Abstract
Mast cells (MC) are stem cell factor-dependent tissue-based hematopoietic cells with substantial functional heterogeneity. Cord blood-derived human MC (hMC) express functional receptors for IL-5, and IL-5 mediates stem cell factor-dependent comitogenesis of hMC in vitro. Although IL-5 is not required for normal hMC development, we considered that it might prime hMC for their high-affinity Fc receptor for IgE (FcɛRI)-dependent generation of cytokines, as previously demonstrated for IL-4. Compared with hMC maintained in stem cell factor alone, hMC primed with IL-5 expressed 2- to 4-fold higher steady-state levels of TNF-α, IL-5, IL-13, macrophage inflammatory protein 1α, and granulocyte-macrophage colony-stimulating factor transcripts 2 h after FcɛRI crosslinking and secreted 2- to 5-fold greater quantities of the corresponding cytokines, except IL-13, at 6 h. Unlike IL-4, IL-5 priming did not enhance FcɛRI-dependent histamine release. Thus, IL-5 augments cytokine production by hMC by a mechanism distinct from that of IL-4 and with a different resultant profile of cytokine production. These observations suggest a potentially autocrine effect of IL-5 on hMC for amplification of allergic immune responses, in addition to its recognized paracrine effects on eosinophils, and implicate both IL-4 and IL-5 in the modulation of the hMC phenotype.
Mast cells (MC) are unique hematopoietic cells that are richly distributed in the skin and the mucosal surfaces of the respiratory and gastrointestinal tracts (1). When stimulated in vitro via their high-affinity Fc receptor for IgE (FcɛRI), they release preformed granule-associated inflammatory mediators such as histamine (2) and protease-proteoglycan complexes (3), generate a panel of arachidonic acid-derived lipid mediators de novo (4), and secrete both induced immunomodulatory and proinflammatory cytokines (5–8). These cytokines include TNF-α (5), IL-13 (6, 7), IL-5 (8), and chemokines such as macrophage inflammatory protein (MIP) 1α (9). The generation of cytokines is likely an important feature of MC behavior in allergic and asthmatic inflammation.
IL-4 and IL-5 are prominently expressed by T cells and other cell types in foci of allergic tissue inflammation. IL-4 has pleotrophic actions that include the induction of FcɛRI expression by human MC (hMC) (10), which is associated with a markedly augmented capacity for hMC to generate IL-5, IL-13, and other cytokines de novo after FcɛRI crosslinkage (6, 11). IL-5 is the major mediator of the expansion of the eosinophil population in allergen-challenged mice (12). Its hematopoietic activity in humans was thought to be selective for eosinophils (13) and basophils (14), which share a common developmental lineage (15, 16). However, we found recently that hMC derived in vitro from umbilical cord blood mononuclear cells express cytofluorographically detectable IL-5 receptors (17) and that IL-5 is comitogenic with stem cell factor (SCF) for hMC. Because there is no known requirement for IL-5 in hMC development, we considered that IL-5 might have functional effects on hMC directed to their FcɛRI-dependent generation of cytokines, as was previously demonstrated for IL-4 (6, 10, 11). In this study, we show that when hMC maintained in SCF were primed with IL-5, they increased their steady-state expression of mRNA encoding TNF-α, IL-5, IL-13, MIP-1α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) at 2 h after passive sensitization and challenge with anti-IgE and the corresponding secreted proteins at 6 h, with the exception of IL-13. In contrast to the priming effects of IL-4, those of IL-5 were independent of increased FcɛRI expression or changes in the exocytosis of histamine. Thus, IL-5 is an amplification factor for the IgE-dependent generation by hMC of cytokines that orchestrate allergic inflammation and may even permit amplification of this process through both autocrine and paracrine mechanisms.
Materials and Methods
Cytokines and Antibodies.
Recombinant human SCF was kindly provided by Amgen Biologicals. IL-6, IL-3, IL-4, IL-5, and IL-10 were purchased from Endogen (Cambridge, MA). Mouse anti-human monoclonal antibodies against the following epitopes were obtained: c-kit [SR-1, IgG2a, V. Broudy, University of Washington, Seattle, WA (18)]; FcɛRIα [22E7, IgG1, R. Chizzonite, Hoffman–LaRoche (19)]; CD13 (WM15, IgG1, PharMingen), β3 integrin (VIP-L2, IgG1, PharMingen), and tryptase (IgG1, Chemicon). Additional antibodies included an irrelevant mouse IgG2a (PharMingen); human myeloma IgE (Chemicon); an affinity-purified rabbit IgG raised against recombinant human chymase [N. Schechter, University of Pennsylvania, Philadelphia (20)]; mouse IgG1 hybridoma culture supernatant (P3, M. Hemler, Harvard Medical School, Boston); rabbit serum (Sigma); and rabbit anti-human IgE (ICN).
Culture and Characterization of hMC.
hMC were derived from cord blood mononuclear cells cultured in the presence of SCF, IL-6, and IL-10, as described (17). The mononuclear cell fraction from umbilical cord blood was cultured in RPMI-1640 medium (GIBCO/BRL) with 10% FBS (Sigma), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 0.2 μM 2-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 μg/ml gentamycin, 100 ng/ml SCF, 50 ng/ml IL-6, and 10 ng/ml IL-10. The entire volume of cytokine-supplemented medium was replaced and the nonadherent cells were transferred to fresh culture flasks on a weekly basis. Portions of 2 × 104 cultured cells were spun onto glass slides in a cytocentrifuge (Cytospin 2, Shandon, Pittsburgh) and stained with toluidine blue. The cells were harvested for study when more than 95% stained positively with toluidine blue (6–9 wk of culture).
Flow cytometry was performed as described (17). After exposure to monoclonal antibodies, the cells were stained with fluorescein isothiocyanate-conjugated sheep anti-mouse IgG (Calbiochem) and then analyzed with FACSort (Becton Dickinson). The results were analyzed as overlaid histograms and in the case of FcɛRI are expressed as the percent change in the ratio of mean fluorescence intensity elicited by 22E7 divided by the isotype-matched control.
For immunocytochemistry, cytocentrifugation slides were prepared as described above, air dried, and fixed in Carnoy's fluid (60% ethanol/30% chloroform/10% glacial acetic acid) for 10 min at room temperature. After being washed with PBS three to four times, the slides were blocked with 2% chicken egg albumin (Sigma) for 30 min at room temperature and were incubated with appropriate dilutions of antitryptase or antichymase antibodies or with the corresponding isotype-matched negative controls. After application of the appropriate secondary antibodies, alkaline phosphatase was used as a chromogenic reporter (17).
Cell Stimulation and Functional Assays.
hMC were extensively washed and resuspended at a concentration of 1.0 × 106 hMC/ml in fresh medium containing SCF (100 ng/ml) alone or with recombinant IL-5 (5 ng/ml) or recombinant IL-4 (10 ng/ml) for 5 days. Human myeloma IgE (2 μg/ml) was added during the final 16 h of cytokine priming. After extensive washing, the hMC were resuspended at a concentration of 5 × 105 hMC/ml in fresh medium containing SCF (100 ng/ml) and were activated in triplicate 200-μl portions by adding anti-IgE (1 μg/ml). The supernatants were collected at 6 h and frozen at −70°C until analyses were performed. The quantities of the cytokines were measured with ELISA (Endogen, Cambridge, MA) according to the manufacturer's protocol.
For histamine release, supernatants from triplicate cell samples were separated from the corresponding pellets by centrifugation of the samples 30 min after activation. The pellets were disrupted by three rounds of freeze thawing followed by sonication. The histamine content of the acylated samples was quantitated with a commercial ELISA (ICN) according to the manufacturer's protocol. The percentage of histamine released [histamine in supernatant/(histamine in supernatant + histamine in pellet) × 100] was expressed as the mean ± SEM for each individual experiment.
For cytokine mRNA studies, the hMC were stimulated in single portions of 5–10 × 106 cells. Total RNA was extracted 2 h after cell activation (a time frame established for maximal induction in preliminary experiments) with TRI Reagent (Molecular Research Center, Cincinnati) according to the manufacturer's protocol. Then 10–15 μg of total RNA were loaded into the wells of 1.3% agarose gels in 1 × MESA buffer (Sigma), with 20% formaldehyde in an equal volume of a sample buffer consisting of 50% standard sodium phosphate with ethylenediaminetetraacetic acid (SSPE), 25% formamide, 15% formaldehyde, 9% blue juice (GIBCO/BRL), and 1% ethidium bromide, and resolved by electrophoresis at 80 mV. The RNA was transferred to nylon membranes overnight by capillary action and processed as described (21) for hybridization under high-stringency conditions with PCR-generated gene-specific DNA fragments encoding TNF-α, MIP-1α, IL-5, IL-13, and GM-CSF, each confirmed by automated sequencing. The membranes were probed with an 18S ribosomal RNA probe (CLONTECH) as a control for RNA loading and intactness. Laser densitometry was performed on each blot, and the relative densities corresponding to each band were calculated as a percentage of the signal for the 18S probe.
Statistical Analysis.
The quantities of the cytokines generated and the percentages of histamine released by primed and unprimed hMC are presented as means ± SEM of at least three experiments each and are compared with the one-tailed Student's t test for matched pairs. Because it was not possible to prime cells with both IL-4 and IL-5 in every experiment, the priming effects of IL-5 and IL-4 were compared with one another by expressing the amounts of each cytokine generated under the priming conditions as a percentage of control (SCF alone). The priming effects were then compared with the one-tailed Student's t test for samples with unequal variances.
Results and Discussion
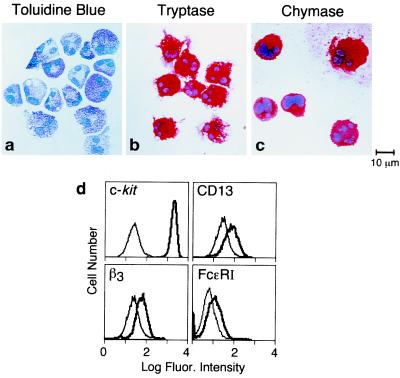
IL-5 is strongly expressed by T cells, along with some eosinophils and mast cells, in bronchial biopsy specimens from patients with asthma (22), and it plays a pivotal role in regulating eosinophilia (12). Although IL-5 in humans was known to have effects only on eosinophils and basophils, we anticipated that IL-5 might prime hMC for FcɛRI-mediated induction of cytokine expression and secretion, a potentially important effector function of hMC, in association with its recently identified comitogenic effects (17, 23). The hMC developed in vitro in the presence of SCF, IL-6, and IL-10 were ≥95% toluidine blue positive (shown for one experiment in Fig. 1a). Most of the cells possessed multiple nuclear lobes typical of hMC grown from cord blood in vitro, an effect attributed to IL-6 (24). The hMC stained strongly positive for tryptase (Fig. 1b) and chymase (Fig. 1c), showed high cytofluorographic expression for c-kit (Fig. 1d), and expressed CD13 in a monophasic distribution (17, 23) (Fig. 1d). Basophils, which like hMC are metachromatic with toluidine blue, possess functional IL-5 receptors (25), and generate cytokines (especially IL-13 and IL-4) on FcɛRI crosslinking (26, 27), were excluded by the high-level expression of c-kit and the uniform β3 integrin expression of the cultured cells (28) (Fig. 1d). Moreover, basophils do not express chymase, and only a few basophils express tryptase (29, 30). Each of these immunocytochemical markers remained positive in our cultures after priming by IL-5 (n = 2, data not shown). We are thus confident that basophil differentiation was not elicited by 5 days of IL-5 priming and that the induction of cytokines reflects effects on hMC and not basophils.
Figure 1.
Morphologic, immunocytochemical, and cytofluorographic characteristics of cultured hMC. hMC harvested at 6–9 wk of culture were ≥95% metachromatic with toluidine blue (a) and were positive for tryptase (b) and chymase (c). Cytofluorographic assays (d) demonstrated strong c-kit expression, positive expression of CD13 and β3 integrin, and low expression of FcɛRI. Light tracings represent staining with negative controls; bold tracings represent staining for the designated markers.
SCF, acting through c-kit, is essential for all MC development in mice (31, 32) and for hMC development in vitro from various progenitor sources (23, 24, 33, 34). IL-4 priming enhances cytokine generation, along with FcɛRI expression and IgE-dependent exocytosis, by SCF-dependent hMC derived in vitro from cord blood (6) or purified from dispersed human intestinal tissue (11, 35). The IL-4-induced increases in FcɛRI expression involve de novo synthesis of the FcɛRIα subunit and are amplified by simultaneous incubation with IgE, reflecting stabilization of the receptors by IgE at the cell membrane (36). As expected, compared with hMC maintained in SCF alone, hMC primed with IL-4 exhibited enhanced FcɛRIα expression (55 ± 26% increase over hMC maintained in SCF alone, P = 0.002, n = 3) and IgE-mediated histamine release (53 ± 4% vs. 26 ± 2% release, n = 3, P = 0.01). In contrast, IL-5 priming of hMC did not change membrane expression of FcɛRIα (0.6 ± 3.0% increase, P = 0.30) and did not enhance exocytosis of histamine by hMC after passive sensitization and challenge (31 ± 4% release, P = 0.10). Thus, the inductive effects of IL-5 on FcɛRI- mediated production of cytokines by hMC reflect events independent of the magnitude of FcɛRI expression or histamine exocytosis.
We examined a panel of cytokines (TNF-α, IL-5, IL-13, MIP-1α, GM-CSF, IL-4, and eotaxin) that are present in bronchoalveolar lavage fluids from humans with asthma (37, 38), are localized to hMC in bronchial biopsy specimens from persons with asthma (39, 40), or are released by hMC (7–9) or mouse MC (41) stimulated ex vivo. Each cytokine mediates one or more of the central features of allergic inflammation in animal models or humans in vivo, including endothelial and epithelial cell activation (TNF-α, IL-4, IL-13) (42, 43), tissue eosinophilia (eotaxin, IL-5, GM-CSF) (37, 44), lymphocyte recruitment (MIP-1α) (37), mucus secretion (IL-4, IL-13) (42, 43), dendritic cell recruitment and maturation (MIP-1α, GM-CSF) (45, 46), and bronchial hyperreactivity (IL-13, IL-5) (42, 43, 47). SCF was included in all priming experiments to maintain optimal hMC viability. Preliminary studies established that maximal concentrations of TNF-α, MIP-1α, IL-5, IL-13, and GM-CSF were detected 6 h after activation and that IL-5 was maximally active at a concentration of 5 ng/ml. Unlike investigators working with hMC purified from dispersed lung tissue (48), we did not identify IL-4 production by hMC. This observation is consistent with that of other groups who did not recognize IL-4 as a secreted product of intestinal hMC stimulated ex vivo (11) and suggests that, unlike basophils, hMC are not obligate IL-4 producers after IgE-dependent activation. Our further studies, therefore, did not include assays for induced IL-4 or eotaxin secretion and focused on the priming effect of IL-5 (5 ng/ml), compared with a maximally effective dose of IL-4 (10 ng/ml), for the generation of TNF-α, MIP-1α, IL-5, IL-13, and GM-CSF.
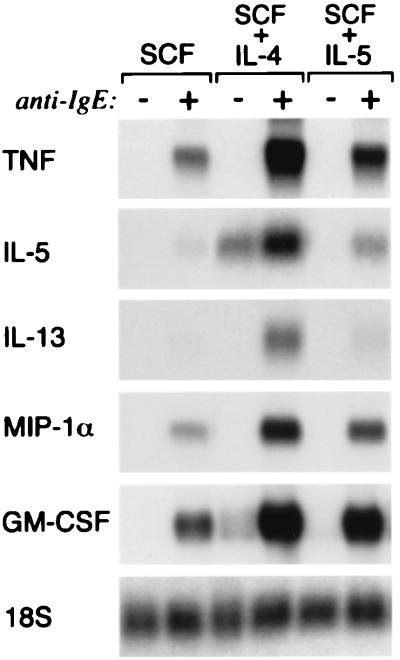
None of the cytokine transcripts tested were detected in hMC maintained in SCF alone before activation. Stimulation with anti-IgE after passive sensitization induced steady-state expression of TNF-α, GM-CSF, and MIP-1α transcripts at 2 h by the hMC maintained without priming (73 ± 17%, 170 ± 5%, and 101 ± 20% of 18S RNA signal, n = 3). The transcripts for IL-5 were also weakly induced (9 ± 3%), whereas IL-13 transcripts remained below limits of detection (Fig. 2). Priming of the hMC by IL-4 (but not by IL-5) in the continued presence of SCF induced the steady-state expression of the mRNAs encoding IL-5 and GM-CSF (to 79 and 45% of the 18S RNA signal, respectively, n = 1; Fig. 2), even without activation. The steady-state expression of both IL-5 and GM-CSF mRNA induced by IL-4 priming alone was associated with IL-5 secretion (16 ± 2 pg/106 hMC, mean ± SEM for triplicate samples, n = 1) but not with GM-CSF secretion, as measured just before hMC activation with anti-IgE. Non-FcɛRI-dependent secretion of IL-5 has been reported previously for intestinal hMC primed in vitro with IL-4 (35). IL-4-mediated induction of steady-state IL-5 and GM-CSF mRNA expression, independent of FcɛRI-mediated activation, may relate to the immunolocalization of cytokines to hMC in bronchial biopsies even from individuals with nonallergic asthma (40) and may be reflected by regional differences in IL-5 immunoreactivity among hMC subpopulations in vivo (49). The steady-state expression of TNF-α (380 ± 172% of 18S RNA signal), IL-5 (142 ± 44%), IL-13 (234 ± 105%), MIP-1α (278 ± 111%), and GM-CSF (425 ± 182%) was strong after anti-IgE challenge of the IL-4-primed hMC (n = 3; shown for one experiment, Fig. 2).
Figure 2.
Effects of IL-4 and IL-5 priming of hMC in the presence of SCF on steady-state levels of cytokine mRNA expression with and without anti-IgE activation. Total RNA samples from sensitized hMC maintained in SCF alone or primed with IL-4 or IL-5 were compared without (−) or with (+) cell activation with anti-IgE for 2 h. Results are from a single experiment that is representative of three independent experiments, with quantitative data provided in the text.
IL-5 priming of hMC followed by passive sensitization and challenge with anti-IgE induced de novo steady-state expression of mRNAs for IL-5 (32 ± 12%) and IL-13 (31 ± 9%) and substantially augmented the steady-state expression of TNF-α (151 ± 36%), MIP-1α (191 ± 46%), and GM-CSF (312 ± 105%) mRNAs compared with their expression by nonprimed, FcɛRI-activated hMC (n = 3; shown for one experiment in Fig. 2). The mRNA signals for every cytokine reverted to undetectable levels by 24 h (n = 1, data not shown). Thus, priming of hMC with either IL-4 or IL-5 augments the IgE-dependent induction of steady-state mRNA expression of each cytokine tested. Although IL-4 and IL-5 are nearly equal in their priming effect for the activation-dependent expression of MIP-1α and GM-CSF mRNA, IL-4 is dominant for induction of IL-5 and IL-13 and, to a lesser extent, TNF-α. It is likely that IL-4 and IL-5 act by both distinct and overlapping mechanisms to control cytokine gene expression by hMC. Although the receptors for IL-4 and IL-5 couple to different respective STAT proteins, both activate the same nuclear factor of activated T cell transcription factors in human peripheral blood eosinophils (50). The fact that both GM-CSF and MIP-1α are regulated by transcript stability-dependent mechanisms (51, 52) suggests that IL-5 may act by altering available RNA-stabilizing and RNA-destabilizing factors. In contrast, IL-13 and IL-5 are controlled by transcription-dependent mechanisms, and their transcription in T cells depends on long-term changes in chromatin structure that are induced by IL-4 during Th2 differentiation (53).
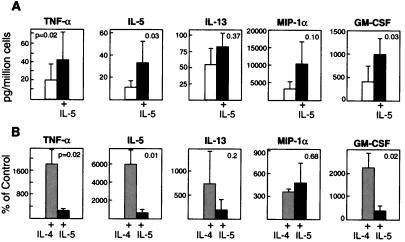
In experiments with the cells from seven donors, hMC maintained in SCF generated small variable quantities of TNF-α, IL-5, and IL-13, and more substantial quantities of GM-CSF (range 54–1282 ng/106 hMC) and MIP-1α (range 499–11,446 ng/106 hMC) after challenge; such hMC had low levels of FcɛRI expression (Fig. 1) and modest histamine release. Priming the hMC with IL-5 increased their activation-dependent generation of TNF-α (20 ± 13 vs. 43 ± 26 pg/106 hMC, P = 0.02), IL-5 (10.3 ± 6.6 vs. 32.9 ± 19.4 pg/106 hMC, P = 0.03), GM-CSF (400 ± 296 vs. 988 ± 606 pg/106 hMC, P = 0.03), and MIP-1α (3161 ± 2075 vs. 10,197 ± 6991 pg/106 hMC, P = 0.10) 6 h after activation with anti-IgE in every experiment performed (n = 5 for each cytokine; Fig. 3). In contrast, IL-5 priming was relatively ineffective for activation-induced IL-13 generation (56 ± 27 vs. 84 ± 23 pg/106 hMC, P = 0.37), with no effect on IL-13 production in two of the three experiments in which it was measured. IL-4 priming strongly induced or up-regulated the activation-dependent generation of TNF-α (24 ± 16 vs. 276 ± 70 pg/106 hMC, P = 0.04), IL-5 (3.7 ± 1.65 vs. 81.7 ± 41.9 pg/106 hMC, P = 0.02), GM-CSF (304 ± 186 vs. 3,918 ± 1,230 pg/106 hMC, P = 0.04), and MIP-1α (3,375 ± 2,472 vs. 14,114 ± 12,976 pg/106 hMC, P = 0.12) (n = 5 for each), and unlike IL-5 also increased the production of IL-13 (42 ± 21 vs. 240 ± 40 pg/106 hMC P = 0.04, n = 4). When cytokine production was expressed as a percentage of that obtained from hMC maintained in SCF alone, the IL-4 priming effect was significantly greater than the IL-5 priming effect for TNF-α, IL-5, and GM-CSF production and approached significance for IL-13 (P = 0.2). However, the two cytokines were equivalent in priming for MIP-1α generation (P = 0.68; Fig. 3); each resulted in large quantities of MIP-1α (>10 ng/106 hMC), which is an important chemoattractant for CCR5-bearing leukocytes such as lymphocytes, monocytes, and dendritic cells.
Figure 3.
Effects of IL-5 priming on the generation of TNF-α, IL-5, IL-13, MIP-1α, and GM-CSF 6 h after anti-IgE activation of sensitized hMC. (A) Results are expressed as pg/106 hMC and are the mean ± SEM of five experiments (except for IL-13, where n = 3). Note differences in scale. (B) Comparison of IL-4 and IL-5 priming effects for each cytokine. The results are expressed as a percentage of the amounts of each cytokine generated by hMC maintained in SCF alone (control) and are the mean ± SEM of five experiments, except for IL-13 (n = 3 for IL-5 priming; n = 4 for IL-4 priming).
Our study makes two observations. First, IL-5, like IL-4, primes hMC for IgE-dependent cytokine generation but with a somewhat different profile that favors GM-CSF and MIP-1α and does not involve IL-13. Second, in contrast to IL-4, the priming effect of IL-5 for cytokine generation is not associated with substantially up-regulated FcɛRI expression or any augmented IgE-dependent exocytosis of histamine. Furthermore, as with IL-4 for T cells, IL-5 production by hMC may serve both autocrine functions (comitogenic and priming effects) and paracrine functions (modulation of local eosinophilia). IL-5 is expressed reportedly even in circumstances where IL-4 is not abundant, as has been reported for severe glucocorticoid-dependent asthma (54). The quantities of IL-5 and GM-CSF produced by activated IL-5-primed hMC (40 pg and nearly 1 ng/106 hMC, respectively) are well within the bioactive range for supporting the survival of eosinophils (44, 55) and for augmenting their functional capacity for cytotoxicity (56). Additionally, because immature dendritic cells express the MIP-1α receptor (CCR5) (45) and mature in response to GM-CSF (46), the large quantities of these two mediators generated by IL-5-primed hMC suggest a mechanism by which dendritic cells might be recruited to the asthmatic bronchial mucosa for their presentation of antigen and activation of lymphocytes. Our study expands the functions of IL-5 to include the modification of the hMC cytokine-producing phenotype, thereby providing a potential autocrine effect on hMC and a paracrine link to eosinophils, which are observed together prominently in allergic tissue inflammation.
Acknowledgments
This work was supported by National Institutes of Health grants AI-01305, AI-31599, AI-22531, and HL-36110, and by a grant from the Hyde and Watson Foundation. F.H.H. is the recipient of grants from Glaxo–Wellcome Pharmaceuticals.
Abbreviations
- MC
mast cells
- FcɛRI
high-affinity receptor for IgE
- hMC
human mast cells
- SCF
stem cell factor
- MIP-1α
macrophage inflammatory protein 1α
- GM-CSF
granulocyte/macrophage colony-stimulating factor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180318697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180318697
References
- 1.McNeil H P, Austen K F. In: Samter's Immunologic Diseases. 5th Ed. Frank M M, Austen K F, Claman H M, Unanue E R, editors. Baltimore: Williams & Wilkins; 1995. pp. pp.185–198. [Google Scholar]
- 2.Paterson N A M, Wasserman S I, Said J W, Austen K F. J Immunol. 1976;117:1356–1362. [PubMed] [Google Scholar]
- 3.Goldstein S M, Leong J, Schwartz L B, Cooke D. J Immunol. 1992;148:2475–2482. [PubMed] [Google Scholar]
- 4.Peters S P, MacGlashan D W, Jr, Schulman E S, Schleimer R P, Hayes E C, Rokach J, Adkinson N F, Jr, Lichtenstein L M. J Immunol. 1984;132:1972–1979. [PubMed] [Google Scholar]
- 5.Walsh L J, Trinchieri G, Waldorf H A, Whitaker D, Murphy G F. Proc Natl Acad Sci USA. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toru H, Pawankar R, Ra C, Yata J, Nakahata T. J Allergy Clin Immunol. 1998;102:491–502. doi: 10.1016/s0091-6749(98)70140-x. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Okayama Y, Ishizuka T, Pawankar R, Ra C, Mori M. Clin Exp Allergy. 1998;28:1219–1227. doi: 10.1046/j.1365-2222.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 8.Okayama Y, Petit-Frere C, Kassel O, Semper A, Quint D, Tunon-de-Lara M J, Bradding P, Holgate S T, Church M K. J Immunol. 1995;155:1796–1808. [PubMed] [Google Scholar]
- 9.Yano K, Yamaguchi M, de Mora F, Lantz C S, Butterfield J H, Costa J J, Galli S J. Lab Invest. 1997;77:185–193. [PubMed] [Google Scholar]
- 10.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Int Immunol. 1996;8:1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 11.Lorentz A, Schwengberg S, Sellge G, Manns M P, Bischoff S C. J Immunol. 2000;164:43–48. doi: 10.4049/jimmunol.164.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Mould A W, Matthaei K I, Young I G, Foster P S. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clutterbuck E J, Hirst E M, Sanderson C J. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 14.Denburg J A, Silver J E, Abrams J S. Blood. 1991;77:1462–1468. [PubMed] [Google Scholar]
- 15.Denburg J A, Telizyn S, Messner H, Jamal B L N, Ackerman S J, Gleich G J, Bienenstock J. Blood. 1985;66:312–318. [PubMed] [Google Scholar]
- 16.Boyce J A, Friend D, Matsumoto R, Austen K F, Owen W F. J Exp Med. 1995;182:49–57. doi: 10.1084/jem.182.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochi H, Hirani W M, Yuan Q, Friend D S, Austen K F, Boyce J A. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briddell R A, Broudy V C, Bruno E, Brandt J E, Srour E F, Hoffman R. Blood. 1992;79:3159–3167. [PubMed] [Google Scholar]
- 19.Riske F, Hakimi J, Mallamaci M, Griffin M, Pilson B, Tobkes N, Lin P, Danho W, Kochan J, Chizzonite R. J Biol Chem. 1991;266:11245–11251. [PubMed] [Google Scholar]
- 20.Wang Z M, Rubin H, Schechter N M. Biol Chem Hoppe-Seyler. 1995;376:681–684. doi: 10.1515/bchm3.1995.376.11.681. [DOI] [PubMed] [Google Scholar]
- 21.Boyce J A, Lam B K, Penrose J F, Friend D S, Parsons S, Owen W F, Austen K F. Blood. 1996;88:4338–4347. [PubMed] [Google Scholar]
- 22.Ying S, Humbert M, Barkans J, Corrigan C J, Pfister R, Menz G, Larche M, Robinson D S, Durham S R, Kay A B. J Immunol. 1997;158:3539–3544. [PubMed] [Google Scholar]
- 23.Kirshenbaum A S, Goff J P, Semere T, Foster B, Scott L M, Metcalfe D D. Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 24.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, Iikura Y, Awaji T, Tsujimoto G, Yanagida M, et al. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 25.Sarmiento E U, Espiritu B R, Gleich G J, Thomas L L. J Immunol. 1995;155:2211–2221. [PubMed] [Google Scholar]
- 26.Ochensberger B, Daepp G C, Rihs S, Dahinden C A. Blood. 1996;88:3028–3037. [PubMed] [Google Scholar]
- 27.Schroeder J T, MacGlashan D W, Jr, MacDonald S M, Kagey-Sobotka A, Lichtenstein L M. J Immunol. 1997;158:5448–5454. [PubMed] [Google Scholar]
- 28.Sperr W R, Agis H, Czerwenka K, Klepetko W, Kubista E, Boltz-Nitulescu G, Lechner K, Valent P. Ann Hematol. 1992;65:10–16. doi: 10.1007/BF01715119. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Li Y, Reddel S W, Cherrian M, Friend D S, Stevens R L, Krilis S A. J Immunol. 1998;161:5079–5086. [PubMed] [Google Scholar]
- 30.Castells M C, Irani A M, Schwartz L B. J Immunol. 1987;138:2184–2189. [PubMed] [Google Scholar]
- 31.Kitamura Y, Go S, Hatanaka K. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 32.Kitamura Y, Go S. Blood. 1979;53:492–497. [PubMed] [Google Scholar]
- 33.Rottem M, Okada T, Goff J P, Metcalfe D D. Blood. 1994;84:2489–2496. [PubMed] [Google Scholar]
- 34.Irani A A, Nillson G, Miettinen U, Craig S S, Ashman L K, Ishizaka T, Zsebo K M, Schwartz L B. Blood. 1992;80:3009–3021. [PubMed] [Google Scholar]
- 35.Bischoff S C, Sellge G, Lorentz A, Sebald W, Raab R, Manns M P. Proc Natl Acad Sci USA. 1999;96:8080–8085. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi M, Sayama K, Yano K, Lantz C S, Noben-Trauth N, Ra C, Costa J J, Galli S J. J Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- 37.Ohnishi T, Kita H, Weiler D, Sur S, Sedgwick J B, Calhoun W J, Busse W W, Abrams J S, Gleich G J. Am Rev Respir Dis. 1993;147:901–907. doi: 10.1164/ajrccm/147.4.901. [DOI] [PubMed] [Google Scholar]
- 38.Holgate S T, Bodey K S, Janezic A, Frew A J, Kaplan A P, Teran L M. Am J Respir Crit Care Med. 1997;156:1377–1383. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 39.Bradding P, Roberts J A, Britten K M, Montefort S, Djukanovic R, Mueller R, Heusser C H, Howarth P H, Holgate S T. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 40.Sousa A R, Lams B E, Pfister R, Christie P E, Schmitz M, Lee T H. Am J Respir Crit Care Med. 1997;156:1384–1389. doi: 10.1164/ajrccm.156.5.9702072. [DOI] [PubMed] [Google Scholar]
- 41.Hogaboam C, Kunkel S L, Strieter R M, Taub D D, Lincoln P, Standiford T J, Lukacs N W. J Immunol. 1998;160:6166–6171. [PubMed] [Google Scholar]
- 42.Grunig G, Warnock M, Wakil A E, Venkayya R, Brombacher F, Rennick D M, Sheppard D, Mohrs M, Donaldson D D, Locksley R M, et al. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben T Y, Karp C L, Donaldson D D. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 44.Simon H U, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. J Immunol. 1997;158:3902–3908. [PubMed] [Google Scholar]
- 45.Cochand L, Isler P, Songeon F, Nicod L P. Am J Respir Cell Mol Biol. 1999;21:547–554. doi: 10.1165/ajrcmb.21.5.3785. [DOI] [PubMed] [Google Scholar]
- 46.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J J, McGarry M P, Farmer S C, Denzler K L, Larson K A, Carrigan P E, Brenneise I E, Horton M A, Haczku A, Gelfand E W, et al. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradding P, Feather I H, Howarth P H, Mueller R, Roberts J A, Britten K, Bews J P, Hunt T C, Okayama Y, Heusser C H, et al. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradding P, Okayama Y, Howarth P, Church M, Holgate S T. J Immunol. 1995;155:297–307. [PubMed] [Google Scholar]
- 50.Jinquan T, Quan S, Jacobi H H, Reimert C M, Millner A, Hansen J B, Thygesen C, Ryder L P, Madsen H O, Malling H-J, et al. J Immunol. 1999;163:21–24. [PubMed] [Google Scholar]
- 51.Aharon T, Schneider R J. Mol Cell Biol. 1993;13:1971–1980. doi: 10.1128/mcb.13.3.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S W, Pawlowski J, Wathen S T, Kinney S D, Lichenstein H S, Manthey C L. Inflamm Res. 1999;48:533–538. doi: 10.1007/s000110050499. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal S, Rao A. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 54.Vrugt B, Wilson S, Underwood J, Bron A, de Bruyn R, Bradding P, Holgate S T, Djukanovic R, Aalbers R. Eur Respir J. 1999;13:1245–1252. doi: 10.1183/09031936.99.13612539. [DOI] [PubMed] [Google Scholar]
- 55.Owen W F, Rothenberg M E, Petersen J, Weller P F, Silberstein D, Sheffer A L, Stevens R L, Soberman R J, Austen K F. J Exp Med. 1989;170:343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothenberg M E, Petersen J, Stevens R L, Silberstein D S, McKenzie D T, Austen K F, Owen W F., Jr J Immunol. 1989;143:2311–2316. [PubMed] [Google Scholar]