Abstract
Two studies used puzzles that required participants to find a word that satisfied a set of constraints. The first study used a remote-association task, where participants had to find a word that would form compound words with 3 other words. The second study required participants to complete a word fragment with an associate of another word. Both studies produced distinct patterns of activity in the lateral inferior prefrontal cortex (LIPFC) and the anterior cingulate cortex (ACC). Activation in the LIPFC rose only as long as the participants were trying to retrieve the solution and dropped off as soon as the solution was obtained. However, activation in the ACC increased upon the retrieval of a solution, reflecting the need to process that solution. The data of the second experiment are fit by an information-processing model that interprets the activity in the LIPFC as reflecting retrieval operations and the activity in the ACC as reflecting subgoal setting.
Keywords: control, memory, ACT-R
Studies of cognitive neuroimaging have shown consistently that medial and lateral areas of the prefrontal cortex are active when participants are engaged in cognitively demanding tasks (1–5). However, the field is still trying to articulate the precise roles of different prefrontal regions. The current work uses event-related functional magnetic resonance imaging (fMRI) to investigate 2 particular components of cognitive demand: the need to retrieve specific information and the need to focus on specific subgoals of the task. The studies reported will test whether a region in the lateral inferior prefrontal cortex (LIPFC) reflects memory retrieval demand, and whether a region in dorsal anterior cingulate cortex (ACC) reflects subgoal setting. The studies use special properties of a class of insight problems to separate the functions of these 2 regions. We will test predictions that follow from the Adaptive Control of Thought-Rational (ACT-R) theory (6, 7).
The human prefrontal cortex is a large structure and consists of many distinct areas, both in terms of structure and function (8, 9). The LIPFC region has been associated with retrieval factors in imaging studies (10–13). It is also active in many tasks, particularly those involving language. It has been suggested (14) that the involvement of this region in such tasks can be understood in terms of retrieving the information needed to perform the tasks. Similar to this suggestion, we have proposed (15) that this region serves the role of maintaining the retrieval cues for accessing information stored elsewhere in the brain. The longer it takes to complete the retrieval successfully, the longer the cues will have to be maintained, and hence the greater the activation. Focused studies that manipulate retrieval difficulty produce systematic differences in the activation of this region. This region tends to respond to manipulations of fan or associative interference (16, 17), retention delay (18), and repetition (15). These are all factors that influence the duration of a single retrieval from declarative memory. Perhaps the major competing interpretation of this prefrontal region is that it is activated in conditions that require difficult selections among retrieved information (19, 20). On the other hand, it has been argued that these effects are due to greater retrieval demands in the more difficult conditions (13, 21). The research reported here will be relevant to adjudicating this difference.
We have proposed (3, 22) that the ACC region is responsible for setting subgoals that enable different courses of information processing to be taken when participants are in otherwise identical problem states. It thus enables internal control of cognition independently of external circumstances. The subgoals determine which branch is taken at decision points in information processing. This sense of “control” is similar to some theories of the ACC (23–25). However, other theories relate ACC activity to error detection (26, 27), response conflict (1, 28, 29), or the likelihood of an error (30). In the ACT-R theory, ACC activity reflects the effort in setting subgoals. Thus, ACC activity will reflect the number and timing of subgoal changes. Again, the research to be reported here will be relevant to distinguishing this conception from alternative theories.
Cognitively demanding tasks can be characterized as having multiple cycles of a retrieval operation followed by a subgoal setting. For example, the cognitive system may have the subgoal of solving an equation such as 2x − 3 = 5, and this will lead to retrieval of the information that 5 + 3 = 8. With the retrieval of this information, the system may change its subgoal to solving the equation to 2x = 8. This, in turn, can evoke another retrieval request (e.g., what is 8 divided by 2?). Thus, the cycle is one in which the current subgoal evokes requests for retrieval, and the retrieved information leads to a change in the subgoal. According to the ACT-R theory, the retrieval operations will be reflected in the activity of the LIPFC, and the subgoal changes in the activity of the ACC. Many researchers have noticed that these regions tend to activate together, and this is would be expected, given this information-processing cycle (5, 31, 32).
The research reported here will capitalize on a feature of certain insight puzzles that allows us to pull apart these normally highly correlated retrieval and goal activities. The first experiment will use remote-association problems (33–36). Participants saw 3 words (e.g., “pine,” “crab,” and “sauce”) and attempted to produce a single solution word (i.e., “apple”) that can form compound words with each of the hint words (i.e., “pineapple,” “crabapple,” and “applesauce”). In the ACT-R model for this task, a subgoal is set to find a solution, and the retrieval module is continuously engaged until the problem is solved. The important characteristic about these problems is that it takes a long time to retrieve a solution, if one is retrieved at all. This produces a sustained demand on the retrieval module while the subgoal module remains unchanged. However, once the problem is solved, activity will stop in the retrieval module while picking up in the goal module to set the subgoals to process the solution. Thus, ACT-R predicts a cross-over, with activation higher in the LIPFC during problem solution but higher in the ACC after problem solution. This cross-over should not be seen in trials where there is no solution.
Results
Experiment 1.
The average time for a solution was about 12 s, but there was a distribution of times with a standard deviation of more than 7 s. A response-locked analysis was used to deal with this variability. We set the scan of the response as scan 0 and focused on the 5 scans (10 s) before and the 5 scans (10 s) after. We used this scan designation to average all solution trials for each participant to get an 11-scan blood-oxygen-level-dependent (BOLD) response that began 5 scans before the response. To have a contrast with solution trials, a baseline is needed from the trials in which no solution was produced. In these trials, no response was generated during the 15-scan trial. Which scan should correspond to scan 0 in the response-locked analysis for the solution trials? We averaged these nonsolution scans together for each participant and then produced a weighted average of them to reflect a comparable set of positions for scan 0, as in the solution trials. We calculated the proportion, pn, of the responses that occurred on the nth scan from onset for solution trials. We then calculated the average baseline, Bi, for the ith scan of that participant's nonsolution trials as:
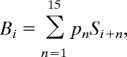 |
where i varies from −5 to 5 and Si+n is the average response during scan i + n. Thus, the distribution of scan i locations for nonsolution trials is the same as the distribution of scan i locations for solution trials.
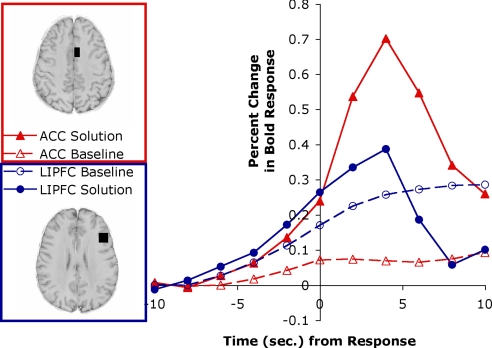
Fig. 1 compares the responses of the predefined LIPFC and ACC regions for the baseline and the solution trials. The patterns are quite different in these 2 regions. Reflecting a residual correlation with task structure, the correlation between ACC and LIPFC was a modest 0.56. Nonetheless, a 3-way ANOVA using the factors of region (LIPFC or ACC), time (before or after solution—scans from −6 to 0 s vs. scans from 4 to 10 s), and condition (solution or baseline) showed a significant 3-way interaction [F(1,19) = 52.80; P < 0.0001]. In solution trials, the activation in the LIPFC was nonsignificantly greater than the ACC before response generation [t(19) = 0.91], whereas after response generation, LIPFC showed significantly less activation than ACC [t(19) = −5.28, P < 0.001]. This is because retrieval efforts in the LIPFC cease upon finding an answer, whereas ACC activity picks up to set subgoals governing answer generation. In contrast, in nonsolution trials, LIPFC activity was greater throughout [t(19) = 2.93 before, 5.15 after; both P < 0.001], reflecting the sustained effort to retrieve an answer. All comparisons were in the predicted direction, and in 3 cases the effects were significant. The 1 nonsignificant comparison will be significant in the second experiment.
Fig. 1.
Response-locked BOLD signal in experiment 1 for the left LIPFC and the left ACC. The ACC region is a 9 × 16 × 13-mm3 region centered at −6, 10, 39 in Talairach coordinates involving Brodmann's area (BA) 24 and 32. The LIPFC is a 16 × 16 × 13-mm3 region centered at −42, 23, 24 in Talairach coordinates involving BA 9 and 46.
Experiment 2.
The differences between LIPFC and ACC in the first experiment were quite robust and qualitatively as predicted. However, the ACT-R theory does more than make qualitative predictions about the effects in these 2 regions. It makes predictions for the exact BOLD responses. Unfortunately, such predictions are difficult to test in the first experiment, with its highly variable response times. Also, the fact that a response was generated immediately upon solution raises the question of whether some effects are associated with response generation. To alleviate these difficulties, the design of the second experiment was such that it allowed more tightly distributed response times and incorporated a delay between problem solution and response generation.
This experiment involves a somewhat different word puzzle task, and so it also tests how well the results of the first experiment generalize. Participants were shown a word fragment like “-a-a-a” and an associate like hockey and were given 10 s to complete the fragment—the intended answer is “Canada.” In a behavioral pilot, participants were asked to generate the response as soon as they thought of it. They were able to solve about 32% of the problems in 10 s, and when they solved the problem, they took an average of 2.98 s, with a standard deviation of 1.77 s. Thus, although these problems took multiple seconds to solve, they were not as long or as variable as the problems in the previous experiment. The 90th percentile for solution times was 5.43 s, and the 95th percentile was 6.72 s. Therefore, we felt confident that most of the solutions would occur early in the 10-s interval before the response was required. A comparison of activity during the early part of that interval versus the later part would offer a test that was free of effects of response generation.
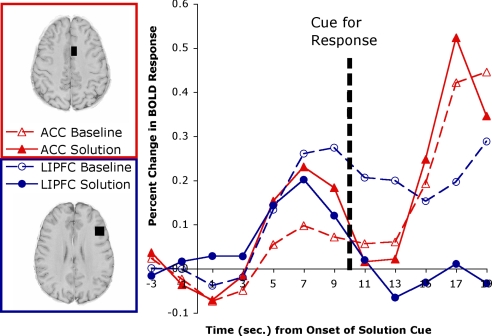
A total of 38% of the problems were solved with the hint, and 90% of these were solved with the intended word. Our analysis will compare those that were solved with the intended word with those that were not solved at all. Fig. 2 presents the results for the same 2 regions as Fig. 1. It uses as a baseline the average of the 2 scans before the appearance of the cue. It plots the percent change from this baseline for the 10 scans that involve 10 s to process the cue and the 10 s to respond and process the feedback.
Fig. 2.
Stimulus-locked BOLD signal in experiment 2 for the left LIPFC and the left ACC.
This experiment again shows major differences in the BOLD response of the LIPFC and the ACC. Reflecting a residual correlation with task structure, the correlation between the LIPFC and ACC was a rather small 0.32. To be able to compare their activity before response generation, we used scans at 1 and 3 s as reflecting presolution activity and scans at 7 and 9 s as reflecting postsolution activity. A 3-way ANOVA using the factors of region (LIPFC or ACC), time (before or after solution), and condition (solution or baseline) found a significant 3-way interaction [F(1,19) = 15.26; P < 0.001]. The pattern is very similar to experiment 1. In solution trials, the activation in the LIPFC was significantly greater than the ACC before solution [t(19) = 2.21, P < 0.05], whereas after solution, LIPFC showed nonsignificantly less activation than ACC [t(19) = −1.08]. This result replicates the direction of effects of experiment 1, although which effect was significant was reversed. In contrast, in nonsolution trials, LIPFC activity was greater throughout; in one case significantly so, and in the other case not [t(19) = 1.18 before, 3.04 after]. These are the effects predicted by the ACT-R model described below. The achievement of a solution ends retrieval and evokes subgoal-setting activity in the ACC. The failure to achieve a solution means that retrieval efforts continue in the LIPFC and produce sustained activation.
To find what other regions might be associated with solution of these problems, the fMRI data were analyzed looking for voxels that showed a significant interaction between scan and solution. Eight regions were found with 15 or more contiguous voxels that had significant interactions greater than P < 0.01 (lower bound corrected). Table 1 lists these (regions 3–10), along with the predefined ACC and LIPFC (regions 1 and 2). A very large prefrontal region was found that included most of our predefined LIPFC and much more (Table 1, region 1). Raising the significance threshold to P < 0.0025 (lower bound corrected), we were able to break this into 2 smaller regions: region 4a is higher and more posterior than our predefined region, and it is quite close to the prefrontal regions reported previously (20), whereas region 4b is lower and more anterior, between our predefined region and the anterior region discussed previously (14).
Table 1.
Regions of interest
| Region of interest | Brodmann area(s) | Voxel count | Tailarach coordinates of center, mm |
Correlation with regions |
Correlation with modules |
||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | LIPFC | ACC | Retrieval | Goal | |||
| 1. Predefined ACC | 24, 32 | 60 | −6 | 10 | 39 | 0.318 | 1.00 | 0.349 | 0.927 |
| 2. Predefined LIPFC | 9, 46 | 100 | −42 | 23 | 24 | 1.00 | 0.318 | 0.956 | 0.157 |
| 3. Supplementary motor | 8 | 32 | −5 | 15 | 50 | 0.866 | 0.697 | 0.871 | 0.529 |
| 4. Left prefrontal | 9, 46, 6 | 481 | −42 | 18 | 23 | 0.983 | 0.233 | 0.925 | 0.092 |
| 4a. Posterior prefrontal | 6 | 55 | −42 | 3 | 35 | 0.960 | 0.455 | 0.926 | 0.308 |
| 4b. Anterior prefrontal | 9/46 | 92 | −46 | 24 | 13 | 0.905 | −0.044 | 0.824 | −0.161 |
| 5. Left parietal | 7 | 35 | −23 | −56 | 44 | 0.684 | 0.820 | 0.664 | 0.763 |
| 6. Right parietal | 7 | 44 | 23 | −58 | 41 | 0.624 | 0.603 | 0.548 | 0.588 |
| 7. Left visual | 18/19 | 48 | −29 | −82 | 6 | 0.719 | 0.743 | 0.704 | 0.666 |
| 8. Right visual | 19 | 18 | 32 | −79 | 9 | 0.729 | 0.764 | 0.710 | 0.695 |
| 9. Right fusiform | 37 | 51 | 40 | −60 | −3 | 0.545 | 0.595 | 0.488 | 0.616 |
| 10. Left fusiform | 37 | 30 | −42 | −58 | −4 | 0.604 | 0.660 | 0.531 | 0.612 |
Table 1 reports the correlation of the regions with our predefined ACC and with our predefined LIPFC, as well as correlations with the predictions of the retrieval and goal modules in the ACT-R model. The left prefrontal region (region 4) and its 2 highly responsive subregions (region 4a and region 4b) all show strong correlations with the predefined LIPFC and low correlations with the predefined ACC. The 2 highly active subregions are also fairly strongly correlated with each other (r = 0.832). The posterior exploratory regions 5–10 are left and right homologues in the parietal cortex, the visual cortex, and the fusiform region. They all show modest correlations with both the LIPFC and ACC patterns and are strongly intercorrelated (r range, 0.805–0.988, mean r, 0.905).
Model of the Task.
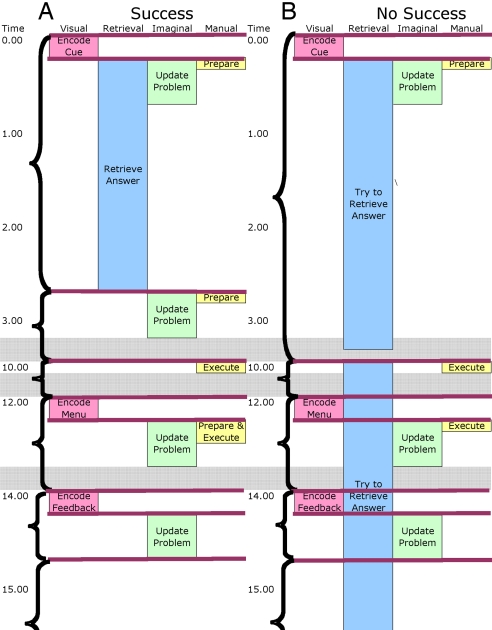
We developed an ACT-R model to perform this task that involved a minimal set of assumed processes.*Fig. 3 compares a trace of this model solving a problem with a trace of the model not solving the problem. The time is traced down the figure from presentation of the critical cue (e.g., “hockey” for “–a-a-a”) to the end of processing the feedback. The figure represents the engagement of the relevant ACT-R modules (see footnote 1 for tests of predictions of all of these modules). Our focus is on the retrieval module, whose engagement is indicated by the box in the second column, and the goal module indicated by the brackets. The brackets reflect periods of time when the goal module was dormant, operating under a single subgoal. The goal module was called upon to change goals between the bracketed periods. From the response times in the pilot experiment, we assumed a 2.5-s time period to retrieve an answer in a successful trial. We estimated that participants gave up trying to retrieve after 7 s. The other retrieval difference between success and no-success is that we assumed participants resumed their retrieval efforts when they were queried for an answer if they had not yet retrieved an answer. There was a single subgoal controlling this phase of the experiment. Upon successful retrieval of a solution, the goal state was changed, and the problem representation was updated. There were additional subgoals associated with the motor actions and encoding the feedback. The retrieval module was engaged for a much longer period on no-solution trials, whereas the goal module had to set an extra subgoal on solution trials. The observed data agree with the predictions of greater ACC activity during solution trials but greater LIPFC activity on nonsolution trials.
Fig. 3.
Engagement of the modules in the ACT-R model for experiment 2. (A) Success. (B) No success. Time is given in seconds. Lengths of boxes (for visual, retrieval, imaginal, and manual modules) reflect approximate times the modules are engaged. The horizontal lines represent the firing of production rules. Brackets indicate subtasks of activity controlled by a setting of a goal. Gray bars indicate compressed periods of time.
Beyond this general qualitative prediction, we can make predictions for the exact time course of the BOLD response. One can take a pattern of module engagement, like that in Fig. 3, and predict the BOLD response in each of the predefined regions of interest (7). From Fig. 3, one can extract for each module a demand function, d(x), which has a value of 1 when the module associated with that region is engaged and a value of 0 when it is not. Whenever there is demand for a module, this demand will drive a hemodynamic response described by b(t), which is a standard gamma function used in previous studies to represent the hemodynamic response (37–40):
In this function, m is the magnitude of the response, s is a time-scaling parameter, and a determines the steepness of the BOLD response—the greater the value of a, the steeper the function. We used values of a = 6 and s = 0.8 s for both regions but estimated different magnitude parameters for the 2 regions.
Functions d(x) and b(t) were convolved to produce the complete BOLD response function:
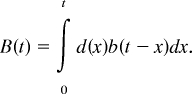 |
We fit the BOLD responses in Fig. 3 by estimating separate magnitude parameters for the ACC and LIPFC. The deviations from predictions can be evaluated according to the following χ2 statistic:
which is a sum of the squared deviations divided by the variance of the participants around that data point. There are 24 data points for each region (12 scans by success vs. no success). Because a single magnitude parameter is estimated for each region, the χ2 statistic has 23 degrees of freedom. A χ2 value greater than 36 reflects significant deviations at a threshold of P = 0.05. This does not take into account correlation in error of measurement in the BOLD function. Correcting for this correlation raises the level for a significant deviation from 36 to 52 (41).
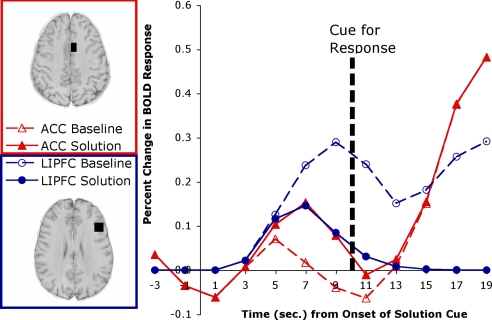
Fig. 4 shows the predicted responses for the LIPFC and the ACC. The correlation with the actual data in Fig. 2 is quite strong (r = 0.929). The χ2 measure of deviation between LIPFC and prediction is 29.44, which is within the acceptable threshold, with or without correction for correlation. The model captures the fact that the BOLD response will initially show the same rise whether there is a solution or not, but the BOLD response will drop off upon retrieval of a solution. The χ2 measure of deviation for the ACC is 45.00, which is below the corrected threshold. Interestingly, the model predicts that in the solution condition, the ACC activity will become greater than the LIPFC activity only after the response cue. We had failed to get a significant difference after solution and before response. This illustrates how a model can serve to show that a qualitative prediction from analysis of the theory (ACC activity greater than LIPFC activity after solution) may need some adjustment when exact parameters of the process are incorporated (the difference should only appear after the response cue).
Fig. 4.
Predictions for the LIPFC and ACC activity in experiment 2 based on the module engagement in Fig. 3. Compare with data in Fig. 2.
Discussion
The experiments confirmed the predicted distinct responses in the LIPFC and ACC. Activity in the LIPFC region continued only as long as the participants were trying to retrieve the solution and ceased as soon as the solution was obtained. In contrast, activation in the ACC increased upon the retrieval of a solution, reflecting the need to process that solution. There are striking similarities between the patterns found in our data and the patterns reported in studies of perceptual recognition (42, 43). Just as the difficulty of the insight problems extended the retrieval period in our research, this research extended the period of perceptual recognition by slowly revealing pictures. Regions involved in perceptual recognition, like temporal regions, became active with problem onset and stayed active until the identification of the picture. A prefrontal region very close to our predefined LIPFC was among these regions. In contrast, control regions like the ACC became active only at the point of identification. As in our experiment 2, this research showed that these patterns emerged in advance of response generation. These studies suggest that such paradigms are an excellent means of decoupling processes that are normally close together.
As discussed in the introduction, the principal alternative interpretation of this LIPFC area is that it supported selection among retrieved alternatives (19, 20). If this were the correct interpretation, activation should only have increased close in time with the identification of a solution, when there might be the need to select among alternatives. Rather, activation dropped off upon identification of a solution and continued to rise in the absence of a solution. A number of studies (14, 15, 44) have found an anterior-to-posterior gradient, with more posterior regions more sensitive to interference or competition factors. However, our exploratory study indicated a rather similar response in this task across a wide area of the left inferior prefrontal cortex. This does not seem to be a task that reflects the anterior-to-posterior gradient.
In both experiments, the ACC was more active when the problem was solved. This fact causes difficulty for alternative theories of the ACC. The fact that the ACC response was stronger in the presence of success is particularly hard to reconcile with a view that claims ACC activation is associated with errors—either the actual occurrence of errors or the likelihood of errors. With respect to the response-conflict view, one could argue that in the first experiment, because participants only generated a response if they solved the problem, response conflict could only occur in solution cases. However, this cannot be the cause of the difference in the second experiment, because the response requirements were equated for solution and no solution, and the critical contrast involved scans before the response.
Materials and Methods
Procedures for Experiment 1.
Twenty right-handed members of the Pittsburgh community (11 women) ages 18 to 32 years (mean, 23.2 years) completed the study. Participants were presented with 3 hint words that could be combined with a common word to produce a new word. For example, the words “print,” “berry,” and “bird” can all be combined with “blue” (i.e., “blueprint,” “blueberry,” “bluebird”). Participants had 30 s to determine the common word. As soon as they thought of the common word, they pressed a button on a data glove and were taken to a solution screen. They were then given 5 s in which to speak the target word. After this, they were presented with a screen that asked whether they had solved the problem with insight, and they had up to 5 s to respond. The insight screen instructed them to respond “yes” by pressing their index finger button and “no” by pressing their middle finger button. After making their insight response, they were presented with a fixation for 9.5–11.5 s (to the start of a new 2-s scan of a volume), and then a new trial began.
If unable to solve the problem in the 30 s, the participant was then taken to a screen that presented the target word as well as the 3 hint words. This screen lasted for 5 s and was followed by an 11-s fixation before the next set of hint words was presented. Participants were given instruction and 20 practice trials during structural scans. The instruction included 1 example of 3 cue words and a solution word. Participants were asked during 1 scan session to solve 63 problems, which were broken into blocks of 9–10 min. These 83 problem–solution combinations were randomly selected from a pool of 144 found in Bowden and Jung-Beeman (36).
Images were acquired by using gradient echo–echo planar image acquisition on a Siemens 3T Allegra Scanner using a standard RF head coil (quadrature birdcage), with a 2-s repetition time (TR), a 30-ms echo time, a 70° flip angle, and a 20-cm field of view. The experiment acquired 34 axial slices on each TR by using a 3.2-mm-thick, 64 × 64 matrix. The anterior commissure–posterior commissure line was on the 11th slice from the bottom scan slice.
Acquired images were analyzed by using the NeuroImaging Software (NIS; http://kraepelin.wpic.pitt.edu/nis/) system. Functional images were motion-corrected by using 6-parameter 3D registration (AIR; ref. 45). All images were then coregistered to a common reference structural MRI by means of a 12-parameter 3D registration (45) and were smoothed with an 8-mm full-width-half-maximum 3D Gaussian filter to accommodate individual differences in anatomy. Spatial F maps were generated by using random-effects ANOVA. Participants were relatively good at controlling head motion during verbal reports—10 kept their maximum movement during the experiment under 3 mm, and 8 had maximum movement under 6 mm.
Procedures for Experiment 2.
Twenty right-handed members of the Pittsburgh community (10 women) ages 19 to 30 years (mean, 22.4 years) completed the study. Participants were presented with a fragment of a word that was between 5 and 11 letters long, with approximately half of the letters replaced with hyphens, always including the first letter. The participants would then have 10 s to study this word and try to identify the word. If the participants could solve the puzzle within the 10-s period, they would press a button on the data glove and be taken to a solution screen. This first 10-s period was included to eliminate any problems that could be solved without the cue word, and our analysis is limited to the 90% of the problems not solved in this interval. If the participants could not complete the fragment, they would then be presented with both the fragment and a cue word for 10 s. After that 10 s, the participants would be asked whether they believed they knew the answer to the word fragment, which they would indicate by pressing a button on the data glove. The participants had 2 s in which to respond. Independently of how they responded, the participants would be taken to a solution screen, which presented the puzzle word along with 5 choices for its first letter. The participants would have 2 s in which to select the letter that they believed to be the first letter of the word, with 1 corresponding to the thumb button, 2 to the index finger, etc. After this, the participants were given feedback on their response, and the correct word was presented. This screen remained for 6 s before returning to the first screen with a new word to solve.
Participants were presented with 68 randomly ordered words. They solved these problems in scan blocks that lasted from 9.5 to 10 minutes. During structural scans, participants were trained both on responding with the data glove to the numbers 1–5 correctly, and they were given 10 practice problems drawn from a different set of words. The same scanning parameters were used as in the first experiment. Participants were relatively good at controlling head motion—16 kept their maximum movement during the experiment under 3 mm, and the other 4 had maximum movement under 5 mm.
Acknowledgments.
This research was supported by Institute of Education Sciences Award R305H030016 (to J.R.A.).
Footnotes
The authors declare no conflict of interest.
A running version of this model may be downloaded from http://act-r.psy.cmu.edu/models under the title of this paper.
References
- 1.Botvinick MM, Braver TS, Carter CS, Barch DM, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 2.Bunge SA, Wallis JD. The Neuroscience of Rule-Guided Behavior. New York: Oxford Univ Press; 2007. [Google Scholar]
- 3.Fincham JM, Anderson JR. Distinct roles of the anterior cingulate and prefrontal cortex in the acquisition and performance of a cognitive skill. Proc Natl Acad Sci USA. 2006;103:12941–12946. doi: 10.1073/pnas.0605493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of dorsolateral prefrontal cortex and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1837. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 5.Schneider W, Cole MW. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;16:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JR, et al. An integrated theory of Mind. Psychol Rev. 2004;111:1036–1060. doi: 10.1037/0033-295X.111.4.1036. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JR. How Can the Human Mind Occur in the Physical Universe? New York: Oxford Univ Press; 2007. [Google Scholar]
- 8.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 9.Petrides M. In: From Monkey Brain to Human Brain: A Fyssen Foundation Symposium. Dehane S, et al., editors. Cambridge, MA: MIT Press; 2005. pp. 293–314. [Google Scholar]
- 10.Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 11.Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 13.Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 14.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Danker JM, Gunn P, Anderson JR. A rational analysis of memory predicts left prefrontal activation during controlled retrieval. Cereb Cortex. 2009;18:2674–2685. doi: 10.1093/cercor/bhn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn MH, Goode A, Stenger VA, Carter CS, Anderson JR. Competition and representation during memory retrieval: Roles of the prefrontal cortex and the posterior parietal cortex. Proc Natl Acad Sci USA. 2003;100:7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn MH, et al. An information-processing model of three cortical regions: Evidence in episodic memory retrieval. NeuroImage. 2005;25:21–33. doi: 10.1016/j.neuroimage.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JR, Byrne D, Fincham JM, Gunn P. Role of prefrontal and parietal cortices in associative learning. Cereb Cortex. 2008;18:904–914. doi: 10.1093/cercor/bhm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss HE, et al. Selecting among competing alternatives: Selection and controlled retrieval in the left prefrontal cortex. Cereb Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin RC, Cheng Y. Selection demands versus association strength in the verb generation task. Psychon Bull Rev. 2006;13:396–401. doi: 10.3758/bf03193859. [DOI] [PubMed] [Google Scholar]
- 22.Sohn MH, et al. Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:10330–10334. doi: 10.1073/pnas.0703225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Esposito M, et al. The neural basis of the central executive of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 24.Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 25.Posner MI, DiGirolamo GJ. In: The Attentive Brain. Parasuraman R, editor. Cambridge, MA: MIT Press; 1998. pp. 401–423. [Google Scholar]
- 26.Falkenstein M, Hohnbein J, Hoorman J. In: Perspectives of Event-Related Potentials Research. Karmos G, Molnar M, Csepe V, Czigler I, Desmedt JE, editors. Amsterdam: Elevier; 1995. pp. 287–296. [Google Scholar]
- 27.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 28.Carter CS, et al. Parsing executive processes: Strategic versus evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 30.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 31.Cabeza R, et al. Attention-related activity during episodic memory retrieval: A crossfunction fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 32.Dosenbach NUF, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mednick SA. The associative basis of the creative process. Psychol Rev. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 34.Jung-Beeman M, et al. Neural activity when people solve verbal problems with insight. PLoS Biol. 2004;2:500–510. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kounios J, et al. The prepared mind: Neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol Sci. 2006;17:882–890. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- 36.Bowden EM, Jung-Beeman M. Normative data for 144 compound remote associate problems. Behav Res Methods Instrum Comput. 2003;35:634–639. doi: 10.3758/bf03195543. [DOI] [PubMed] [Google Scholar]
- 37.Boyton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 39.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Map. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JR, Carter CS, Fincham JM, Ravizza SM, Rosenberg-Lee M. Using fMRI to test models of complex cognition. Cogn Sci. 2009;32:1323–1348. doi: 10.1080/03640210802451588. [DOI] [PubMed] [Google Scholar]
- 42.Ploran EJ, et al. Evidence accumulation and the moment of recognition: Dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27:11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheeler ME, Petersen SE, Velanova K, Nelson SM, Ploran EJ. Identifying early and late error signals in perceptual recognition. J Cogn Neurosci. 2008;20:2211–2225. doi: 10.1162/jocn.2008.20155. [DOI] [PubMed] [Google Scholar]
- 44.Race EA, Shanker S, Wagner AD. Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21132. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intraparticipant intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]