Abstract
Chemokines and chemokine receptors contribute to the migration of hepatic stellate cells (HSCs) and Kupffer cells, two key cells during fibrogenesis. Here, we investigate the role of CCR2, the receptor for MCP-1, MCP-2 and MCP-3, in hepatic fibrosis. Hepatic CCR2, MCP-1, MCP-2 and MCP-3 mRNA expression was increased after bile duct ligation (BDL). Both Kupffer cells and HSCs, but not hepatocytes, expressed CCR2. BDL- and CCl4-induced fibrosis was markedly reduced in CCR2-/- mice as assessed by collagen deposition, α smooth muscle actin expression and hepatic hydroxyproline content. We generated CCR2 chimeric mice by the combination of clodronate, irradiation and bone marrow (BM) transplantation allowing fully reconstitution of Kupffer cells, but not of HSCs, with BM cells. Chimeric mice containing WT-BM displayed increased macrophage recruitment, whereas the chimeric mice containing CCR2-/- BM showed less macrophage recruitment at 5 days after BDL. Although CCR2 expressed in the BM enhanced macrophage recruitment in early phases of injury, CCR2 expression on resident liver cells including HSCs, but not on the BM, was required for fibrogenic responses in chronic fibrosis models. In vitro experiments demonstrated that HSCs deficient in CCR2-/- or its downstream mediator p47phox-/- did not display ERK and AKT phosphorylation, chemotaxis or ROS production in response to MCP-1, MCP-2 and MCP-3.
Conclusion
Our results indicate that CCR2 promotes HSC chemotaxis and the development of hepatic fibrosis.
Keywords: chemokine, chemokine receptor, hepatic stellate cell, Kupffer cell, reactive oxygen species
INTRODUCTION
Hepatic fibrosis results from many chronic liver diseases, including hepatitis B and C, autoimmune hepatitis, alcoholic liver disease and non-alcoholic steatohepatitis (NASH) 1. Hepatic stellate cells (HSCs) are the principal liver cells that promote hepatic fibrosis2, 3. Upon the activation of HSCs by various stimuli, such as transforming growth factor (TGF)-β and platelet-derived growth factor (PDGF), HSCs transdifferentiate into myofibroblasts and then produce excessive extracellular matrix (ECM) proteins, including collagen type I, III and IV, resulting in hepatic fibrosis. Cirrhosis, the end stage of hepatic fibrosis, may result in hepatic dysfunction, portal hypertension and hepatocellular carcinoma (HCC)1,4. Kupffer cells, hepatic resident macrophages, induce acute and chronic liver inflammation by producing inflammatory cytokines, including TNF-α, IL-6, CC-chemokine ligand (CCL) 2/Monocyte chemoattractant protein (MCP)-1, IL-1 and TGFβ and by activating HSCs during hepatic fibrosis3,5-7.
Recruitment of immune cells including Kupffer cells and HSCs to the site of injury and inflammation is important event in regeneration, wound healing and hepatic fibrosis1. Chemokines and chemokine receptors have a central role in the regulation of cell migration and local inflammation8. CC-chemokine receptor 2 (CCR2) which is mainly expressed on the surface of monocytes and macrophages is a functional receptor for MCP-1, MCP-2 and MCP-3 and is involved in the migration of monocytes and macrophages in peritonitis, autoimmune encephalitis, rheumatoid arthritis, tuberculosis, atherosclerosis and obesity9-16. Genetic or pharmacological inactivation of CCR2 inhibits pulmonary and renal fibrosis17-19. The CCR2 ligand MCP-1 is produced by Kupffer cells and HSCs, which promotes hepatic fibrosis by recruitment of macrophages that are associated with HSC activation7, 20-22.
The present study examines the role of CCR2 in two different experimental models of hepatic fibrosis and identifies the CCR2-expressing cell types in the liver. We characterize the distinct roles of CCR2 in Kupffer cells and HSCs between the early phase of liver injury and chronic liver fibrosis. In addition, we assess the function of CCR2 in HSC activation by stimulating primary cells with CCR2 ligands, MCP-1, MCP-2 and MCP-3 and then investigate the requirement of NADPH oxidase in HSC activation and migration.
METHODS
Animals and Models of hepatic fibrosis
Specific pathogen-free Wild type (WT)(C57BL/6J), CCR2-/- (Jackson Laboratories, Bar Harbor, Maine) and p47phox-/- mice (Taconic, Hudson, NY) were used for this study. For BDL model, the 8-12 week-old mice were anesthetized7, 23. After laparotomy, the common bile duct was ligated twice and closed the abdomen. The sham operation was performed similarly without bile duct ligation. For CCl4 model, the mice were injected with CCl4 (Sigma-Aldrich, St. Louis, MO, diluted 1:3 in corn oil) or vehicle (corn-oil) intraperitoneally at a dose of 0.5 μl CCl4/g body weight twice a week for a total 12 injections7. The mice received humane care according to US National Institutes of Health recommendations outlined in the “Guide for the Care and Use of Laboratory Animals”. All animal experiments were approved by the Columbia University and University of California San Diego Institutional Animal Care and Use Committee.
Bone Marrow Transplantation
BMT experiments were performed as previously described7, 24. Because only 30% of Kupffer cells are reconstituted by donor-derived BM cells six months after BMT, mice received liposomal clodronate injection (200 μl) before irradiation to deplete Kupffer cells and accelerate tissue macrophage turnover in order to obtain fully-reconstituted BM-derived cells25, 26. 1×107 BM cells from the tibias and femurs of donor mice were injected into the tail vein of lethally irradiated (11Gy) recipient mice. As we previously reported, the mice were transplanted with BM isolated from β-actin promoter-driven GFP transgenic (GFP-Tg) mice and the liver tissue was fixed and stained with macrophage marker (F4/80) or HSC marker (Desmin) confirming the reconstitution of Kupffer cells by GFP-positive cells and GFP-negative HSCs (Suppl. Fig. 1A,B)7. We also demonstrated that GFP-positive Kupffer cells in GFP-Tg BM-transplanted mice was the same as that in GFP-Tg mice by FACS analysis (FACSCanto, BD bioscience)(Suppl. Fig. 1C-E). The CCR2-chimeric mice were generated by WT mice transplanted CCR2-/- BM and vice versa. They were subjected to BDL or CCl4 treatment 12 weeks after BMT. To demonstrate the success of BMT in CCR2-/- and WT mice, spleen cells were isolated from all CCR2-chimeric mice and CCR2 mRNA expression was measured by qPCR.
HSC and Kupffer cell isolation and culture
HSCs were isolated by a collagenase-pronase perfusion of livers followed by 8.2% Nycodenz (Accurate Chemical and Scientific corporation, Westbury, NY) two-layer discontinuous density gradient centrifugation7, 23, resulting in 99% purity of HSCs as confirmed by retinoid autofluorescence. To avoid Kupffer cell contamination in HSCs isolated from BDL-mice, contaminated Kupffer cells were depleted by magnetic antibody sorting (MACS; Miltenyi Biotec, Auburn, CA) using F4/80 (eBiosience, San Diego, CA) and CD-11b (Miltenyi Biotec) antibodies27. The method of Kupffer cell isolation was described previously28, 29.
Immunohistochemistry and immonofluorescence
Liver specimens were fixed in 10% buffered formalin and incubated with monoclonal antibody to αSMA (DakoCytomation, Carpinteria, CA) using MOM kit (Vector Laboratories, Burlingame, CA), monoclonal antibody to F4/80 (eBioscience, San Diego, CA) or rabbit anti 4-hydroxy-nonenal (4-HNE) antibody (Alpha Diagnostic, San Antonio, TX)7, 23. For immunofluorescent staining, liver specimens were fixed in 4% paraformaldehyde and subsequently incubated in PBS containing 30% sucrose and frozen at -80°C. Frozen section were incubated with antibody for CCR2 (Novus Biologicals, Littleton, CO), desmin (Neomarkers, Fremont, CA), F4/80 and pan-CK (Biolegend, San Diego, CA) and imaged with confocal microscopy. Immunocytofluorescence against CCR2 was performed as described previously23.
Western blot
Protein extracts were electrophoresed and subsequently blotted7, 23. Blots were incubated with antibodies for αSMA (Sigma-Aldrich, St. Louis, MO), phospho-ERK, phospho-AKT, AKT (Cell signaling, Danvers, MA) and ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA), with secondary horseradish peroxidase-conjugated antibody, and visualized by the enhanced chemiluminescence light method (Amersham Biosciences).
Measurement of hepatic collagen content
Hepatic hydroxyproline content was measured as previously described7, 23. Hepatic collagen content was also quantitated by Sirius red staining of paraffin-embedded sections. Sirius red positive area was analyzed in six random fields (100x) on each slide and quantified using NIH imaging software.
Real time quantitative PCR and RT-PCR
RNA was extracted and real time quantitative PCR by using primer-probe sets(Applied Biosystems, Foster City, CA) was performed as described previously7.
RT-PCR for CCR2 and β-actin was performed using primers 5′-AGAGGTCTCGGTTGGGTTGT-3′ and 5′-ATCATAACGTTCTGGGCACC-3′ for 33 cycles and primers 5 ′-GATGACGATATCGCTGCGCTG-3 ′ and 5′-GTACGACCAGAGGCATACAGG-3′ for 27 cycles at 95°C (45sec), 60°C(45sec) and 72°C(60sec), respectively.
Cell chemotaxis assay
Cell migration assays was performed using a modified Boiden-Chamber as described previously7, 30. Briefly, HSCs isolated from WT, CCR2-/- or p47phox-/- mice were placed onto the upper chamber (4×104 cells/well) in DMEM without serum and exposed to vehicle, MCP-1, MCP-2 or MCP-3 (R&D systems, Minneapolis, MN) placed in the lower chamber. After 24 hours of incubation at 37°C, cells migrated to the lower side of the chamber were counted in eight randomly chosen (100x) fields. In some experiments, the NADPH-oxidase inhibitors diphenilene-iodonium (DPI) (Sigma-Aldrich, St. Louis, MO) was used. 10μM DPI was incubated for 30 minutes before treatment with chemokines.
Measurement of intracellular ROS
HSCs were preincubated with the redox-sensitive dye DCFDA (8μM)(Molecularprobe, Eugene, OR) for 20 minutes and then stimulated with MCP-1, MCP-2 or MCP-3 (100ng/ml)31. DCFDA fluorescence was measured by a multi-well fluorescence scanner (Fluostar Optima, BMG).
Statistical Analysis
All data are expressed as mean ± standard error of mean. Multiple groups were compared using one-way ANOVA with post-hoc Bonferroni′s correction (GraphPad Prism 4.02; GraphPad Software). Two groups were compared using an unpaired Student′s t test (two-tailed). p values less than 0.05 were considered statistically significant.
RESULTS
Expression of CCR2 and its ligands in hepatic fibrosis
Although the expression of MCP-1 is increased in acute liver injury and hepatic fibrosis7, 20, 22, 32, its receptor CCR2 and the other CCR2 ligands, MCP-2 and MCP-3, have not been investigated. Expression of hepatic CCR2, MCP-1, MCP-2 and MCP-3 mRNA levels were elevated after BDL (Fig. 1A) and CCl4 treatment (Fig. 1B). Next, we examined what cell types express CCR2 in the liver. We co-stained CCR2 with desmin (HSC marker, Fig. 1C), F4/80 (Kupffer cell marker, Fig. 1D) or pan-CK (hepatocyte and cholangiocyte marker, Fig. 1E) and found that CCR2 is expressed both in HSCs and Kupffer cells, but not in hepatocytes and billiary epithelial cells, in bile duct ligated liver. We also demonstrated CCR2 expression in mouse primary HSCs (Suppl. Fig.1A-C) and Kupffer cells (Suppl. Fig. 1D,E) by immunofluorescence and CCR2 mRNA by RT-PCR (Fig. 1F).
Figure 1. Expression of CCR2 and CCR2 ligands are increased in hepatic fibrosis.
Livers were analyzed from mice after BDL (n=6) or 12 times repeated CCl4 treatment (n=6) at indicated time points. (A, B) Hepatic mRNA levels of CCR2, MCP-1, MCP-2 and MCP-3 were measured after BDL (A) or CCl4 treatment (B) by qPCR. (C-E) CCR2 expression (in green) was detected by co-stained with desmin (in red, C), F4/80 (in red, D) and pan-Cytokeratin (in red, E), which was visualized by confocal microscopy. Arrows indicate CCR2-positive and desmin- (C) or F4/80-positive cells (D). The dashed lines indicate intrahepatic bile ducts (E). (F) CCR2 mRNA in Kupffer cells, HSCs and hepatocytes was measured by RT-PCR. β-actin mRNA served as an internal control.
CCR2 mediates hepatic fibrosis after BDL
21 days after BDL, WT mice had significant hepatic fibrosis, as demonstrated by Sirius red staining and hydroxyproline content. In contrast, CCR2-/- mice had less fibrosis as demonstrated by reduced collagen deposition and hydroxyproline level (Fig. 2A,B). Expression of α-SMA, a marker of HSC activation, was increased in WT mice, but not in CCR2-/- mice as assessed by immunohistochemistry and immunoblotting (Fig. 2C,D). Early fibrogenic responses were demonstrated at five days after BDL, as represented by mRNA expression of markers for fibrogenesis including collagenα1(I), α-SMA, TGF-β1 and tissue inhibitor of metalloproteinases (TIMP)-1. WT mice showed an increased expression of these genes whereas CCR2-/- mice had suppression in fibrogenic gene expression (Fig. 2E). Gene expression of proinflammatory mediators, such as TNF-α, MCP-1, RANTES, MIP-1β, IL-1β and IL-6 were decreased in CCR2-/- mice compared to WT mice (Fig. 3A). Moreover, CCR2-/- mice showed less hepatic macrophage infiltration, as assessed by expression of CD68 mRNA and immunohistochemistry for F4/80 (Fig. 3B,C). Lipid peroxidation product 4-HNE is a marker for the generation of reactive oxygen species (ROS). The level of 4-HNE was increased in WT, but not in CCR2-/- mice (Fig. 3D). Serum ALT level at 21 days after BDL was suppressed in CCR2-/- mice compared with WT mice, whereas we did not find significant differences in ALP and T-Bil levels between WT and CCR2-/- mice after BDL (Fig. 3E).
Figure 2. Reduction of hepatic fibrogenesis in CCR2-/- mice after BDL.
WT (n=6) and CCR2-/- (n=6) mice underwent BDL. (A) Fibrillar collagen deposition was evaluated by Sirius red staining and its quantification 21 days after BDL. (B) Hepatic hydroxyproline content was measured. (C, D) Expression of α-SMA was determined by immunohistochemistry (C) and western blotting (D). (E) Hepatic mRNA levels of collagen α1(I), α-SMA, TGFβ1 and TIMP-1 were measured in WT (n=5) and CCR2-/- (n=5) 5 days after BDL by qPCR. * p<0.05 for bile-duct ligated CCR2-/- mice versus bile-duct ligated WT mice. Original magnification is ×100 (A, C).
Figure 3. Reduction of hepatic inflammation and macrophage infiltration in CCR2-/- mice after BDL.
(A, B) Hepatic mRNA levels of TNFα, MCP-1, RANTES, MIP-1β, IL-1β and IL-6 (A) and CD68 (B) were measured in WT (n=5) and CCR2-/- (n=5) 5 days after BDL by qPCR. (C) Macrophage infiltration was determined by immunohistochemistry for F4/80 21 days after BDL. (D) 4-hydoxy-nonenal was detected by immunohistochemistry. (E) Serum ALT, ALP and T-Bil levels 5 or 21 days after BDL were measured. * p<0.05 for bile-duct ligated CCR2-/- mice versus bile-duct ligated WT mice. Original magnification is ×200(C, D).
CCR2 mediates CCl4-induced hepatic fibrosis
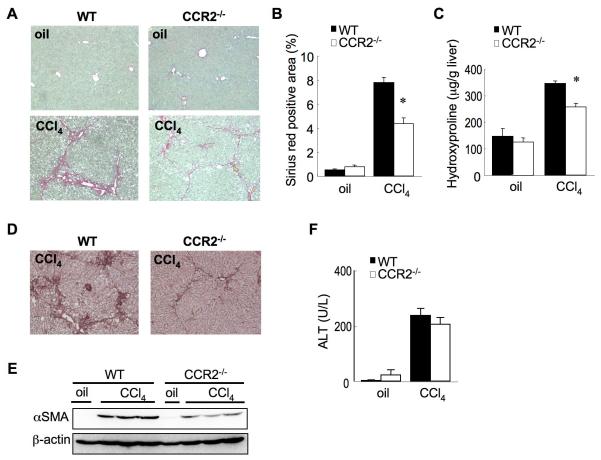
Hepatic fibrosis was induced in WT mice after 12 injections of CCl4, as assessed by Sirius red staining (Fig. 4A, B) and hepatic hydroxyproline content (Fig. 4C). In contrast, CCR2-/- mice showed a marked reduction of hepatic fibrosis (Fig. 4A-C). During CCl4-induced hepatic fibrosis, the expression of αSMA was significantly increased in WT mice, but not in CCR2-/- mice (Fig. 4D, E). However, serum ALT levels after 12 injections of CCl4 were compatible between WT and CCR2-/- mice (Fig. 4F), suggesting a similar hepatocellular injury occurred by CCl4 in WT and CCR2-/- mice.
Figure 4. CCR2 deficiency inhibits hepatic fibrosis after CCl4 treatment.
WT (n=6) and CCR2-/- (n=6) mice were treated with 12 injections of CCl4 (0.5μl/g). (A, B) Fibrillar collagen deposition was evaluated by Sirius red staining (A) and quantitation (B) after 12 injection of CCl4. (C) Hepatic hydroxyproline content was measured. (D, E) Expression of α-SMA was determined by immunohistochemistry (D) and western blotting (E). (F) Serum ALT levels after 12 injection of CCl4 were measured. *: p<0.05 for CCl4-treated CCR2-/- mice versus CCl4-treated WT mice. Original magnification is ×100 (A, D).
CCR2 on resident cells, but not BM-derived cells, are critical for hepatic fibrosis
As CCR2 is important in two models of hepatic fibrosis (Fig. 2-4) and is expressed both in Kupffer cells and HSCs (Fig. 1C-F, Suppl.Fig. 2A-E), we investigated which cell types are critical in CCR2-mediated hepatic fibrosis. We generated CCR2-chimeric mice by using a combination of Kupffer cell depletion, irradiation and bone marrow transplantation (BMT) 7. Because hepatocytes do not express CCR2 (Fig. 1E,F), this protocol reconstitutes Kupffer cells, but not HSCs, with BM-derived cells (Suppl. Fig. 1A-E). Thus, We generated two types of CCR2-chimeric mice; WT mice transplanted with CCR2-/- BM, which contained CCR2-/- Kupffer cells and WT HSCs, and CCR2-/- mice transplanted with WT BM, which contained WT Kupffer cells and CCR2-/- HSCs. We confirmed successful BMT by measuring CCR2 mRNA expression in spleen cells from both types of CCR2-chimeric mice (Fig. 5A). At 5 days after BDL, chimeric mice containing WT BM cells showed increased inflammatory gene expressions (TNF-α, MCP-1, RANTES and MIP-1β) (Fig. 5B) with macrophage accumulation (Fig. 5C, D). In contrast, the mice with CCR2-/- BM cells had reduced inflammatory gene expression (Fig. 5B) and Kupffer cell recruitment (Fig. 5C,D), suggesting that inflammation and macrophage recruitment requires CCR2 expressed on BM cells in the early phase of liver injury. There are no significant differences in serum ALT levels among all types of chimeric mice at 5 days after BDL (Fig. 5E).
Figure 5. CCR2 on bone marrow-derived cells is required for the early phase of macrophage infiltration after BDL.
Chimeric mice were generated by transplanting WT or CCR2-/- BM into irradiated and clodronate-treated WT mice or CCR2-/- mice (n=4-5 each group). Then chimeric mice were subjected to BDL for 5 days. (A) Successful BMT was confirmed by low levels of CCR2 mRNA in splenocytes from WT mice, but normal CCR2 mRNA in CCR2-/- mice transplanted with WT BM. (B) Hepatic mRNA levels of proinflammatory cytokines (TNF-α, MCP-1, RANTES and MIP-1β) in chimeric mice were measured by qPCR. (C, D) Macrophage infiltration was determined by immunohistochemistry for F4/80 (C) and qPCR for CD68 (D). (E) Serum ALT levels in chimeric mice 5 days after BDL were measured. Original magnification is ×200 (C). * p<0.05, ** p<0.01.
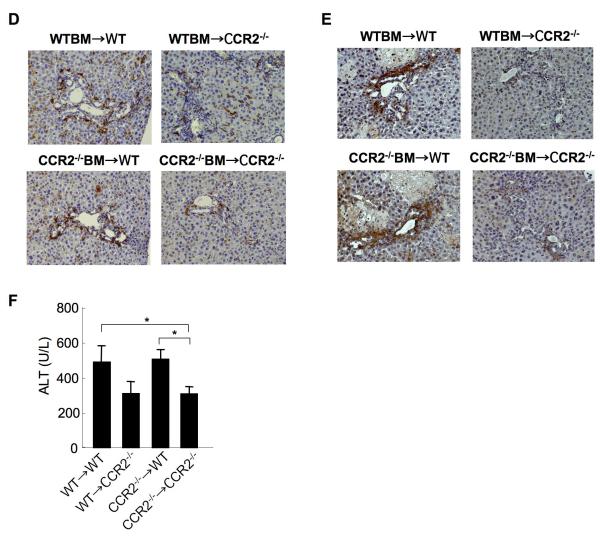
At 21 days after BDL, WT mice containing CCR2-/- BM had increased collagen deposition as assessed by Sirius red staining and hydroxyproline content (Fig. 6A, B). Meanwhile, CCR2-/- mice containing WT BM had reduced fibrosis similar to CCR2-/- mice transplanted with CCR2-/- BM (Fig. 6A, B). In agreement with the results on collagen deposition, WT mice containing CCR2-/- BM showed an increased expression of αSMA comparable to WT mice transplanted with WT BM, whereas CCR2-/- mice containing WT BM decreased αSMA expression comparable to CCR2-/- mice transplanted with CCR2-/- BM (Fig. 6C). Increased macrophage recruitment was observed in WT mice transplanted with either WT or CCR2-/- BM and reduced macrophage infiltration was seen in CCR2-/- mice transplanted with WT or CCR2-/- BM, indicating that macrophage recruitment does not require CCR2 in BM-originated cells in the chronic phase of hepatic fibrosis (Fig. 6D). Moreover, WT mice transplanted with WT or CCR2-/- BM showed increased accumulation of 4-HNE compared with CCR2-/- mice transplanted with WT or CCR2-/- BM (Fig. 6E). Serum ALT levels were increased in WT recipient transplanted with either WT or CCR2-/- BM and reduced in CCR2-/- mice transplanted with WT or CCR2-/- BM (Fig. 6F). To test the importance of CCR2 expressed on the recipient-originated cells including HSCs in a second model of experimental hepatic fibrosis, hepatic fibrosis was induced by chronic injection of CCl4 on CCR2-chimeric mice. Similarly with the results from BDL, CCR2-/- mice containing either WT or CCR2-/- BM cells showed decreased collagen deposition and αSMA expression (Fig. 7A-E) whereas the WT mice containing either WT or CCR2-/- BM cells had increased collagen deposition and αSMA expression (Fig. 7A-E). Serum ALT levels were increased in CCR2-/- recipient transplanted with either WT or CCR2-/- BM and reduced in WT mice transplanted with WT or CCR2-/- BM (Fig. 7F). Taken together, these results suggest that CCR2 expressed on BM cells including Kupffer cells is important in the early phase of hepatic inflammation. However, CCR2 expression on recipient-originated cells including HSCs is required for the late phase of hepatic fibrosis.
Figure 6. Recipient-originated cells, but not bone marrow-derived cells, mediate CCR2-dependent fibrogenic effects after BDL.
(A-F) Chimeric mice were generated by transplanting CCR2-/- BM into irradiated and clodronate-treated WT mice (n=6) or CCR2-/- mice (n=6) and vice versa (n=6 each group). 3 months after BMT, the mice were subjected to BDL and hepatic fibrosis was judged at 21 days after BDL. (A-B) Hepatic fibrosis in chimeric mice was assessed by Sirius red staining (A, left), quantitation (A, right), and hydroxyproline measurement (B). (C) α-SMA expression was shown by immunohistochemistry (C, upper) and western blotting (C, lower). (D) Macrophage infiltration was determined by immunohistochemistry for F4/80. (E) The accumulation of 4-hydoxy-nonenal was measured by immunohistochemistry. (F) Serum ALT levels in chimeric mice 21 days after BDL were measured. Original magnification is ×100 (A, C) and ×200 (D, E). * p<0.05, ** p<0.01.
Figure 7. CCR2 on recipient-originated cells is crucial for CCl4-induced hepatic fibrosis.
(A-F) Chimeric mice were generated by transplanting CCR2-/- BM into irradiated and clodronate-treated WT mice (n=5) or CCR2-/- mice (n=5) and vice versa (n=5 each group). Hepatic fibrosis was induced by 12 injection of CCl4. (A, B) Hepatic fibrosis in chimeric mice was assessed by Sirius red staining (A), quantitation (B), and measurement of hydroxyproline content (C). (D, E) α-SMA expression was determined by immunohistochemistry (D) and western blotting (E). (F) Serum ALT levels in chimeric mice after 12 injection of CCl4 after BDL were measured. Original magnification is ×100 (A, D). * p<0.05, ** p<0.01.
CCR2 mediates ROS production and migration in HSCs
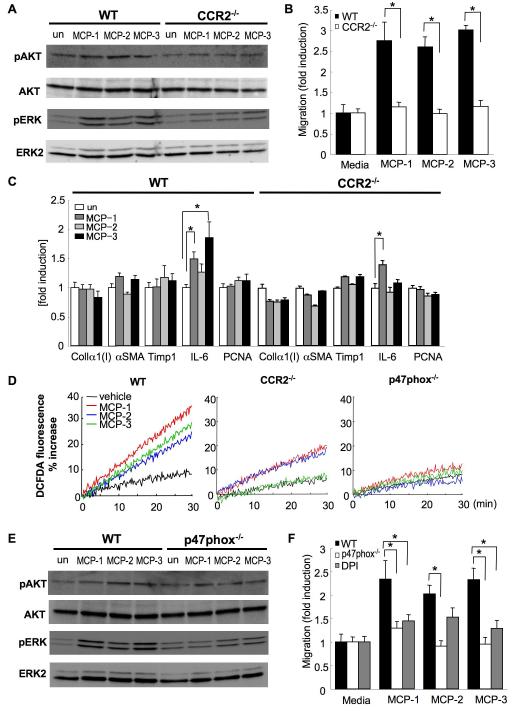
On the basis of the above results, the HSC appears to be the primary cell type for CCR2-mediated hepatic fibrosis. We examined intracellular signaling in HSCs by the phosphorylation of AKT and ERK in response to CCR2 ligands (Fig. 8A). MCP-1, MCP-2 and MCP-3 induce the phosphorylation of AKT and ERK in WT HSCs, and CCR2-/- HSCs showed reduced AKT and ERK phosphorylation by CCR2 ligands. Next, we investigated whether HSC migration depends on CCR2 (Fig. 8B). MCP-1, MCP-2 and MCP-3 significantly induced the HSC chemotaxis. In contrast, these chemotactic activities were abolished in CCR2-/- HSCs. We additionally measured fibrogenic genes (collagenα1(I), αSMA, TIMP-1), inflammatory cytokine (IL-6) and proliferative marker (PCNA) in response to CCR2 ligands in HSCs (Fig. 8C). Fibrogenic genes and PCNA were not increased in WT and CCR2-/- HSCs after the stimulation of all CCR2 ligands. IL-6 mRNA level was elevated in response to MCP-1 and MCP-3 in WT HSCs, and MCP-1 still increased IL-6 mRNA level in CCR2-/- HSCs. CCR2 ligands do not induce fibrogenic and proliferative responses directly on HSCs and may have inflammatory responses that depend on CCR2 by MCP-3 and independently of CCR2 in response to MCP-1 (Fig. 8C). Next, we investigated ROS production in CCR2 signal. ROS was produced by CCR2 ligands in WT HSCs. In contrast, HSCs deficient in CCR2 or p47phox, which is a critical component of NADPH oxidase, produce little ROS after stimulation with MCP-1, MCP-2 or MCP-3 (Fig. 8D). In p47phox-/- HSCs, the phosphorylation of AKT and ERK were suppressed slightly and significantly, respectively, after stimulation with CCR2 ligands (Fig. 8E). Finally, we tested whether NADPH oxidase is involved in HSC migration. We inactivated NADPH-oxidase by using either the inhibitor, DPI or in p47phox-/- HSCs. DPI treatment and p47phox deficiency dramatically inhibited the migration induced by CCR2 ligands (Fig. 8F). These data indicate that CCR2 ligands, MCP-1, MCP-2 and MCP-3, stimulate AKT and ERK activation, ROS production and HSC migration through CCR2 and p47phox.
Figure 8. CCR2-dependent NADPH oxidase-dependent ROS production and chemotaxis in HSCs in response to MCP-1, MCP-2 and MCP-3.
(A-F) HSCs isolated from WT, CCR2-/- and p47phox-/- mice were stimulated with MCP-1, MCP-2 and MCP-3. (A) phospho-AKT (on Ser473), total AKT, phospho-ERK and total ERK were determined in WT and CCR2-/- HSCs by immunoblotting. (B) Serum free media containing MCP-1, MCP-2 or MCP-3 (50ng/ml) was placed in the lower chamber. HSCs isolated from WT or CCR2-/- mice were placed in the upper chamber. Migration of HSCs into the lower chamber was counted 24 hours after stimulation. (C) mRNA expression of collagen α1(I), αSMA, TIMP-1, IL-6 and PCNA were measured in WT or CCR2-/- HSCs 24 hours after stimulation by qPCR. (D) Mouse HSCs from WT, CCR2-/- and p47phox-/- mice were loaded with DCFDA (8μM) for 20 minutes and incubated with MCP-1, MCP-2 and MCP-3 (100ng/ml) and measured fluorescence intensity for 30 minutes. (E) phospho-AKT (on Ser473), total AKT, phospho-ERK and ERK2 were determined in WT and p47phox-/- HSCs by immunoblotting. (F) Serum free media containing MCP-1, MCP-2 or MCP-3 (50ng/ml) was placed in the lower chamber. HSCs isolated from WT or p47phox-/- mice were placed in the upper chamber, with or without pretreatment of DPI (10μM) for 30 minutes. Migration of HSCs into the lower chamber was counted 24 hours after stimulation. *: p<0.05.
DISCUSSION
Chronic inflammation leads to continuous hepatocyte damage and subsequently hepatic fibrosis1-3. The recruitment and migration of Kupffer cells and HSCs are critical events for developing liver inflammation and fibrosis. In human liver diseases, increased MCP-1 is associated with macrophage recruitment and severity of hepatic fibrosis and primary biliary cirrhosis22,32. In animal models, the inactivation of MCP-1 attenuates CCl4-induced liver injury and fibrosis by inhibiting macrophage recruitment20,21. Our current study demonstrates that CCR2 deficiency lessens liver inflammation and fibrosis (Fig. 2-4) and that CCR2 on Kupffer cells is required for the early phase of Kupffer cell infiltration and inflammation, but is dispensable for the chronic phase of Kupffer cell accumulation, HSC activation and collagen deposition (Fig. 5, 6). Intriguingly, CCR2 on recipient-originated cells including HSCs is more important than CCR2 on Kupffer cells in HSC activation and fibrosis in two different models of experimental fibrogenesis (Fig. 6, 7). BDL increases biliary pressure and stimulates the proliferation of biliary epithelial cells, which is accompanied by inflammation and necrosis in the surrounding portal area, resulting in HSC activation and fibrosis33 (Fig. 2, 3). In contrast, intoxication with CCl4 which is converted into hepatotoxic metabolites by p450 CYP2E1, directly damages hepatocytes inducing severe necrosis around central vein 33. Subsequently, necrotic hepatocytes stimulate immune cells and HSCs, resulting in fibrosis bridging between central veins (Fig. 4). CCl4 induces hepatocyte damage independent of CCR2-mediated inflammatory responses as demonstrated by similar ALT levels between WT and CCR2-/- mice (Fig. 4F). However, 3 weeks after BDL, ALT levels are suppressed in CCR2-/- mice, suggesting that cholestasis-mediated liver damage requires a CCR2-dependent inflammatory response (Fig. 3E).
Macrophages and Kupffer cells are a principal source of inflammatory cytokines and chemokines including CCR2 ligands. In addition to Kupffer cells, HSCs, biliary epithelial cells and hepatocytes produce chemokines including CCR2 ligands such as MCP-17, 34-36. Our previous study has shown that HSCs promote TLR4-mediated liver injury and fibrosis7. Liver injury increases the intestinal permeability via the release of cytokines that alter portal circulation and intestinal epithelial tight junctions, allowing intestinal microflora-derived LPS to enter the portal circulation and the portal LPS then activates HSCs via TLR47,37. TLR4 signaling further modulates TGF-β signaling by inhibiting TGF-β pseudoreceptor BMP and activin membrane bound inhibitor (BAMBI) in HSCs. TLR4 also induces the production of chemokines, such as MCP-1, MCP-2, MCP-3, MIP-1α and MIP-1β (data not shown) 7.
CCR2 is mainly expressed on macrophages/Kupffer cells and functions to recruit these cells to the site of inflammation21,38,39. Intriguingly, CCR2 expressed on BM mediates Kupffer cells recruitment in the acute phase (Fig. 5), but Kupffer cell recruitment, HSC activation and fibrogenic responses in chronic injury require CCR2 expression on resident liver cells (Fig. 6-8) with HSCs being the prime candidate as suggested by CCR2 expression and functional studies (Fig. 1, 8). We speculate that there is a biphasic inflammatory response after BDL and that chronic phase of inflammation contributes to fibrosis. The monocyte/macrophage lineage is highly heterogeneous, and consists of a CCR2hi population and CX3CR1hi population40. A recent report demonstrated that CCR2 is not required for monocyte migration into tissue41. Thus, macrophage recruitment might be through other chemokine receptors, such as CX3CR1 in the chronic phase of hepatic fibrosis.
Previous studies have reported that CCR2 is not expressed in human HSCs and rat portal fibroblasts, which are cells primarily activated in cholestasis-mediated hepatic fibrosis22, 35, 42. In contrast, our study demonstrates that both Kupffer cells and HSCs, but not hepatocytes and biliary epithelial cells, express CCR2 in normal and fibrotic liver (Fig. 1C-F, Suppl. Fig. 2A-E). These differences might be explained by the different roles of HSCs or portal fibroblasts among humans, rats and mice. Previous studies suggested that MCP-1 activates human HSCs, rat portal fibroblasts and hepatocytes by an unknown receptor, instead of by CCR235, 42, 43. Our results also support a limited role for a non-CCR2 receptor, since MCP-1 increases IL-6 mRNA in CCR2-/- HSCs. Additionally, we demonstrated that CCR2 leads to ERK and AKT phosphorylation, ROS production and migration in response to MCP-1, MCP-2 and MCP-3 in murine HSCs. We did not find that CCR2 ligands induce proliferation and profirogenic features in murine HSCs (Figure 8 and data not shown)35,42. These results might be explained by less proliferative and profibrogenic characteristics of murine HSCs compared to human and rat HSCs.
NADPH oxidase is a critical component of HSC activation and chemotaxis in hepatic fibrosis31. We have previously shown that RANTES induces ROS production, proliferation and migration via NADPH oxidase30. MCP-1 generates ROS production and migration in vascular smooth muscle cells and monocytes and DPI abolishes migration induced by MCP-144, 45. Here, we showed that CCR2 ligand-induced ROS production, ERK and AKT phosphorylation and chemotaxis were attenuated by the inactivation of NADPH oxidase in the p47phox-/- or DPI-treated HSCs (Fig. 8), indicating that NADPH oxidase is an essential component for CCR2-mediated HSC activation.
In summary, our study demonstrates that CCR2 has distinct roles in Kupffer cells and HSCs in different phase of liver injury. In the early phase of liver injury Kupffer cell migration is mediated by CCR2 expressed in BM, whereas CCR2 expression in resident liver cells promotes macrophage recruitment and hepatic fibrosis in chronic liver injury. In conjunction with our in vitro studies, CCR2 expression on HSCs appears to responsible for its fibrogenic effects. Thus, inhibition of CCR2 in chronic liver disease might represent a potential strategy for the treatment of hepatic fibrosis with minimal suppression of Kupffer cell-mediated immune response.
Supplementary Material
Supplementary Figure 1. (A, B) The liver section from mice transplanted with β-actin promoter driven GFP BM cells was stained with desmin (red, A) or F4/80 (red, B). GFP was not expressed in desmin-expressing HSCs (A) and GFP was expressed in F4/80-expressing Kupffer cells (B). (C-E) GFP-positive Kupffer cells isolated from WT (C), GFP-Tg (D) and GFP-Tg BM cell-transplanted mice (E) were shown by FACS analysis.
Supplementary Figure 2. (A-C) Immunofluorescence of CCR2 in primary HSCs. HSCs were isolated from normal mice (A) and bile duct ligated mice (B) and then fixed at 24 hours after plating with 4% formalin. The cells were incubated with a primary antibody for CCR2 (A, B) or isotype control (C) followed by an Alexa-Fluor 488 conjugated secondary antibody and nuclear staining with DAPI. (D, E) Immunofluorescence for CCR2 in primary Kupffer cells. The cells were stained with a primary antibody for CCR2 (D) or isotype control (E) followed by an Alexa-Fluor 488 conjugated secondary antibody and nuclear staining with DAPI.
Acknowledgments
Grant support: This study was supported by a grant from the American Liver Foundation (to E.S.), an Alimenti e Salute grant from the University of Ancona (to S.D.M.) and NIH R01GM041804 (to D.A.B.).
Abbreviations
- BDL
bile duct ligation
- BM
bone marrow
- BMT
BM transplantation
- CCL
CC-chemokine ligand
- CCl4
carbon tetrachloride
- CCR
CC-chemokine receptor
- DPI
diphenilene-iodonium
- ECM
extracellular matrix
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cells
- MCP
monocyte chemoattractant protein
- NADPH-oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- PCNA
proliferation cell nuclear antigen
- PDGF
platelet-derived growth factor
- ROS
reactive oxygen species
- SMA
smooth muscle actin
- TLR
Toll-like receptor
- TGF
transforming growth factor
- WT
wild-type.
Footnotes
No conflicts of interest exist.
Referrence
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–5. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann MF, Kopf M, Marsland BJ. Chemokines: more than just road signs. Nat Rev Immunol. 2006;6:159–64. doi: 10.1038/nri1776. [DOI] [PubMed] [Google Scholar]
- 9.Feterowski C, Mack M, Weighardt H, Bartsch B, Kaiser-Moore S, Holzmann B. CC chemokine receptor 2 regulates leukocyte recruitment and IL-10 production during acute polymicrobial sepsis. Eur J Immunol. 2004;34:3664–73. doi: 10.1002/eji.200425294. [DOI] [PubMed] [Google Scholar]
- 10.Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, Ernst JD, Charo IF. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol. 2004;172:7647–53. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- 11.Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A, Chenaux G, Reddick RL, Kuziel WA, Ahuja SS. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J Clin Invest. 2004;113:856–66. doi: 10.1172/JCI20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jee Y, Yoon WK, Okura Y, Tanuma N, Matsumoto Y. Upregulation of monocyte chemotactic protein-1 and CC chemokine receptor 2 in the central nervous system is closely associated with relapse of autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol. 2002;128:49–57. doi: 10.1016/s0165-5728(02)00147-9. [DOI] [PubMed] [Google Scholar]
- 13.Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol. 2004;15:145–9. doi: 10.1097/00041433-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H, Kaneko S. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555–63. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 15.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine. 2003;24:266–76. doi: 10.1016/j.cyto.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204:594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–46. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamara E, Galastri S, Aleffi S, Petrai I, Aragno M, Mastrocola R, Novo E, Bertolani C, Milani S, Vizzutti F, Vercelli A, Pinzani M, Laffi G, LaVilla G, Parola M, Marra F. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46:230–8. doi: 10.1016/j.jhep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128:138–46. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, Pinzani M, Romanelli RG, Laffi G, Gentilini P. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152:423–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Uchinami H, Seki E, Brenner DA, D’Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–9. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 24.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–38. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–93. [PubMed] [Google Scholar]
- 26.van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–85. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 27.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–46. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, Adachi K, Futatsugi S, Kuida K, Takeuchi O, Okamura H, Fujimoto J, Akira S, Nakanishi K. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–7. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 29.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–8. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 30.Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–58. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- 31.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–94. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuneyama K, Harada K, Yasoshima M, Hiramatsu K, Mackay CR, Mackay IR, Gershwin ME, Nakanuma Y. Monocyte chemotactic protein-1, -2, and -3 are distinctively expressed in portal tracts and granulomata in primary biliary cirrhosis: implications for pathogenesis. J Pathol. 2001;193:102–9. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH725>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 33.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–48. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efsen E, Bonacchi A, Pastacaldi S, Valente AJ, Wenzel UO, Tosti-Guerra C, Pinzani M, Laffi G, Abboud HE, Gentilini P, Marra F. Agonist-specific regulation of monocyte chemoattractant protein-1 expression by cyclooxygenase metabolites in hepatic stellate cells. Hepatology. 2001;33:713–21. doi: 10.1053/jhep.2001.22761. [DOI] [PubMed] [Google Scholar]
- 35.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G765–71. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 36.Ramm GA, Shepherd RW, Hoskins AC, Greco SA, Ney AD, Pereira TN, Bridle KR, Doecke JD, Meikle PJ, Turlin B, Lewindon PJ. Fibrogenesis in pediatric cholestatic liver disease: Role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment. Hepatology. 2008 doi: 10.1002/hep.22637. [DOI] [PubMed] [Google Scholar]
- 37.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 38.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 39.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, Boring L, Charo IF, Simpson KJ, Lukacs NW, Kunkel SL. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–52. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–7. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 42.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–8. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 43.Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, Meier CA, Negro F. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology. 2008;48:799–807. doi: 10.1002/hep.22404. [DOI] [PubMed] [Google Scholar]
- 44.Lo IC, Shih JM, Jiang MJ. Reactive oxygen species and ERK 1/2 mediate monocyte chemotactic protein-1-stimulated smooth muscle cell migration. J Biomed Sci. 2005;12:377–88. doi: 10.1007/s11373-005-1703-2. [DOI] [PubMed] [Google Scholar]
- 45.Aukrust P, Berge RK, Ueland T, Aaser E, Damas JK, Wikeby L, Brunsvig A, Muller F, Forfang K, Froland SS, Gullestad L. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. J Am Coll Cardiol. 2001;37:485–91. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A, B) The liver section from mice transplanted with β-actin promoter driven GFP BM cells was stained with desmin (red, A) or F4/80 (red, B). GFP was not expressed in desmin-expressing HSCs (A) and GFP was expressed in F4/80-expressing Kupffer cells (B). (C-E) GFP-positive Kupffer cells isolated from WT (C), GFP-Tg (D) and GFP-Tg BM cell-transplanted mice (E) were shown by FACS analysis.
Supplementary Figure 2. (A-C) Immunofluorescence of CCR2 in primary HSCs. HSCs were isolated from normal mice (A) and bile duct ligated mice (B) and then fixed at 24 hours after plating with 4% formalin. The cells were incubated with a primary antibody for CCR2 (A, B) or isotype control (C) followed by an Alexa-Fluor 488 conjugated secondary antibody and nuclear staining with DAPI. (D, E) Immunofluorescence for CCR2 in primary Kupffer cells. The cells were stained with a primary antibody for CCR2 (D) or isotype control (E) followed by an Alexa-Fluor 488 conjugated secondary antibody and nuclear staining with DAPI.