The oceans account for approximately half of global carbon fixation (1), but unlike plant-dominated terrestrial environments, marine photosynthesis is dominated by single-celled microbes, or phytoplankton. These phytoplankton are the engines that drive marine food webs and biogeochemistry. Among the vast variety of phytoplankton found in the open ocean, the non-nitrogen-fixing cyanobacterium Prochlorococcus is the most numerically abundant (2). Thus, understanding the diversity, physiology, and ecology of Prochlorococcus is critical to describing and predicting the biological processes and patterns that originate at the base of the marine food web and their implications for carbon dioxide uptake and climate variability. In this issue of PNAS, Martiny et al. (3) provide compelling evidence that some strains of Prochlorococcus can use nitrate (NO3−), which is the most abundant form of fixed nitrogen in the oceans and often limits production, further expanding the importance of this tiny organism.
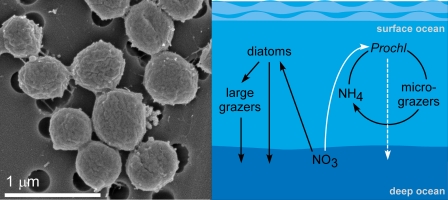
As the smallest known marine phytoplankton, Prochlorococcus has a streamlined genome of roughly 2,000 genes (4), unlike eukaryotic algae, which can have well over 10,000 genes (5). But the tradeoff for this simple complement of genes is a reduced metabolic potential, limiting the ability to use some nitrogen compounds. Nevertheless, Prochlorococcus dominates in most tropical and subtropical open ocean environments because its small diameter and large surface area to volume ratio affords a competitive advantage for the limited light and nutrient resources (Fig. 1Left). The nitrogen requirement for this growth is largely supplied by urea [(NH2)2CO] and ammonium (NH4+), which in turn are largely generated either directly or indirectly (mediated by bacterioplankton) by the small protozoa grazers that feed on Prochlorococcus. Thus the growth of Prochlorococcus is part of a tightly coupled loop dependent on recycled nitrogen (Fig. 1 Right).
Fig. 1.
Prochlorococcus is the smallest marine phytoplankton and important to the marine nitrogen cycle. (Left) Scanning electron micrograph of Prochlorococcus (strain MIT9312). (Right) Conceptual diagram of the uptake of some major nitrogen compounds by various phytoplankton groups and their contribution to export production. Diatoms and Synechococcus, among other types of phytoplankton, can use new NO3− and therefore contribute substantially to the draw-down of carbon dioxide from the surface ocean (and atmosphere). Prochlorococcus was thought to be in a tightly coupled cycle living only on NH4+ and urea, which is supplied directly or indirectly (by bacterioplankton) by micrograzers. However, Martiny et al. (3) provide strong evidence that Prochlorococcus can also use NO3− and therefore may contribute more substantially to carbon export from surface waters.
In certain areas, ocean currents bring new nitrogen in the form of NO3− to the upper sunlit waters, stimulating photosynthesis and primary production. However, this new nitrogen was thought to be unavailable to Prochlorococcus because none of the genome sequences from laboratory strains contain the single gene (assimilatory nitrate reductase) necessary for nitrate utilization. But other, larger types of prokaryotic and eukaryotic phytoplankton, including Synechococcus and diatoms, do have this gene and therefore are able to bloom. Although Prochlorococcus establishes the baseline of primary production driven by recycled NH4+ and urea, only the phytoplankton that can use NO3− such as diatoms can respond to the pulses of high-nitrogen waters, ensuring that they can take advantage of these nitrogen oases.
However, Martiny et al. (3) use an array of metagenomic sequence data from the Global Ocean Survey (6) representing many different oceanographic regions, to provide the first evidence that some types of Prochlorococcus may contain the nitrate reductase gene (narB). Using field RNA samples, they further demonstrate that this putative Prochlorococcus gene is expressed, strongly suggesting that Prochlorococcus has the ability to use NO3−. Although preliminary, this finding has two substantial implications for our understanding of the functioning of the oceans.
The ability to use NO3− alters our conception of how Prochlorococcus fits into the marine food web and in particular what limits and regulates its growth. Although it may use NO3−, Prochlorococcus does not dominate in oceanic regions with high NO3− concentrations such as coastal upwelling, open ocean upwelling (e.g., eddies or equatorial regions), or high latitudes. These observations suggest that other factors control Prochlorococcus growth and biomass in these areas. These factors may include both “bottom-up” processes such as other nutrient limitation (non-nitrogen), allelopathic interactions from other microbes, or competition from faster growing NO3−-using phytoplankton (e.g., Synechococcus, diatoms) and “top-down” processes such as tightly controlled grazing by their protistan predators. Evidence from nutrient addition experiments suggests that Prochlorococcus populations are tightly regulated by grazers, but there is support for bottom-up regulation of these populations as well (7). Interestingly, Synechococcus, which is a close cyanobacterial cousin of Prochlorococcus, utilizes NO3− and does bloom in some of these regions. Although Synechococcus and Prochlorococcus have many similarities in their genomes, cell properties (e.g., size), and physiologies and therefore have overlapping habitats (8), something unique about Prochlorococcus prevents it from flourishing in these high-NO3− waters.
The second implication of this finding is that Prochlorococcus may be more important in exporting carbon from the upper ocean than originally thought. In the tightly coupled growth and grazing cycle driven by NH4+ and urea, nutrients and carbon are recycled continuously in the upper ocean (Fig. 1 Right). Although carbon dioxide is quickly consumed by the phytoplankton, this uptake is balanced by losses to grazing and release to the upper ocean (and atmosphere). NO3− can also be involved in this fast spinning cycle when microbes convert NH4+ to NO3− through a process called nitrification. In nutrient-poor regions of the oceans, approximately half of the NO3− consumed by phytoplankton is recycled through this process (9), thus the ability to use NO3− gives Prochlorococcus access to another part of this rapidly cycling nitrogen pool. But in systems where new nitrogen (such as NO3−) is added, a net export of carbon from the surface ocean can occur, if biomass is removed from the surface layers via direct sinking or packaging of cells in dense zooplankton fecal pellets. This so-called export production (or biological pump that draws down carbon dioxide from the atmosphere into the ocean) was thought to be largely driven by large, fast-sinking algae, but more recent evidence suggests that small cells, such as Prochlorococcus, may be equally important in this process that is significant for the global climate (10). Additive tracer experiments have shown that natural populations of Prochlorococcus can take up NO3− and that it significantly contributes to new production (10). Because the potential of Prochlorococcus to use NO3− has only recently been demonstrated, its contribution to new and export production has not historically been included in models of open ocean marine ecosystems (Fig. 1 Right). Incorporating this finding into these models has the potential to revise and possibly increase our estimate of the contribution of marine photosynthesis to carbon uptake by the oceans.
Some types of Prochlorococcus may contain the nitrate reductase gene (narB).
With the metagenomic data presented in Martiny et al. (3) there are now two compelling and complementary lines of evidence that Prochlorococcus can use NO3− (3, 11), yet none of the ≈50 strains isolated to date has been shown to contain the narB gene. Nevertheless, we already know that the genus Prochlorococcus comprises several different genotypes that can differ in their gene complement and physiology (12) and ecologies (13), each exhibiting its own niche differentiation. Inventories of the known ecological types (or ecotypes) show that the culture collections do not account for all of the Prochlorococcus found in the oceans (14), and this discrepancy is most acute deeper in the water column. This depth is exactly where new NO3− fluxes are highest and narB-containing Prochlorococcus would be expected to be found. In addition to targeting isolates of NO3−-assimilating Prochlorococcus from these areas, direct quantification of the contribution of Prochlorococcus to new (and export) production should be pursued. More challenging, but equally important, areas of future study will be to determine what limits Prochlorococcus growth in areas of high NO3− and why it does not participate in the bloom dynamics characteristic of many other NO3−-using phytoplankton.
Footnotes
The authors declare no conflict of interest.
See companion article on page 10787.
References
- 1.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 2.Partensky F, Hess WR, Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev. 1999;63:106–127. doi: 10.1128/mmbr.63.1.106-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martiny AC, Kathuria S, Berube PM. Widespread metabolic potential for nitrite and nitrate assimilation among Prochlorococcus ecotypes. Proc Natl Acad Sci USA. 2009;106:10787–10792. doi: 10.1073/pnas.0902532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kettler GC, et al. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet. 2007;3:e231. doi: 10.1371/journal.pgen.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armbrust EV, et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science. 2004;306:79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- 6.Rusch DB, et al. The Sorcerer II Global Ocean Sampling Expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 2007;5:e77. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landry MR, et al. Iron and grazing constraints on primary production in the central equatorial Pacific: An EqPac synthesis. Limnol Oceanogr. 1997;42:405–418. [Google Scholar]
- 8.Palenik B, et al. The genome of a motile marine Synechococcus. Nature. 2003;424:1037–1042. doi: 10.1038/nature01943. [DOI] [PubMed] [Google Scholar]
- 9.Yool A, Martin AP, Fernandez C, Clark DR. The significance of nitrification for oceanic new production. Nature. 2007;447:999–1002. doi: 10.1038/nature05885. [DOI] [PubMed] [Google Scholar]
- 10.Richardson TL, Jackson GA. Small phytoplankton and carbon export from the surface ocean. Science. 2007;315:838–840. doi: 10.1126/science.1133471. [DOI] [PubMed] [Google Scholar]
- 11.Casey JR, Lomas MW, Mandecki J, Walker DE. Prochlorococcus contributes to new pro-duction in the Sargasso Sea deep chlorophyll maximum. Geophys Res Lett. 2007;34:L10604. doi: 10.1029/206GL-028725. [DOI] [Google Scholar]
- 12.Rocap G, et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature. 2003;424:1042–1047. doi: 10.1038/nature01947. [DOI] [PubMed] [Google Scholar]
- 13.Johnson ZI, et al. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science. 2006;311:1737–1740. doi: 10.1126/science.1118052. [DOI] [PubMed] [Google Scholar]
- 14.Zinser ER, et al. Prochlorococcus ecotype abundances in the North Atlantic Ocean revealed by an improved quantitative PCR method. Appl Environ Microbiol. 2006;72:723–732. doi: 10.1128/AEM.72.1.723-732.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]