United Sates Army Medical Research and Material Command administered by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: A 30 Year Post-Injury Follow-Up Study, Grant number DAMD17-01-1-0675).
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
References
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Asendorpf JB, Banse R, Mucke D. Double dissociation between implicit and explicit personality self-concept: The case of shy behavior. Journal of Personality and Social Psychology. 2002;83(2):380–393. [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory. second ed. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion–cognition interactions. Journal of Cognitive Neuroscience. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Beer JS, Stallen M, Lombardo MV, Gonsalkorale K, Cunningham WA, Sherman JW. The Quadruple Process model approach to examining the neural underpinnings of prejudice. NeuroImage. 2008;43(4):775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal: A case of ‘acquired sociopathy’. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, et al. Sensitivity of prefrontal cortex to changes in target probability: A functional MRI study. Human Brain Mapping. 2001;13(1):26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Sriram N, Soon CS, Lee KM. Dorsolateral prefrontal cortex and the implicit association of concepts and attributes. NeuroReport. 2000;11:135–140. doi: 10.1097/00001756-200001170-00027. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. [Google Scholar]
- Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. Journal of Personality & Social Psychology. 2003;85(4):639–649. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: A social cognitive neuroscience perspective. Trends in Cognitive Sciences. 2007;11(3):97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychological Science. 2004;15(12):806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioral Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Delis Kaplan, Kramer . Delis–Kaplan executive function system. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Eberhardt JL. Imaging race. American Psychologist. 2005;60(2):181–190. doi: 10.1037/0003-066X.60.2.181. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt 6):1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fazio RH, Olson MA. Implicit measures in social cognition research: Their meaning and use. Annual Review of Psychology. 2003;54:297–327. doi: 10.1146/annurev.psych.54.101601.145225. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, non-patient edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Golby AJ, Gabrieli JD, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nature Neuroscience. 2001;4(8):845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Grafman J, Litvan I. Importance of deficits in executive functions. Lancet. 1999;354(9194):1921–1923. doi: 10.1016/S0140-6736(99)90438-5. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar A. Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR. Implicit social cognition: Attitudes, self-esteem, and stereotypes. Psychological Review. 1995;102:4–27. doi: 10.1037/0033-295x.102.1.4. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR, Rudman LA, Farnham SD, Nosek BA, Mellott DS. A unified theory of implicit attitudes, stereotypes, self-esteem, and self-concept. Psychological Review. 2002;109(1):3–25. doi: 10.1037/0033-295x.109.1.3. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The Implicit Association Test. Journal of Personality and Social Psychology. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the Implicit Association Test. I. An improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85(2):197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. NeuroReport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Hofmann Gawronski, Gschwendner Le, Schmitt A meta-analysis on the correlation between the Implicit Association Test and explicit self-report measures. Personality and Social Psychology Bulletin. 2005;31(10):1369–1385. doi: 10.1177/0146167205275613. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: An fMRI study. Neuropsychologia. 2003;41(14):1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Kleist K. Geistes und nervenkrankheiten. Verlag von Johann Ambrosius Barth; Leipzig: 1922. [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Human Brain Mapping. 2007;28(10):915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KM, Wood JN, Spampinato MV, Grafman J. Politics on the brain: An fMRI investigation. Social Neuroscience. 2006;1:25–40. doi: 10.1080/17470910600670603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nature Neuroscience. 2008;11(2):232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Nakic M, Wheatley T, Richell R, Martin A, Blair RJ. The neural basis of implicit moral attitude—an IAT study using event-related fMRI. NeuroImage. 2006;30(4):1449–1457. doi: 10.1016/j.neuroimage.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry. 2004;161(7):1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using Interactive Automated Software. Behavior Research Methods, Instruments, and Computers. 2002;34(1):6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- McNeil MM, Prescott TE. Revised Token Test. Western Psychological Services; Los Angeles, CA: 1994. [Google Scholar]
- Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K. Neuroanatomy of the self: Evidence from patients with frontotemporal dementia. Neurology. 2001;57(5):817–821. doi: 10.1212/wnl.57.5.817. [DOI] [PubMed] [Google Scholar]
- Milne E, Grafman J. Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. Journal of Neuroscience. 2001;21(RC150):151–156. doi: 10.1523/JNEUROSCI.21-12-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys G. Veening. The medial forebrain bundle of the rat. I. General introduction. Journal of Comparative Neurology. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Nosek BA, Banaji MR, Greenwald AG. Math = male, me = female, therefore math not = me. Journal of Personality and Social Psychology. 2002;83(1):44–59. [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Cannistraci CJ, Cunningham WA. Intact performance on an indirect measure of race bias following amygdala damage. Neuropsychologia. 2003;41:203–208. doi: 10.1016/s0028-3932(02)00150-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography. American Journal of Psychiatry. 2000;157:1772–1781. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frakowiak RS, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. Journal of Cognitive Neuroscience. 1999;11(4):371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Quadflieg S, Turk DJ, Waiter GD, Mitchell JP, Jenkins AC, Macrae CN. Exploring the neural correlates of social stereotyping. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21091. (in press) [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, et al. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6(12):1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Rudman LA, Greenwald AG, McGhee DE. Implicit self-concept and evaluative implicit gender stereotypes: Self and ingroup share desirable traits. Personality and Social Psychology Bulletin. 2001;27(9):1164–1178. [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29(12):1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe). Computer Methods and Programs in Biomedicine. 2007;86(3):245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JT, Helmreich RL. Masculinity and femininity: Their psychological dimensions, correlates and antecedents. University of Texas Press; Austin, TX: 1978. [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128(Pt 12):2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. third ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wood JN. Social cognition and the prefrontal cortex. Behavioral and Cognitive Neuroscience Reviews. 2003;2:97–114. doi: 10.1177/1534582303253625. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: Processing and representational perspectives. Nature Reviews Neuroscience. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration. II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences, USA. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, et al. The neural basis of human social values: Evidence from functional MRI. Cerebral Cortex. 2009;19(2):276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]