Abstract
The melanocortin 1 receptor (MC1R), a Gs protein-coupled receptor (GPCR) expressed in melanocytes, is a major determinant of skin pigmentation and phototype. MC1R activation stimulates melanogenesis and increases the ratio of black, strongly photoprotective eumelanins to reddish, poorly photoprotective pheomelanins. Several MC1R alleles are associated with red hair, fair skin, increased sensitivity to ultraviolet radiation (the RHC phenotype) and increased skin cancer risk. Three highly penetrant RHC variants, R151C, R160W and D294H are loss-of-function MC1R mutants with altered cell surface expression. In this study, we show that forward trafficking was normal for D294H. Conversely, export traffic was impaired for R151C, which accumulated in the endoplasmic reticulum (ER), and for R160W, which was enriched in the cis-Golgi. This is the first report of steady state retention in a post-ER secretory compartment of a GPCR mutant found in the human population. Residues R151 and R160 are located in the MC1R second intracellular loop (il2). Two other mutations in il2, T157A preventing T157 phosphorylation and R162P disrupting a 160RARR163 motif, also caused intracellular retention. Moreover T157 was phosphorylated in wild type MC1R and a T157D mutation mimicking constitutive phosphorylation allowed normal traffic, and rescued the retention phenotype of R160W and R162P. Therefore MC1R export is likely regulated by T157 phosphorylation and the 160RARR163 arginine-based motif functioning as an ER retrieval signal. These elements are conserved in mammalian MC1Rs and in all 5 types of human melanocortin receptors. Thus, members of this GPCR subfamily might share common mechanisms for regulation of plasma membrane expression.
Keywords: Melanocortin 1 receptor, MC1R variants, red hair colour phenotype, export trafficking, intracellular retention, arginine-based sorting motif
INTRODUCTION
The human melanocortin 1 receptor (MC1R) is a G protein coupled receptor (GPCR) expressed in epidermal melanocytes (Bohm et al., 2006; Roberts et al., 2006). MC1R regulates the amount and type of melanin pigment produced and is a major determinant of skin phototype, sensitivity to ultraviolet radiation and skin cancer risk (Rees, 2004; Sturm, 2002). MC1R stimulates cAMP synthesis upon activation by αmelanocyte stimulating hormone (αMSH) or adrenocorticotropin. cAMP is responsible for most actions of αMSH (Busca and Ballotti 2000), including increased activity of the rate-limiting melanogenic enzyme tyrosinase and a switch from production of light-coloured and poorly photoprotective pheomelanins to synthesis of darker and more photoprotective eumelanins (Ito and Wakamatsu 2003).
The MC1R gene is highly polymorphic, and more than 100 non synonymous mutations have been reported (Perez Oliva et al., 2009). Population studies showed that several variant alleles are associated with red hair and fair skin (the RHC phenotype) (Box et al., 1997; Healy et al., 2000; Smith et al., 1998; Valverde et al., 1995). The frequent RHC alleles R151C, R160W, and D294H are highly penetrant, with odds ratios for red hair ranging from 50 to 120 (Duffy et al., 2004; Sturm et al., 2003b), and they are particularly relevant since they account for 60% of all humans with red hair. These variants are also associated with increased risk for melanoma and nonmelanoma skin cancers (Newton Bishop and Bishop 2005; Sturm, 2002; Sturm et al., 2003a). This association is partially independent of the effect on skin pigmentation, and might be related with the ability of wild type MC1R (wtMC1R), but not of RHC variants, to activate DNA repair mechanisms following exposure to ultraviolet radiation (Bohm et al., 2005; Kadekaro et al., 2005). The R151C, R160W, and D294H RHC variants are diminished function forms with reduced coupling to the cAMP cascade (Beaumont et al., 2005; Healy et al., 2001; Newton et al., 2005, 2007; Ringholm et al., 2004; Sanchez-Laorden et al., 2006a, 2007). Residues R151 and R160 are located in the second intracellular loop (il2) (Fig. 1A), a highly conserved region in vertebrates, as well as in the five human melanocortin receptor subtypes (Fig. 1B). D294 lies in the cytoplasmic side of the seventh transmembrane helix and is also highly conserved. D294H is expressed at a cell membrane density higher than wtMC1R. Conversely, the R151C and R160W variants are poorly expressed on the cell surface (Newton et al., 2007; Sanchez-Laorden et al., 2006a, 2007). Therefore, the major RHC variants display an altered cell surface expression that might underlie their functional impairment, but the mechanisms accounting for their aberrant trafficking are unknown.
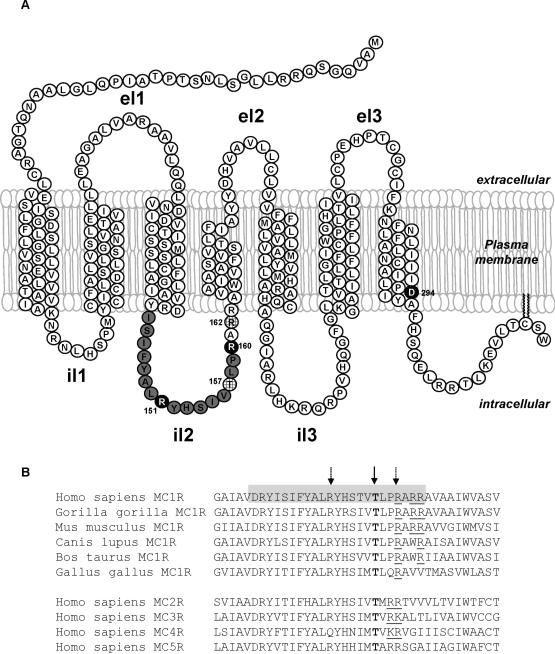
Figure 1. Structure of the human MC1R.
A. Putative MC1R secondary structure, according to the two dimensional model of Ringholm et al (Ringholm et al., 2004) with minor modifications. Residues in il2 are shown in grey. Residues R151, R160 and D294 frequently mutated in red hair individuals are shown in black. Other residues discussed in this study are hatched. el, extracellular loop; il, intracellular loop.
B. Sequence alignment of il2 and flanking residues in melanocortin receptors. The upper block shows the sequence of MC1R from various vertebrate species. Amino acids likely forming the il2 are shown on a grey background. The lower block shows the sequences of the remaining human melanocortin receptors. The invariant T157 is highlighted in bold and by a solid arrow. Residues R151 and R160 are highlighted by broken arrows. Basic residues in the C-terminal vicinity of T157 are underlined.
Cell surface expression of integral plasma membrane proteins reflects the balance between forward traffic from the ER to the cell surface and internalization away from the cell membrane (Polo and Di Fiore 2006). GPCR internalization is mostly accounted for by agonist-mediated phosphorylation by GPCR kinases (GRKs), followed by recruitment of β-arrestins and formation of clathrin-coated endocytic vesicles (DeWire et al., 2007; Pierce et al., 2002). We have shown that D294H is resistant to GRK-dependent desensitization and internalization (Sanchez-Laorden et al., 2007), which accounts for its high plasma membrane expression. However, internalization of R151C and R160W was comparable with wtMC1R (Sanchez-Laorden et al., 2007), suggesting that their low plasma membrane expression was not due to increased endocytic transport, but rather to aberrant forward traffic. Export traffic of membrane proteins is regulated by stringent quality control (QC) mechanisms. Misfolded or incorrectly assembled proteins are retained in the ER, and are frequently retrotranslocated to the cytosol and degraded by proteasomes. At least two types of QC mechanisms have been described. A general system involving ER-resident chaperones tests for broad conformational features such as adequate insertion within the membrane of hydrophobic domains, or correct disulfide bonding (Anelli and Sitia 2008; Ellgaard and Helenius 2003). A second and more specific QC system is thought to recognize specific motifs interacting with proteins that assist correct folding or escort the correctly folded proteins through the ER and the Golgi (Duvernay et al., 2005; Michelsen et al., 2005). Only a limited number of these motifs have been identified. The QC systems are normally considered to reside in the ER. However, certain disease-causing V2 vasopressin receptor mutants have been shown to reach the ER/Golgi intermediate compartment (ERGIC), but are retrieved to the ER where they accumulate (Hermosilla et al., 2004). Similarly, a mutant of the vesicular stomatitis virus G protein seems able to reach the ERGIC (Lotti et al., 1996) and an inactive sucrase-isomaltase mutant has been found in the cis-Golgi (Ouwendijk et al., 1996). These data suggest the occurrence of post-ER control checkpoints. Their nature and the structural elements that determine the ER residency of retained proteins as opposed to those exiting to the Golgi are not well understood.
We now report a study of traffic through the biosynthetic pathway of the major RHC alleles R151C, R160W and D294H. We show that forward traffic is normal for D294H, but impaired for R151C and R160W, resulting in their intracellular retention. However, the sites of retention are different, with R151C preferentially associated with the ER whereas R160W accumulates in post-ER compartments, most likely the cis-Golgi. This provides the first example of retention of a mutant GPCR within this compartment. Moreover, analysis of other natural and artificial mutants suggests that proper forward movement of the MC1R requires phosphorylation of residue T157 and is controlled by an arginine-based motif involving R160.
MATERIALS AND METHODS
Materials
Radioimmunoassay kits for cAMP and [125I]NDP-MSH (2000 Ci/mmol) were from Amersham Pharmacia Biotech (Little Chalfont, UK). The following antibodies were used: anti-Flag M2 monoclonal and anti-Flag M2-Peroxidase conjugate (Sigma, St. Louis, MO), anti-ERK2 and anti-calnexin rabbit polyclonals (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-COPI rabbit polyclonal (Affinity Bioreagents, Golden, CO), anti-GM130 monoclonal (BD Biosciences, Franklin Lakes, NJ), and anti-phosphothreonine-HRP conjugated rabbit polyclonal (StressMarq, Victoria, Canada).
Cell lines and transfection
Cell culture reagents were from Nunc (Roskilde, Denmark) or Gibco (Gaithersburg, MD). HEK293 cells were grown in 12 well dishes using RPMI 1640, supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin sulfate. Cells grown to 75% confluence were transfected with 0.3 μg plasmid and Lipofectamine (Invitrogen, Carlsbad, CA). HBL cells (a gift from Prof. G. Ghanem, Free University of Brussels, Belgium) were grown in DMEM with antibiotics and 10% fetal calf serum. Stable transfectants were obtained as described (Mas et al., 2003), and were cultured in the presence of 800 μg/ml G418 sulfate.
Expression constructs
All expression constructs were prepared in pcDNA3 (Invitrogen). The preparation of the Flag-tagged wtMC1R or variant MC1R has been described (Sanchez-Mas et al., 2005, Sanchez-Laorden et al., 2006a, 2007). All constructs were verified by complete automated sequencing of both strands. EGFP-Rab1 was a gift from Prof. Miguel Seabra, Imperial College, London, UK) and the CFP-β-GT construct encoding for a fusion of the cyan fluorescent protein and galactosyl transferase (designated CFP-GalTr) was from Clontech (Mountain View, CA).
Functional assays
Radioligand binding assays were done with 10−10 M [125I]NDP-MSH, and increasing concentrations of unlabeled NDP-MSH, up to 10−7 M, when required as previously described (Jimenez-Cervantes et al., 2001a). For cAMP production assays, cells were grown in 12 well plates, transfected as required, and serum-deprived for 24 h. They were then incubated with NDP-MSH (10−7 M, 30 min). The medium was aspirated and the cells quickly washed with 800 μl ice-cold phosphate buffered saline (PBS). Cells were lysed with 350 μl preheated 0.1N HCl (70 °C) per well, and scraped. The mix was freeze-dried, washed with 100 μl H2O and dried again. cAMP was measured with a commercial radioimmunoassay, as per instructions. Parallel dishes were used for protein determination performed with the bicinchoninic acid method.
Electrophoresis and Western blot
Cells were washed twice with PBS and solubilized at 4 °C in 200 μl solubilization buffer (50 mM Tris-HCl pH 8, 1% Igepal, 1 mM EDTA, 0.1 mM PMSF, 10 mM iodoacetamide). Samples were centrifuged (105,000×g, 30 min) and a volume of supernatant containing 10 μg protein was mixed (2:1 ratio) with sample buffer (180 mM Tris-HCl, pH 6.8, 15% glycerol, 9% SDS, 0.075% bromophenol blue and 7.5% β-mercaptoethanol). Electrophoresis and Western blotting were performed as described (Sanchez-Laorden et al., 2006a; Sanchez-Mas et al., 2005). Comparable loading was ascertained by stripping and reprobing the membranes with an anti-ERK2 antibody. Stripping was performed by washing the membranes with PBS, followed by treatment with 0.5 N NaOH, 10 min at room temperature, and a 10 min wash with PBS.
Metabolic labelling and endoglycosidase treatment
Cells grown in 100 mm2 dishes were incubated in methionine-free medium for 30 min. The culture medium was then supplemented with 0.5 μCi of 35S-Methionine&Cysteine Redivue Promix mixture (7.15 mCi/500 μl). Cells were further incubated for 30 min and washed twice with PBS. Then, cells were incubated in complete DMEM medium (10 ml) supplemented with 1 mM methionine, for the required times, extensively washed and lysed in 1 ml lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 0.01% SDS). Lysates were precleared with preimmune rabbit serum and protein G-Sepharose, followed by centrifugation. Aliquots of the cleared supernatants were counted for protein-bound radioactivity by TCA precipitation. Volumes corresponding to 8 × 106 cpm were incubated (overnight, 4 °C) with 8 μl anti-Flag, or anti-GAPDH, for control. 20 μl protein G-Sepharose slurry were added and the samples were further incubated for 2 h and centrifuged. The pellets were extensively washed with lysis buffer. Immunoprecipitated protein was released by 5 min incubation at 80 °C in 0.5% SDS and 0.04 M dithiothreitol and digested with endoglycosidase H (EndoH) when required. For this purpose, samples were incubated (37 °C, 4 h) with 15 mU of EndoH in 50 mM phosphate buffer, pH 7.0, 10 mM EDTA 0.1% SDS. Reactions were stopped by addition of sample buffer, and the samples were centrifuged and electrophoresed. Gels were dried and autoradiographed.
Flow Cytometric Analysis
Approximately 500,000 cells were incubated in a final volume of 100 μl with anti-Flag M2 (1:100) for 30 min at 4 °C. Cells were washed twice (2% fetal calf serum, 0.01% NaN3 in PBS), and further incubated with a phycoerythrin-labeled anti-mouse IgG, at a final dilution of 1:50, for 30 min at 4 °C. Cells were washed, resuspended in 500 μl PBS and analyzed in a Becton Dickinson FACScan system.
Confocal microscopy
Cells grown on coverslips were transfected with the Flag epitope-labeled wtMC1R or mutant MC1R constructs. In some experiments, cells were co-transfected with MC1R variants and EGFP-Rab1 or CFP-GalTr. 24 h after transfection, cells were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.5% Igepal. Cells were labeled with anti-Flag (1:7000), followed by an Alexa 568-conjugated secondary antibody, for detection of MC1R. For co-localization of Flag-MC1R and calnexin, GM130 or COPI, cells treated as above were incubated simultaneously with the anti-Flag monoclonal and the appropriate antibody directed against the relevant organelle markers, followed by Alexa 568-conjugated anti-mouse and Alexa 488-conjugated anti-rabbit secondaries. Samples were mounted by standard procedures using a mounting medium from DakoCytomation (Carpinteria) and examined with a Leica laser scanning confocal microscope. The images were acquired in a 1024×1024 pixels format in sequential scan mode between frames for avoid cross-talk. The objective used was HCX PL APO CS 63X and the pinhole value 1, corresponding to 114.73 μm.
RESULTS
Aberrant cell surface expression of major MC1R variants
Previous reports have shown a different plasma membrane expression of the R151C, R160W and D294H MC1R variants (Beaumont et al., 2005, 2007; Sanchez-Laorden et al., 2006a), but these differences have not yet been quantified under identical experimental conditions. Since normal human melanocyte cultures of defined MC1R genotype are extremely difficult to obtain and are not routinely available (Kadekaro et al., 2005, Roberts et al., 2006), we used HEK293 cells and HBL human melanoma cells as model systems for this study. HEK293 cells were selected because they have been widely employed in studies of MC1R function as related with intracellular trafficking (Beaumont et al., 2005, 2007; Sanchez-Laorden et al., 2006a, 2007). HBL melanoma cells were chosen as a suitable melanocytic model because they respond normally to stimulation with melanocortin agonists by increasing cAMP levels and are wild type for MC1R (Sanchez et al., 2009), thus avoiding potential artefacts due to expression of endogenous variant receptors with altered trafficking properties (Beaumont et al., 2007; Sanchez-Laorden et al., 2006a). The number of NDP-MSH binding sites (Bmax) and their affinity (dissociation constant, Kd) were analyzed by saturation radioligand binding experiments. Bmax values in HEK293 cells transiently transfected with MC1R variants were significantly lower for R151C and R160W (p = 0.0071 and p = 0.0055 respectively), but their affinity for the αMSH analogue was not impaired (Table 1). Conversely, D294H was expressed at a higher cell surface density, as shown by a Bmax value approximately 2-fold higher than wtMC1R, but its affinity for the agonist was significantly reduced (p < 0.0001). On the other hand, HBL human melanoma cells were stably transfected with wtMC1R or variant receptors and at least 6 clones for each form were selected and analyzed. Clones were first incubated with 125I-NDP-MSH at a single concentration of 10−10 M. Although some variability was observed, clones expressing R151C and R160W bound less radioligand than cells expressing either wtMC1R or D294H (not shown). One representative clone per MC1R form was selected for further study. Bmax values were lower for clones expressing R151C and R160W, intermediate for wtMC1R and higher for D294H (Table 1), confirming the results obtained in HEK293 cells.
TABLE 1.
Binding parameters of wtMC1R and RHC variants
| Cell system | MC1R variant | Bmax (fmoles/μg protein) | Kd (nM) |
|---|---|---|---|
| HEK 293 cells (transient transfection) | wtMC1R | 3.8 ± 0.6 | 2.2 ± 0.3 |
| R151C | 0.6 ± 0.2 | 0.9 ± 0.2 | |
| R160W | 0.4 ± 0.2 | 0.7 ± 0.3 | |
| D294H | 6.2 ± 0.7 | 16.1 ± 1.4 | |
| HBL melanoma cellsa (stable transfection) | wtMC1R | 2.4 ± 0.1 | 0.7 ± 0.1 |
| R151C | 0.5 ± 0.05 | 0.6 ± 0.1 | |
| R160W | 0.4 ± 0.05 | 0.8 ± 0.3 | |
| D294H | 3.4 ± 0.2 | 1.5 ± 0.4 |
Data are given as mean ± SEM, for at least three independent experiments.
HBL human melanoma cells express endogenous wtMC1R at a density of 0.3 fmoles/μg protein
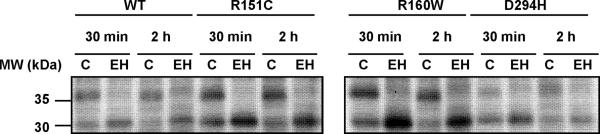
These differences in plasma membrane density could be due to different levels of protein expression or, alternatively, to aberrant trafficking of the RHC variants. To distinguish between these possibilities, we compared MC1R levels and processing by means of metabolic labelling experiments. Clones of HBL melanoma cells were metabolically labelled with 35S, and chased for 30 min or 2 h. Then MC1R was immunoprecipitated using an anti-Flag monoclonal antibody, treated with EndoH and analyzed by SDS-PAGE and autoradiography (Fig. 2). EndoH efficiently cleaves high mannose glycans found in incompletely processed glycoproteins present in the ER. However, upon further processing to complex glycans in the medial Golgi, glycoproteins most often become resistant to EndoH. Consistent with previous results, MC1R appeared as a doublet with two bands of 30 and 36 kDa, corresponding to de novo and glycosylated forms respectively (Perez Oliva et al., 2009). The intensity of both bands was slightly higher for the R151C and R160W mutants and comparable for wtMC1R and D294H. This showed that reduced plasma membrane expression of R151C and R160W, and high levels of cell surface D294H were not accounted for by parallel changes in the levels of protein expression. Moreover, the relative intensity of the 30 kDa band compared with the 36 kDa protein was less at the longer chase time (2 h), consistent with a precursor-product relationship for these forms. Finally, EndoH fully digested the 36 kDa proteins for all MC1R forms and switched their mobility to 30 kDa, as previously reported (Sanchez et al., 2002). This confirmed that MC1R belongs to a restricted group of proteins of the biosynthetic-secretory pathway with EndoH-sensitive mature forms (Perez Oliva et al., 2009), and excluded the use of this enzyme to compare wtMC1R and mutant MC1R trafficking. On the other hand, the finding of substantial amounts of unprocessed protein after a 2 h chase showed that MC1R maturation is a slow process.
Figure 2. Expression and processing of wild type and variant MC1R in human melanoma cells.
Clones of HBL melanoma cells stably expressing epitope-tagged wtMC1R or variant receptors were metabolically labelled with 35S, chased for 30 min or 2 h as indicated and immunoprecipitated for MC1R. Immunoprecipitated proteins were digested with EndoH (EH), solubilized and electrophoresed. C stands for undigested control samples.
The R151C MC1R variant is retained within the ER
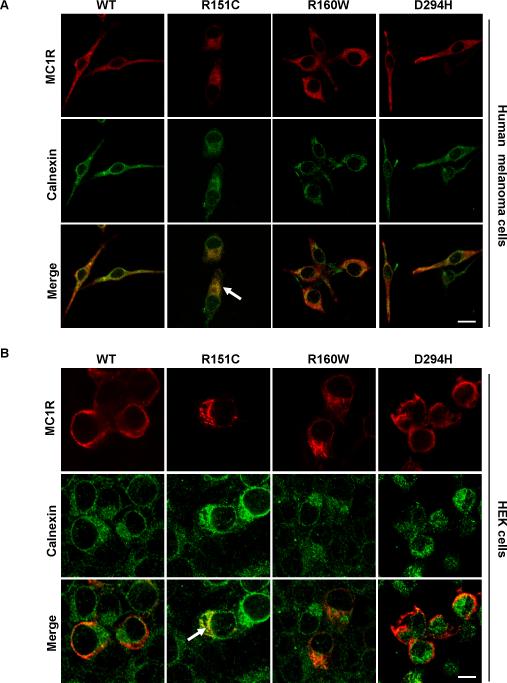
In order to assess the trafficking defects of the RHC variants, we studied their co-localization with specific organelle markers by confocal laser scanning microscopy. Clones of HBL melanoma cells stably expressing wtMC1R or variant MC1R were permeabilized and stained for the receptor and calnexin, an ER-resident chaperone that assists the folding of nascent proteins of the secretory pathway (Anelli and Sitia 2008). In permeabilized cells, cell surface MC1R staining was low but detectable for wtMC1R and D294H but almost absent for R151C and R160W (Fig. 3A), consistent with the binding experiments described above. Conversely, high levels of intracellular staining were observed for wtMC1R and mutant receptor (Fig. 3A), as previously reported by others (Beaumont et al., 2005). This is consistent with slow maturation of the protein on one hand, and intracellular retention of mutant forms on the other. For all variants, intracellular MC1R partially co-localized with calnexin as expected for a protein of the biosynthetic-secretory. Nevertheless, co-localization with the ER marker calnexin was lower for wtMC1R, R160W or D294H (Fig. 3A), and extensive for R151C, suggesting its retention within the ER. We confirmed these data using HEK cells, where wtMC1R was also present on the plasma membrane and in intracellular locations (Fig. 3B). D294H was mostly found on the cell surface, and intracellular staining was the lowest for this variant, consistent with binding data (Table 1). The R151C variant showed a reticular distribution with strong perinuclear staining and extensive co-localization with calnexin. R160W presented a different and more concentrated staining pattern with poor co-distribution with the ER marker.
Figure 3. Retention of the R151C variant of MC1R in the ER.
A. HBL melanoma cells expressing wtMC1R or the indicated variants were fixed, permeabilized, stained for MC1R and calnexin and examined in a confocal microscope. MC1R is shown in red (upper) and calnexin in green (middle). In merged images (lower) co-localization is shown in yellow. Note the higher co-localization of R151C with the ER marker (arrow). Scale bar: 20 μm
B. Same as in panel A, except that HEK293 cells transiently transfected with wtMC1R or mutant MC1R were used. Co-localization of R151C with calnexin is highlighted (arrow). Scale bar: 15 μm
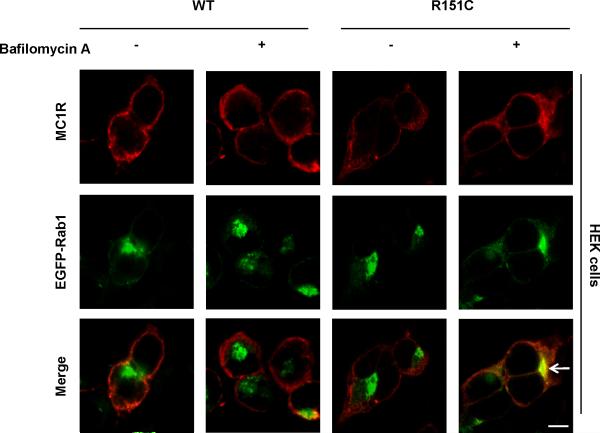
These data suggested that at least R151C was specifically retained within the ER. In order to analyze whether this was due to its inability to move forward to the Golgi or, alternatively, to efficient retrieval, we used bafilomycin A (bafA). This drug inhibits retrograde traffic and in its presence cycling proteins accumulate in post-ER compartments where they become detectable (Oueslati et al., 2007). wtMC1R showed a very similar staining pattern in control and in bafA-treated cells (Fig. 4). The distribution of R160W or D294H was also unaffected by the drug (not shown). However, bafA treatment caused a change in the intracellular staining of R151C from its reticular pattern to a more concentrated one (Fig. 4). Moreover, we found a significant co-localization with a Rab1-EGFP construct co-expressed with the MC1R, which was absent in control cells. Rab1 is a small GTPase that regulates forward traffic from the ER to the Golgi (Donaldson et al., 1992; Duvernay et al., 2005) and can be used as a marker of the ERGIC and the cis side of the Golgi. Therefore, R151C was detected in post-ER sites upon bafA treatment, showing that its accumulation within the ER was due to efficient retrieval.
Figure 4. Bafilomycin A-promoted accumulation of the R151C variant in a post-ER site.
HEK293 cells co-transfected with wtMC1R or R151C and the small GTPase Rab1 fused to the fluorescent protein EGFP (EGFP-Rab1) were treated with bafilomycin A (1 μM) for 4 h. Cells were stained for MC1R (red, upper row). The EGFP-Rab1 signal was detected directly (green, middle row). Merged images are shown in the lower row. The higher co-localization with the proximal Golgi marker Rab1 following bafilomycin treatment is highlighted by an arrow. Scale bar: 10 μm
Steady-state accumulation of R160W in a post-ER compartment
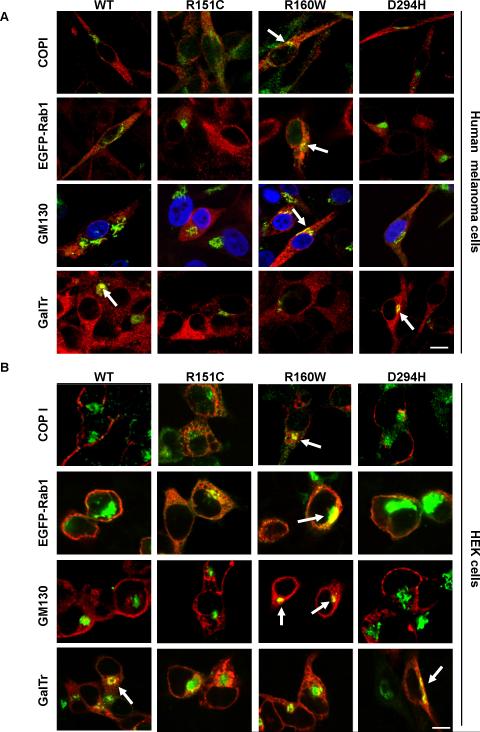
We next analyzed the co-distribution of MC1R variants with a panel of Golgi markers. The Golgi consists of multiple disk-shaped cisternae, designated cis, medial or trans to indicate the sequence of their function in the secretory pathway (Losev et al., 2006; Matsuura-Tokita et al., 2006). These Golgi compartments are differentially enriched in proteins that can be used as suitable markers. In addition to Rab1, we used COPI, GM130 and galactosyl transferase (GalTr). COPI is a protein complex essential for the coating of vesicles involved in retrograde intra-Golgi and Golgi-to-ER transport, mainly found in association with the ERGIC and the cis-Golgi (Lippincott-Schwartz and Liu 2003, 2006). GM130 is a peripheral membrane protein involved in the stacking of cis and medial cisternae and in the tethering of forward-trafficking vesicles to the cis-Golgi (Moyer et al., 2001; Rottger et al., 1998). Finally, GalTr is an enzyme involved in the modification of carbohydrate chains on proteins and is enriched in trans-Golgi cisternae (Rottger et al., 1998). We used tagged versions of Rab1 and GalTr fused with fluorescent proteins. Co-expression of these constructs and wtMC1R did not alter the number of binding sites available on the cell surface of HEK cells, thus excluding abnormal distribution of cargo as a consequence of marker overexpression (not shown). On the other hand, endogenous COPI and GM130 were detected with specific antibodies. In stably transfected melanoma cells, R160W co-localized markedly with the ERGIC/cis-Golgi markers COPI and Rab1, as well as with the cis-medial Golgi protein GM130 (Fig. 5A). However, co-localization of the other MC1R forms with these markers was low or undetectable. Neither R160W nor R151C co-distributed significantly with the trans-Golgi marker GalTr, consistent with their retention in upstream compartments of the secretory pathway. Conversely, a clear co-localization was detected for wtMC1R and D294H, thus showing that these forms were able to reach the trans-Golgi, in keeping with their presence on the cell surface. The same trend was observed in HEK cells (Fig. 5B), with strong co-localization of R160W with COPI, Rab1 and GM130, and a much weaker co-distribution of these markers with wtMC1R, R151C and D294H. Only wtMC1R and D294H displayed significant co-distribution with GalTr.
Figure 5. Selective steady-state enrichment of R160W MC1R variant in the proximal Golgi.
A. HBL melanoma cells stably expressing wtMC1R or variant MC1R were transfected with EGFP-Rab1, CFP-GalTr, or empty vector. Cells were stained for MC1R and, if appropriate, for GM130 or COPI, as indicated. Occasionally, nuclei were labelled with DAPI (shown in blue). Cells were examined in a confocal microscope. MC1R is shown in red, all markers in green and co-localization in yellow. Only merged images are shown. Stronger co-localization signals are shown with arrows. Scale bar: 15 μm
B. Same as in A, except that HEK293 cells were analyzed. Scale bar: 15 μm
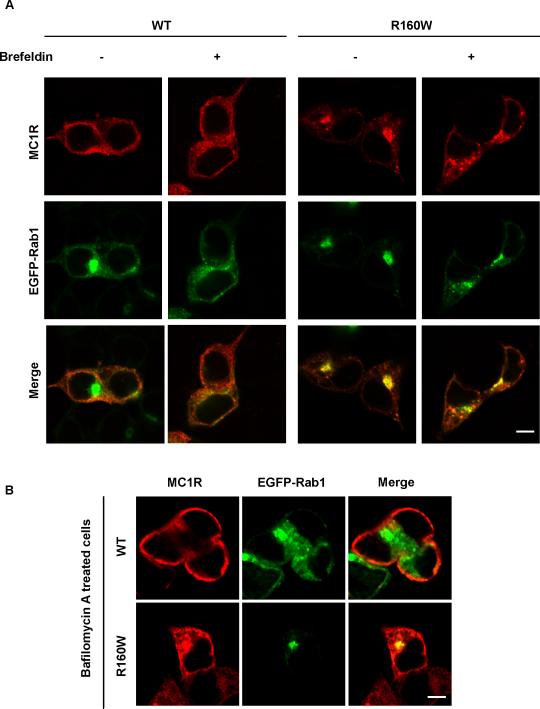
These results, together with the absence of co-localization of R160W with calnexin, suggested that the site of intracellular accumulation of R151C and R160W was different, with retention of R151C in the ER and of R160W in a post-ER compartment, most likely the proximal Golgi. To confirm the accumulation of R160W in the Golgi, we used brefeldin A. This drug specifically causes the breakdown of the Golgi apparatus (Donaldson et al., 1992; Lippincott-Schwartz and Liu 2003). Thus, disruption of the staining pattern of intracellular proteins by brefeldin A treatment is diagnostic of association with this organelle. The drug clearly disorganized the intense and compact R160W staining in HEK cells and yielded a more diffuse and vesicular pattern (Fig. 6A). Conversely, bafA had no effect on R160W staining (Fig. 6B). This confirmed the specificity of the effect of brefeldin A and the steady-state association of the R160W with a post-ER site.
Figure 6. Disruption of the staining pattern of the R160W MC1R variant by brefeldin treatment.
A. HEK293 cells transfected with wtMC1R or R160W and EGFP-Rab1 were treated with brefeldin (1 μg/ml, 30 min), permeabilized and stained for MC1R (red). Disruption of the Golgi apparatus is shown by comparison of the EGFP-Rab1 signal (green) in treated and control cells. Scale bar: 10 μm
B. Same as in panel A except that cells were treated with baf A (1 μM, 4 h). Scale bar: 10 μm
T157 phosphorylation is a major determinant for MC1R trafficking
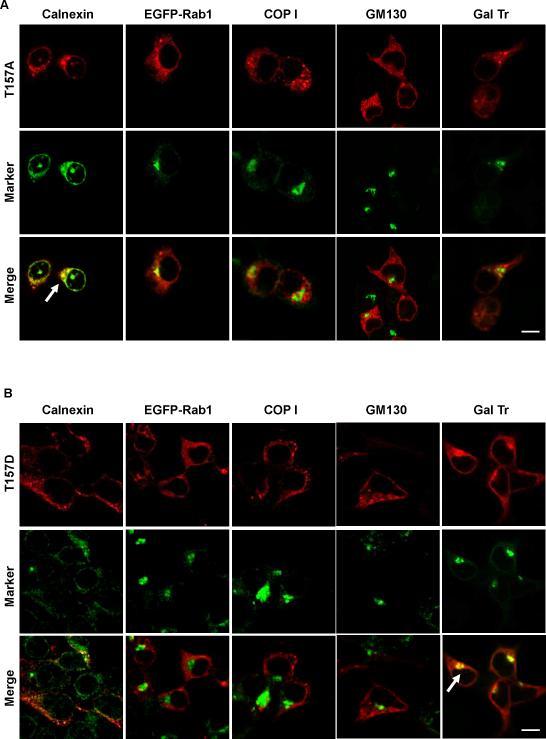
R160 is located within a 157TLPR160 PKC target sequence of MC1R (Siegrist et al., 1994) whose sensitivity to phosphorylation should be reduced or abolished by the R160W mutation. We have reported that a phosphorylation incompetent T157A mutant is non-functional and does not bind significant levels of the specific radioligand 125I-NDP-MSH (Sanchez-Laorden et al., 2007), suggesting its inability to reach the cell surface. We prepared a T157D mutant which mimics the phosphorylated state of T157 due to the introduction of a negatively charged residue, and we analyzed the trafficking of T157A and T157D. Since the previous results showed that the trafficking pattern of MC1R variants was the same in HEK293 and in melanoma cells, we used transient expression in HEK cells as a convenient model system. T157A co-localized strongly with calnexin, but not with Rab1, COPI, GM130 or GalTr (Fig. 7A). This pattern was the same as for R151C. Interestingly, T157D was clearly detected on the plasma membrane in addition to intracellular locations (Fig. 7B). Moreover, it displayed poor co-localization with calnexin, Rab1, COPI and GM130, but significant co-distribution with GalTr. This pattern resembled wtMC1R behaviour.
Figure 7. Distribution of the T157A and T157D MC1R mutants.
HEK293 cells were transiently transfected to express the T157A (panel A) or T157D (panel B) mutants, alone or in combination with EGFP-Rab1 or CFP-GalTr. Permeabilized cells were stained for MC1R (red, upper row in each panel), and for calnexin, COPI or GM130, when needed. The EGFP-Rab1 and CFP-GalTr signals were detected directly. All organelle markers are shown on green (middle row in each panel). Strong co-localization signals are highlighted by arrows. Scale bar: 15 μm
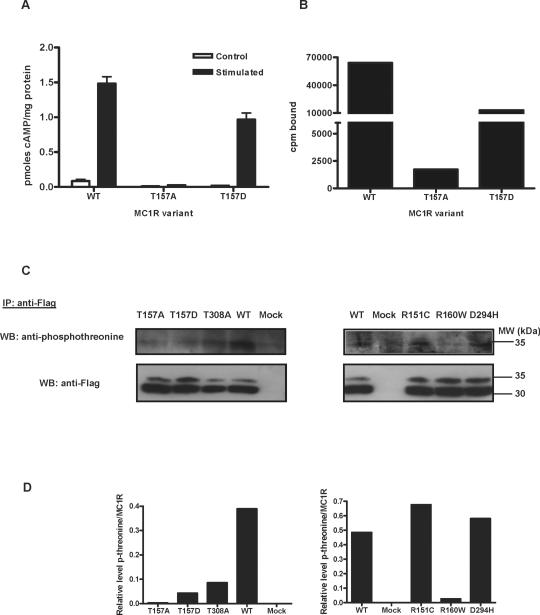
Cells expressing the T157A variant did not display functional coupling to cAMP synthesis or detectable radioligand binding, consistent with extensive intracellular retention and with previous reports (Fig. 8A, B). Conversely, cells expressing T157D responded to an agonist challenge with a strong increase of cAMP intracellular concentration, thus indicating receptor expression on the cell surface (Fig. 8A). This was confirmed by binding studies, where cells expressing the T157D mutant bound significant amounts of ligand (Fig. 8B). Therefore, comparison of the phenotype of T157A and the less conservative T157D mutation strongly suggested that loss of function of the T157A mutant was due to intracellular retention away from the cell surface as a result of its inability to undergo a phosphorylation event required for forward traffic. To test whether T157 is phosphorylated in vivo, extracts from cells expressing wtMC1R, T157A or T157D were immunoprecipitated for MC1R with anti-Flag, followed by Western blot of the pellets with an anti-phosphothreonine antibody. A T308A mutant was included in this experiment, since T308 has been shown to undergo phosphorylation (Sanchez-Laorden et al., 2007). The clear phosphothreonine signal present in wtMC1R was significantly reduced in the T157A, T157D and T308A mutants, thus showing that these residues were indeed phosphorylated in vivo (Fig. 8C). This signal was also markedly reduced in R160W. In contrast, T157 phosphorylation was comparable for wtMC1R, D294H and R151C. We did not perform similar experiments aiming at the detection of T157 phosphorylation in untransfected human melanocytic cells, since the levels of expression of the receptor protein in those cells are most often below the limit of detection by Western blot (Roberts et al., 2006).
Figure 8. Functional analysis of the T157A and T157D mutants.
A. Functional coupling to cAMP generation. wtMC1R, T157A or T157D were transiently expressed in HEK293 cells. Cells were challenged with 10−7 M NDP-MSH for 30 min, lysed and their cAMP content was determined by radioimmunoassay. Empty bars represent the cAMP concentration in resting controls and solid bars the cAMP levels after stimulation.
B. Binding of [125 I]NDP-MSH. Cells were incubated with 10−10 M [125 I]NDP-MSH for 1 h, washed and the radioactivity associated was determined.
C. Phosphorylation of T157. HEK293 cells expressing the MC1R form indicated on top of each lane were washed with PBS and solubilized in 200 μl solubilization buffer supplemented with 1% phosphatase inhibitor mix (Calbiochem). Extracts were immunoprecipitated with a resin for affinity capture of Flag-tagged proteins. Released proteins were electrophoresed and immunoblotted with an anti-phosphothreonine antibody (upper). Comparable input was checked by staining for MC1R with anti-Flag (lower). A representative blot out of three experiments with similar results is shown.
The T157D mutation rescues plasma membrane localization of R160W and R162P
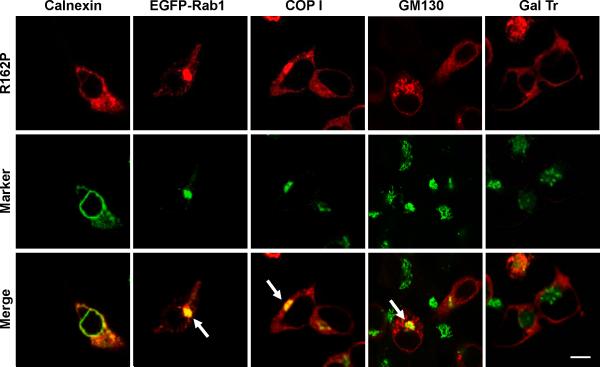
The R160W mutation not only abolishes the 157TLPR160 kinase target sequence but also impacts on a 160RARR163 tetrapeptide resembling several previously described arginine-based ER sorting motifs. These motifs maintain proteins in the ER by retention or retrieval until they are inactivated by specific protein-protein interactions (Michelsen et al., 2005; Nufer and Hauri 2003; O'Kelly et al., 2002). Another natural MC1R variant, R162P, also interferes with the 160RARR163 arginine-rich motif. This variant was originally described as wtMC1R (Mountjoy et al., 1992) but later on it was shown to be a rare mutation yielding an inactive protein (Jimenez-Cervantes et al., 2001b) unable to reach the plasma membrane and retained within a still uncharacterized subcellular compartment (Sanchez-Laorden et al., 2006b). Therefore, it was of interest to assess the effect on forward traffic of the R162P mutation as well as the T157D mutation introduced on a R160W or R162P background. A R151C/T157D double mutant was also obtained and analyzed.
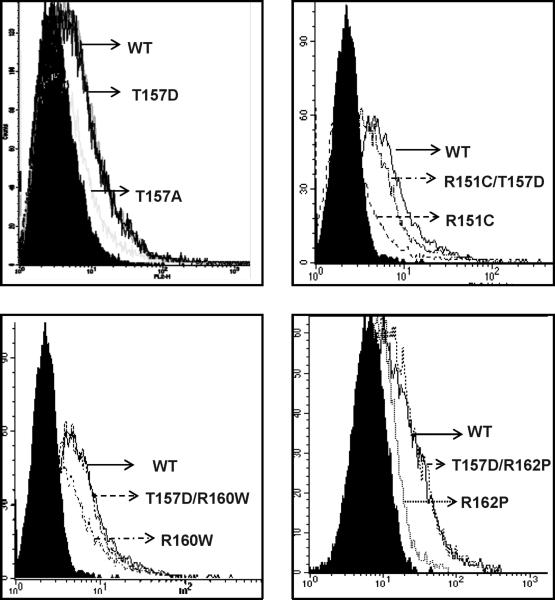
R162P co-localized partially with calnexin, strongly with Rab1, COPI and GM130 but not significantly with GalTr (Fig. 9). On the other hand, the behaviour of the T157D/R160W and T157D/R162P mutants was clearly different from the corresponding single mutants, with a higher plasma membrane expression, a decreased co-localization with intracellular markers and a clear co-localization with the trans-Golgi marker GalTr (Fig. 10A-C). Concerning T157D/R151C, the mutant displayed a less clear-cut distribution pattern with detectable co-localization with GalTr, but strong co-distribution with calnexin, comparable with the R151C single mutant. Low specific radioligand binding of the double mutants excluded the determination of Bmax values by saturation binding experiments. Therefore, their plasma membrane expression was further analyzed by flow cytometry (FACS) using anti-Flag (Fig. 11). In non permeabilized cells, this antibody would only bind to cells expressing the receptor on the plasma membrane, since the Flag epitope was introduced at the extracellular N-terminus of the protein. The FACS signal was reduced for cells expressing R151C, T157A, R160W and R162P, thus confirming previous results (Sanchez-Laorden et al., 2006a, 2006b). However, cells expressing either T157D or the T157D/R160W and T157D/R162P double mutants bound as much antibody as cells expressing wtMC1R (Fig. 11). Therefore, the T157D mutation rescued R160W and R162P from intracellular retention. Again, the situation for the R151C mutant was intermediate. Introduction of the T157D mutation on a R151C background increased cell surface expression, but plasma membrane levels of the double mutant remained lower than wtMC1R.
Figure 9. Distribution of the R162P mutant.
HEK293 cells were transiently transfected to express the R162P mutant, alone or in combination with EGFP-Rab1 or CFP-GalTr. Permeabilized cells were stained for MC1R (red, upper row), and for calnexin, COPI or GM130, when needed. Organelle markers are shown in green (middle row). Stronger co-localization signals are highlighted by arrows. Scale bar: 15 μm
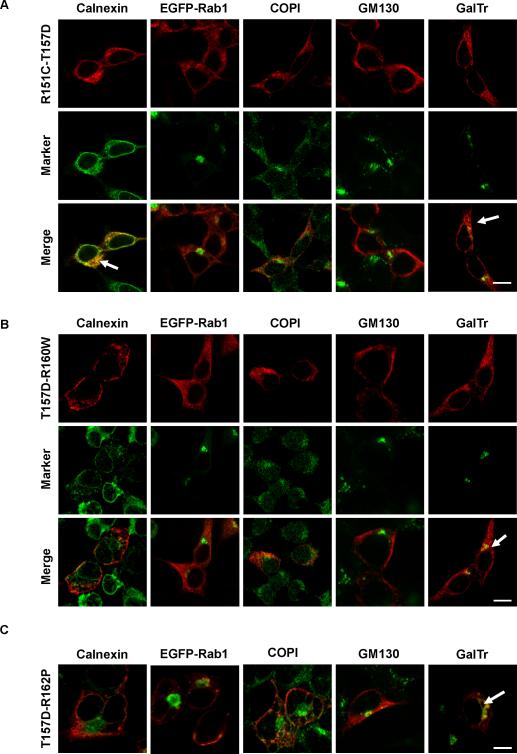
Figure 10. The T157D mutation rescued normal forward traffic of R160W and R162P.
HEK293 cells were transfected to express the T157D/R151C, T157D/R160W or T157D/R162P double mutants alone or in combination with EGFP-Rab1 and CFP-GalTr. Permeabilized cells were stained for MC1R (red, upper row in each panel), and for organelle markers when needed. Markers are shown in green (middle row). Arrows point to positive co-localization signals.
A. Behaviour of the T157D/R151C mutant. Note the significant co-localization with calnexin and the noticeable co-distribution with CFP-GalT. Scale bar: 15 μm
B. Behaviour of T157D/R160W. Note the detectable plasma membrane expression and the lack of the strong co-localization with Rab1, COPI or GM130 observed for R160W. Scale bar: 15 μm
C. Staining of T157D/R162P. For convenience only merged images are shown. Bar: 10 μm
Figure 11.
Flow cytometric analysis of HEK293 cells transfected with Flag-tagged wtMC1R or variant MC1R. Histograms display cell number (counts) as a function of MC1R surface staining, on a logarithmic scale. The blank (filled curve) refers to cells transfected with a native wtMC1R construct lacking the Flag epitope. Representative histograms out of triplicates are shown.
DISCUSSION
The RHC variant alleles of the human MC1R gene are associated with a complex phenotype characterised by fair skin, poor tanning ability, freckling, red hair colour and increased risk of skin cancers (Box et al., 1997; Duffy et al., 2004; Healy et al., 2000; Newton Bishop and Bishop 2005; Smith et al., 1998; Sturm, 2002; Sturm et al., 2003b). These mutant alleles encode for partial loss-of-function proteins, with diminished ability to activate the cAMP pathway (Beaumont et al., 2005, 2007; Healy et al., 2001; Newton et al., 2005, 2007; Ringholm et al., 2004; Sanchez-Laorden et al., 2006a, 2007). The results presented in this study prove that the major RHC alleles R151C and R160W show an aberrant forward traffic with intracellular retention, which accounts for their functional impairment, as suggested by previous reports (Beaumont et al., 2005, 2007; Sanchez-Laorden et al., 2006a, 2007). This conclusion was based on equilibrium binding, confocal microscopy and flow cytometry experiments. Unfortunately, wtMC1R and the RHC mutants were equally sensitive to EndoH digestion, thus excluding endoglycosidase analysis as a tool to examine misprocessing. The sensitivity of wtMC1R to EndoH has been previously demonstrated (Perez-Oliva et al, 2009) and might be related to lack of modification of the high mannose glycans acquired in the ER. This suggests that oligosaccharide processing in the Golgi is not essential for MC1R traffic and activity. Although this is not a common situation, it has been found for a few proteins of the secretory pathway, such as the human corticosteroid binding globulin (Ghose-Dastidar et al., 1991).
R151C and R160W were mistrafficked to similar extents, as shown by comparable steady-state density of binding sites on the plasma membrane of cells expressing the mutant MC1Rs. However, their pattern of intracellular retention was remarkably different. Intracellular R151C was preferentially found in the ER as shown by extensive co-localization with calnexin, and lack of co-distribution with a panel of Golgi markers. Retention was not due to saturation of the ER export machinery since it was detected in melanoma cells stably expressing moderate levels of the variant and was not seen for the other forms of MC1R expressed at similar levels. Moreover, treatment of HEK293 cells with bafA, a drug blocking retrograde Golgi-to-ER transport (Oueslati et al., 2007), promoted a redistribution of R151C to a post-ER site. Therefore, ER retention of R151C was a characteristic of this variant rather than an artefact of overexpression-related aggresome formation.
On the other hand, R160W was able to leave the ER but was trapped within a post-ER compartment, most likely the cis-Golgi. This was shown in stably transfected melanoma cells and in transiently transfected HEK293 cells, by poor co-localization with calnexin and strong co-segregation with proximal Golgi markers. Since steady state retention in the Golgi is a highly uncommon event, we confirmed this finding by means of pharmacological treatments. The staining pattern of R160W was insensitive to bafA but was disorganized by brefeldin A, an antibiotic that disrupts the Golgi stacks and promotes relocalization of Golgi-resident proteins (Donaldson et al., 1992). The escape of R160W from the ER was not due to excessive overexpression that overwhelmed saturable ER QC checkpoints, since total R151C or R160W protein levels were similar, yet R151C accumulated in the ER. Moreover, accumulation of R160W in the Golgi was also seen in HBL cells stably expressing moderate levels of the variant receptor. Several inactive mutants of the V2 vasopressin receptor causing nephrogenic diabetes insipidus have been shown to reach a post-ER compartment. However, in the absence of pharmacological treatments those mutants accumulated in the ER rather than in the ERGIC/Golgi, due to efficient retrieval (Hermosilla et al., 2004). Therefore, to our knowledge this is the first report of specific retention in a post-ER compartment of GPCR mutants found in the human population, in the absence of pharmacological perturbations. The consequences of accumulating a mutant GPCR in a compartment of the secretory pathway different from the ER deserve further investigation. ER-retained proteins can trigger the unfolded protein response (Rutkowski and Kaufman 2004), or can be retrotranslocated to the cytosol where they are degraded by proteasomes (Ahner and Brodsky 2004). It will be important to learn if similar mechanisms operate in the Golgi, and the MC1R mutants might provide unique models for these studies.
Remarkably, two mutations in residues near R151 and R160 had phenotypic effects consistent with the R151C and R160W changes. The T157A artificial variant was retained in the ER, whereas the R162P mutant displayed a proximal Golgi accumulation similar to R160W. These observations, together with the facts that T157 lies in a 157TLPR160 PKC target and is phosphorylated in vivo, suggest a model for the regulation of MC1R export traffic. In this model, T157 phosphorylation would be a key regulatory event. By disrupting the 157TLPR160 kinase target consensus, the R160W mutation would prevent T157 phosphorylation, thus accounting for the intracellular retention of the mutant. Conceivably, the R162P mutation might have a similar effect. Consistent with this hypothesis a T157D mutation mimicking the phosphorylated state of T157 was compatible with receptor export and function and restored forward traffic of R160W and R162P, as shown by high plasma membrane expression of the T157D/R160W and T157D/R162P double mutants.
Within this scenario, the cause of R151C intracellular retention remains unclear, since we have not found evidence for an effect of this mutation on T157 phosphorylation. Moreover, the T157D mutation only restores partially R151C export to the cell surface. Therefore, the QC mechanisms responsible for retention of R151C must be different from those accounting for the R160W phenotype. In any case, the different location of R151C on one hand, and R160W or R162P on the other can be reasonably explained by the effects of the mutations on the basic 160RARR163 tetrapeptide matching the consensus for the arginine-based ER-localization signals. These motifs mediate ER residency either by retention or retrieval, until they are masked by mechanisms such as multimeric assembly, phosphorylation and/or specific interactions with 14−3−3 or PDZ-domain proteins (Michelsen et al., 2005; Nufer and Hauri 2003). Its possible relationship with MC1R export traffic has recently been suggested (Conn et al., 2007). The 160RARR163 tetrapeptide is highly similar to the RSRR motif responsible for ER retention of the GABAB1 in the absence of the GABAB2 subunit (Couve et al., 1998; Margeta-Mitrovic et al., 2000). Similar motifs also regulate intracellular traffic of the invariant chain lip35 of major histocompatibility complex class II (Schutze et al., 1994), and the vasopressin receptor 2 (Hermosilla et al., 2004). Conceivably, the 160RARR163 motif would function as a retrieval motif disrupted by the R160W and R162P mutations. Therefore, the R160W and R162P mutants would be unable to reach the plasma membrane due to lack of T157 phosphorylation, but would not be efficiently recycled to the ER because of the loss of the arginine-based retrieval signal. Conversely, the R151C mutant conserving an intact 160RARR163 motif might be rapidly retrieved to the ER. Accumulation of R151C in the ER would therefore result from slow forward movement to the ERGIC/Golgi coupled to rapid retrieval back to the ER, rather than inability to leave the ER, consistent with the effects of bafA.
The hypothesis that phosphorylation of T157 might be a key step in MC1R forward traffic not only accounts for the behaviour of the different mutants analyzed here, but also suggests a mechanism for the rapid modulation of plasma membrane expression of receptor molecules in response to extracellular signals. Stimuli leading to the activation of the putative kinase responsible for T157 phosphorylation would promote MC1R export to the cell surface. Interestingly, ultraviolet radiation was shown to activate a PKC isoform in epidermal cells (Huang et al., 2000), and to induce a redistribution of internal αMSH binding sites to the plasma membrane (Chakraborty et al., 1991). Nevertheless, a role for a specific PKC isoform in MC1R forward trafficking remains to be proven.
In summary, we have shown that the loss-of-function R151C and R160W MC1R alleles are export-deficient forms retained within the ER and the proximal Golgi, respectively. For R160W, intracellular retention is most likely due to impaired phosphorylation at T157, whereas accumulation in the Golgi might be caused by disruption of an arginine-based motif functioning as an ER retrieval signal. The 157TLPRARR163 sequence containing the phosphorylation target and the dibasic motif is perfectly conserved in the human, mouse, gorilla and many other vertebrate MC1Rs. Moreover, T157 is invariant in most species as well as in the five human melanocortin receptors, which also share the presence of immediately C-terminal basic residues that make this threonine a likely kinase target (Fig. 1). These homology considerations suggest that our model for MC1R forward trafficking might also apply to other receptors of the family. In any case, our results provide the first example of specific retention of a GPCR in a post-ER compartment of the secretory pathway, thus making the MC1R variants associated with skin cancer excellent models to study the physiological consequences of mutant protein accumulation in post-ER sites.
ACKNOWLEDGMENTS
This work was partially supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. BLS-L holds fellowships from the Ministry of Education (Spain). We thank Prof. G. Ghanem (Free University of Brussels, Belgium) for gift of HBL human melanoma cells, Dr C. Olivares for helpful discussions and A. B. Pérez Oliva for skilful collaboration and technical support.
Contract grant sponsor: Ministry of Science and Technology, Spain and FEDER funds, EC
Contract grant number: SAF2006−11206
Contract grant sponsor: CARM, Plan de Ciencia y Tecnología 2007−2010
Contract grant number: 464/2008
LITERATURE CITED
- Ahner A, Brodsky JL. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 2004;14(9):474–478. doi: 10.1016/j.tcb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27(2):315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14(15):2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet. 2007;16(18):2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126(9):1966–1975. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280(7):5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6(11):1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13(2):60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Orlow SJ, Bolognia JL, Pawelek JM. Structural/functional relationships between internal and external MSH receptors: modulation of expression in Cloudman melanoma cells by UVB radiation. J Cell Physiol. 1991;147(1):1–6. doi: 10.1002/jcp.1041470102. [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59(3):225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J Biol Chem. 1998;273(41):26361–26367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360(6402):350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13(4):447–461. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17(12):1457–1465. doi: 10.1016/j.cellsig.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4(3):181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Ghose-Dastidar J, Ross JB, Green R. Expression of biologically active human corticosteroid binding globulin by insect cells: acquisition of function requires glycosylation and transport. Proc Natl Acad Sci U S A. 1991;88(15):6408–6412. doi: 10.1073/pnas.88.15.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy E, Flannagan N, Ray A, Todd C, Jackson IJ, Matthews JN, Birch-Machin MA, Rees JL. Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet. 2000;355(9209):1072–1073. doi: 10.1016/S0140-6736(00)02042-0. [DOI] [PubMed] [Google Scholar]
- Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ. Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet. 2001;10(21):2397–2402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- Hermosilla R, Oueslati M, Donalies U, Schonenberger E, Krause E, Oksche A, Rosenthal W, Schulein R. Disease-causing V(2) vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5(12):993–1005. doi: 10.1111/j.1600-0854.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Huang C, Li J, Chen N, Ma W, Bowden GT, Dong Z. Inhibition of atypical PKC blocks ultraviolet-induced AP-1 activation by specifically inhibiting ERKs activation. Mol Carcinog. 2000;27(2):65–75. [PubMed] [Google Scholar]
- Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16(5):523–531. doi: 10.1034/j.1600-0749.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Germer S, Gonzalez P, Sanchez J, Sanchez CO, Garcia-Borron JC. Thr40 and Met122 are new partial loss-of-function natural mutations of the human melanocortin 1 receptor. FEBS Lett. 2001a;508(1):44–48. doi: 10.1016/s0014-5793(01)03025-3. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Olivares C, Gonzalez P, Morandini R, Ghanem G, Garcia-Borron JC. The Pro162 variant is a loss-of-function mutation of the human melanocortin 1 receptor gene. J Invest Dermatol. 2001b;117(1):156–158. doi: 10.1046/j.0022-202x.2001.01393.x. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65(10):4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Liu W. Membrane trafficking: coat control by curvature. Nature. 2003;426(6966):507–508. doi: 10.1038/426507a. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Liu W. Insights into COPI coat assembly and function in living cells. Trends Cell Biol. 2006;16(10):e1–e4. doi: 10.1016/j.tcb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441(7096):1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Lotti LV, Torrisi MR, Erra MC, Bonatti S. Morphological analysis of the transfer of VSV ts-045 G glycoprotein from the endoplasmic reticulum to the intermediate compartment in vero cells. Exp Cell Res. 1996;227(2):323–331. doi: 10.1006/excr.1996.0281. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27(1):97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Mas JS, Gerritsen I, Hahmann C, Jimenez-Cervantes C, Garcia-Borron JC. Rate limiting factors in melanocortin 1 receptor signalling through the cAMP pathway. Pigment Cell Res. 2003;16(5):540–547. doi: 10.1034/j.1600-0749.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441(7096):1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- Michelsen K, Yuan H, Schwappach B. Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 2005;6(8):717–722. doi: 10.1038/sj.embor.7400480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis--Golgi tethering. Traffic. 2001;2(4):268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- Newton Bishop JA, Bishop DT. The genetics of susceptibility to cutaneous melanoma. Drugs Today (Barc ) 2005;41(3):193–203. doi: 10.1358/dot.2005.41.3.892524. [DOI] [PubMed] [Google Scholar]
- Newton RA, Roberts DW, Leonard JH, Sturm RA. Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides. 2007;28(12):2387–2396. doi: 10.1016/j.peptides.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA. Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells. Peptides. 2005;26(10):1818–1824. doi: 10.1016/j.peptides.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Nufer O, Hauri HP. ER export: call 14−3−3. Curr Biol. 2003;13(10):R391–R393. doi: 10.1016/s0960-9822(03)00318-x. [DOI] [PubMed] [Google Scholar]
- O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14−3−3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111(4):577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- Oueslati M, Hermosilla R, Schonenberger E, Oorschot V, Beyermann M, Wiesner B, Schmidt A, Klumperman J, Rosenthal W, Schulein R. Rescue of a nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutant by cell-penetrating peptides. J Biol Chem. 2007;282(28):20676–20685. doi: 10.1074/jbc.M611530200. [DOI] [PubMed] [Google Scholar]
- Ouwendijk J, Moolenaar CE, Peters WJ, Hollenberg CP, Ginsel LA, Fransen JA, Naim HY. Congenital sucrase-isomaltase deficiency. Identification of a glutamine to proline substitution that leads to a transport block of sucrase-isomaltase in a pre-Golgi compartment. J Clin Invest. 1996;97(3):633–641. doi: 10.1172/JCI118459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Oliva AB, Fernández LP, DeTorre C, Herraiz C, Martínez-Escribano JA, Benítez J, Lozano Teruel JA, García-Borrón JC, Jiménez-Cervantes C, Gloria Ribas R. Identification and functional analysis of novel variants of the human melanocortin receptor found in melanoma patients. Human Mutation. 2008 doi: 10.1002/humu.20971. in press. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124(5):897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Rees JL. The genetics of sun sensitivity in humans. Am J Hum Genet. 2004;75(5):739–751. doi: 10.1086/425285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholm A, Klovins J, Rudzish R, Phillips S, Rees JL, Schioth HB. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J Invest Dermatol. 2004;123(5):917–923. doi: 10.1111/j.0022-202X.2004.23444.x. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Newton RA, Beaumont KA, Helen LJ, Sturm RA. Quantitative analysis of MC1R gene expression in human skin cell cultures. Pigment Cell Res. 2006;19(1):76–89. doi: 10.1111/j.1600-0749.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- Rottger S, White J, Wandall HH, Olivo JC, Stark A, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T. J Cell Sci. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. 1998;111(Pt 1):45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14(1):20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sanchez MJ, Olivares SC, Ghanem G, Haycock J, Lozano Teruel JA, Garcia-Borron JC, Jimenez-Cervantes C. Loss-of-function variants of the human melanocortin-1 receptor gene in melanoma cells define structural determinants of receptor function. Eur J Biochem. 2002;269(24):6133–6141. doi: 10.1046/j.1432-1033.2002.03329.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Jimenez-Cervantes C, Garcia-Borron JC. Regulation of human melanocortin 1 receptor signaling and trafficking by Thr-308 and Ser-316 and its alteration in variant alleles associated with red hair and skin cancer. J Biol Chem. 2007;282(5):3241–3251. doi: 10.1074/jbc.M606865200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, Martinez-Menarguez JA, Garcia-Borron JC, Jimenez-Cervantes C. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol. 2006a;126(1):172–181. doi: 10.1038/sj.jid.5700036. [DOI] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Sanchez-Mas J, Turpin MC, Garcia-Borron JC, Jimenez-Cervantes C. Variant amino acids in different domains of the human melanocortin 1 receptor impair cell surface expression. Cell Mol Biol (Noisy-le-grand) 2006b;52(2):39–46. [PubMed] [Google Scholar]
- Sanchez-Mas J, Guillo LA, Zanna P, Jimenez-Cervantes C, Garcia-Borron JC. Role of G protein-coupled receptor kinases in the homologous desensitization of the human and mouse melanocortin 1 receptors. Mol Endocrinol. 2005;19(4):1035–1048. doi: 10.1210/me.2004-0227. [DOI] [PubMed] [Google Scholar]
- Schutze MP, Peterson PA, Jackson MR. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994;13(7):1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist W, Stutz S, Eberle AN. Homologous and heterologous regulation of alpha-melanocyte-stimulating hormone receptors in human and mouse melanoma cell lines. Cancer Res. 1994;54(10):2604–2610. [PubMed] [Google Scholar]
- Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, Jacques JP, Rogers S, Turner R, Jackson IJ, Birch-Machin MA, Rees JL. Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol. 1998;111(1):119–122. doi: 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- Solca F, Siegrist W, Drozdz R, Girard J, Eberle AN. The receptor for alpha-melanotropin of mouse and human melanoma cells. Application of a potent alpha-melanotropin photoaffinity label. J Biol Chem. 1989;264(24):14277–14281. [PubMed] [Google Scholar]
- Sturm RA. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 2002;12(5):405–416. doi: 10.1097/00008390-200209000-00001. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, Chen W, Smit DJ, Brown DL, Stow JL, Leonard JH, Martin NG. The role of melanocortin-1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Res. 2003a;16(3):266–272. doi: 10.1034/j.1600-0749.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, Newton RA, Shepherd AG, Chen W, Marks LH, Leonard JH, Martin NG. Genetic association and cellular function of MC1R variant alleles in human pigmentation. Ann N Y Acad Sci. 2003b;994:348–358. doi: 10.1111/j.1749-6632.2003.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]